Abstract
Gastric cancer (GC) is among the most common types of human cancer and is associated with recurrence and metastasis, despite comprehensive surgical and medical treatment. Previous studies observed downregulation of T-cadherin expression in GC tissues, suggesting that this protein may act as an oncosuppressor. The current study investigated the activity of T-cadherin in GC tissues. In a follow-up study of 81 patients with GC, a Kaplan-Meier analysis of overall survival revealed a strong association of T-cadherin overexpression with increased overall survival (P<0.01). Furthermore, stable T-cadherin-overexpressing cell lines were established from HGC-27 cells via transfection of a pcDNA3.1-T-cadherin plasmid and in vitro growth and cell cycle of these cells were measured using MTT and flow cytometry assays, respectively. MTT assays revealed that proliferation of engineered T-cadherin-overexpressing cells was significantly inhibited and flow cytometry demonstrated that T-cadherin overexpression in HGC-27 cells induced cell cycle arrest in the G0/G1 phase. Transwell assays demonstrated that T-cadherin-overexpressing HGC-27 cells exhibited reduced invasiveness and metastatic potential. Phosphorylated (p)-protein kinase B (AKT) and p-mammalian target of rapamycin (mTOR) protein levels were reduced in T-cadherin overexpressing HGC-27 cells, suggesting that the AKT/mTOR signaling pathway was involved in the gastric tumor inhibitory effect of T-cadherin. Administration of AKT-activator, insulin-like growth factor-1, to T-cadherin-overexpressing HGC-27 cells significantly affected the proliferation phenotype. In conclusion, the current study provided clinical evidence and revealed a potential mechanism supporting that T-cadherin inhibits gastric tumorigenesis through inhibition of the AKT/mTOR signaling pathway.
Keywords: gastric cancer, T-cadherin, overexpression, migration, invasion
Introduction
Gastric cancer (GC) is one of the most common types of cancer and the second most common cause of cancer-associated mortalities worldwide (1). In certain cases, recurrence and metastasis may occur despite comprehensive surgical and medical treatment (2). Although previous studies of GC tumorigenesis demonstrated close associations between overexpression of oncogenes/oncoproteins and inactivation of anti-oncogenes/anti-oncoproteins (3), the underlying mechanisms remain to be investigated. Exploration of molecular mechanisms, prognosis and rational clinical/medical treatments are important for the discovery of new genes involved in GC development and behavior and for improvements in treatment.
Members of the cadherin superfamily of cell adhesion factors are expressed as cell surface glycoproteins and regulate calcium-mediated cell adhesion, influence cell polarity and morphogenesis and direct cell recognition and signal transduction mechanisms (4). Classical cadherins, including E- and N-cadherin, comprise an extracellular calcium-binding domain and a transmembrane domain (5). By contrast, the non-classical truncated (T)-cadherin lacks the transmembrane domain and binds cytomembranes via glycosyl-phosphatidyl inositol (GPI) (6). Previous studies have reported associations between deletion or mutation of classical cadherins and proliferation, migration and invasion of GC, breast cancer and lung cancer cells (7–10). Tryndyak et al (11) observed that transfection of tumor cells with E-cadherin decreased proliferation and invasiveness significantly. Ivanov et al (12) revealed that T-cadherin upregulation correlates with cell cycle progression and promotes proliferation of vascular cells. Notably, T-cadherin downregulation was observed in GC (13), breast cancer (14), lung cancer (15), colon cancer (16), skin squamous carcinoma (17) and other types of cancer (18), suggesting a potential role as an anti-oncoprotein.
Defects, including aberrant promoter methylation and improper histone modification of CDH13, which encodes T-cadherin, may contribute to downregulation of protein expression (16,19). A study on non-small cell lung cancer cell lines and tumor tissues revealed that T-cadherin deletion increased tumorigenicity (15). Furthermore, a defect in CDH13 was demonstrated to promote tumor progression in human prostate cancer cells, whereas restoration of T-cadherin expression inhibited both cell proliferation and invasion (20). In neuroblastoma, transduction with CDH13 was revealed to inhibit tumor growth by reducing endothelial growth factor receptor expression (21). An in vitro study by Lee (22) demonstrated that transduction of CDH13 cDNA into breast cancer cells reduced growth and invasiveness of tumor cells. Furthermore, tumor volumes observed in mice implanted with T-cadherin-overexpressing MCF-7 human breast cancer cells were significantly reduced, suggesting that T-cadherin expression inhibits tumorigenesis in vivo (22,23). In a previous study, it was demonstrated that mRNA levels and T-cadherin protein expression were significantly downregulated in GC tissues compared with adjacent noncancerous tissues, suggesting that T-cadherin may be important in GC cell proliferation and metastasis and serve as a target for treatment of GC (24).
The current study aimed to investigate functions and underlying mechanisms of T-cadherin and to provide a basis for usage of this protein in clinical diagnosis and treatment of GC. A 5-year follow-up study of survival among patients with GC was conducted to determine the association between T-cadherin expression and GC prognosis. A T-cadherin-overexpressing cell line was generated from HGC-27 cells and used to investigate associations between T-cadherin expression and GC cell proliferation, invasiveness and metastasis.
Materials and methods
Patients
Eighty-one patients with Stage I–III GC who underwent surgical treatment at the Department of Surgical Oncology, Second Affiliated Hospital of Fujian Medical University (Quanzhou, China) between August 2011 and August 2015, were followed for 2–60 months. Overall survival was estimated using the Kaplan-Meier method, as described in a previous report (25). T-cadherin-negative disease was defined as the tissue section exhibiting ≤10% or no positive cancer cells. Patients included 49 males and 32 females with a mean age of 62.5±18.6 years (range, 26–76 years). All experiments were performed in accordance with the relevant guidelines and written informed consent was obtained from all patients. Study protocols were approved by the Ethical Committee of the Second Affiliated Hospital of Fujian Medical University.
HGC-27 cell culture and transfection
Human gastric carcinoma cell line HGC-27 was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), penicillin (100 U/ml) and streptomycin (100 µg/ml) and incubated in a 5% CO2 incubator at 37°C.
Plasmids and cell transfection
The plasmids for pcDNA3.1 (400 ng/µl) and pcDNA-T-cadherin (400 ng/µl; pcDNA-Tcad; Invitrogen; Thermo Fisher Scientific, Inc.) were purchased and respectively transfected into HGC-27 cell lines using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Cell transfection efficiency was determined as the percentage of fluorescent cells among total cells in ten regions, using a 200-fold phase contrast fluorescence microscope. Two weeks following transfection, positive colonies (with GFP expression) were obtained and cells were resuspended in RPMI-1640 medium and cultured to yield T-cadherin overexpression cells. Cells transfected with pcDNA3.1 (400 ng/µl; empty vector) were used as negative controls. Untransfected HGC-27 cells were used as blank controls.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA from transfected HGC-27 cells following 24 h incubation according to the manufacturer's protocol. RNA was quantified spectrophotometrically as ratio of absorbance at 260 over 280 nm. cDNA was synthesized from total RNA using the TIANScriptII cDNA first chain synthesis kit (Tiangen Biotech, Co., Ltd., Beijing, China) according to the manufacturer's protocol. Primers for qPCR are listed in Table I. RT-qPCR was performed using a PCR-iQ5 detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with a SYBR-Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.; 4309155). The thermocycling conditions were as follows: 1 min at 95°C, followed by 40 cycles of 95°C for 5 sec and 60°C for 20 sec. Gene expression was normalized to the expression of β-actin using the 2−ΔΔCq method (26). Each experiment was performed in triplicate.
Table I.
Primer sequences.
| Name | Sequence (5′-3′) |
|---|---|
| T-cadherin_Forward | GATGTTGGCAAGGTAGTCGAT |
| T-cadherin_Reverse | GCTCCCTGTGTTCTCATTGAT |
| β-actin_Forward | GACGATATCGCTGCGCTG |
| β-actin_Reverse | GTACGACCAGAGGCATACAGG |
Western blotting
Proteins were extracted from tissue samples according to the manufacturer's protocol in HGC-27 cells using the Total Protein Extraction kit (BestBio, Co., Shanghai, China) and were separated on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes, which were subsequently blocked with 5% skimmed milk for 1 h at 37°C. Membranes were incubated overnight at 4°C with rabbit antibodies specific for phosphorylated (p)-S6K (1:1,000; ab32529; Abcam), β-actin (1:5,000; ab6276; Abcam), anti-p-protein kinase B (AKT; 1:1,000; 9271; Cell Signaling Technology, Inc.), anti-AKT (1:1,000; 9272; Cell Signaling Technology, Inc.), anti-mammalian target of rapamycin (mTOR; 1:1,000; 2972; Cell Signaling Technology, Inc.) or anti-p-mTOR (1:1,000; 5536; Cell Signaling Technology, Inc.). Following washing, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:3,000; ab6721; Abcam). Membranes were washed, incubated for 2 h at room temperature with an enhanced chemiluminescence substrate (Abcam) and analyzed. To quantify, signal intensities of specific bands were measured using Image Lab 3.0 software (Bio-Rad Laboratories, Inc.).
Flow cytometry analysis of cell cycle
HGC-27 cells transfected with pcDNA3.1-Tadherin or pcDNA3.1 were harvested following 24 h transfection by trypsinization, fixed and permeabilized at 4°C for 30 minusing Cytofix/Cytoperm™ Fixation/Permeabilization Solution kit (BD Biosciences, San Jose, CA, USA) and stored at 4°C. At the time of analysis, cells were stained with propidium iodide (Sigma-Aldrich, Merck KGaA) at room temperature for 15 min and analyzed on a flow cytometer (Aria II, BD Biosciences). Data were analyzed using FlowJo Software version 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Invasion and migration assay
pcDNA3.1-Tadherin- and pcDNA3.1-transfected HGC-27 cells were harvested in the logarithmic growth phase following 24 h transfection and resuspended at a density of 5×10 cells/ml in RPMI-1640 serum-free medium. Matrigel matrix (BD Biosciences) was used to coat 24-well invasion chambers and rehydrated with serum-free culture medium for 30 min at 37°C prior to adding 200 µl of cell suspension to upper chambers and 600 µl of RPMI-1640 medium with 10% FBS to lower chambers. Plates were incubated for 22 h at 37°C. Following discarding of excess matrix and non-invading cells, cells that had invaded the Matrigel were fixed with 95% ethanol and stained with crystal violet at room temperature for 10 min according to the manufacturer's instructions (BD Biosciences). Invaded and migrated cells were observed using a microscope (magnification, ×10). Cell migration assays were performed using the same procedure in the absence of Matrigel coating.
Cell proliferation assay
A suspension of HGC-27 cells transfected with pcDNA3.1-Tadherin and pcDNA3.1 (200 µl; density, 1×104/ml) were added to each well of a 96-well plate. Following a 24-h incubation at 37°C, cell viability was quantified every day over 5 days using an MTT assay (American Type Culture Collection, Manassas, VA, USA). MTT reagent (10 µl) was added to each well and cells were incubated at room temperature in the dark for 2 h. Detergent reagent (100 µl, American Type Culture Collection) was added to each well and following an additional 2-h incubation at room temperature, absorbance at 570 nm was measured using a microplate reader. Insulin-like growth factor-1 (IGF-1) was added to the RPMI-1640 medium. The growth inhibition rates were defined as the OD negative value-OD positive value/OD value negative.
Statistical analysis
All data are presented as the mean ± standard deviations of at least five independent repeats. Survival rates of patients with GC were estimated using the Kaplan-Meier method and differences in survival were compared using the log-rank test. Data were analyzed by analysis of variance (ANOVA) using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). In the event that ANOVA justified post hoc comparisons, these were conducted using Neuman-Keuls or Tukey's multiple-comparisons test. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between T-cadherin expression and survival
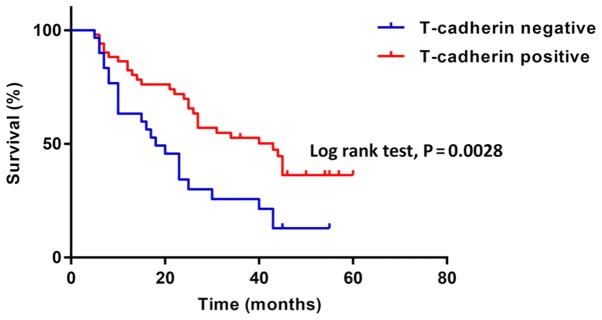
To investigate how T-cadherin expression affected the prognosis of patients with GC, the Kaplan-Meier method was used to evaluate the association of overall survival and T-cadherin expression levels (Fig. 1). A total of 81 patients with GC, including 30 with T-cadherin-negative disease (≤10% or no positive cancer cells in tissue sections) and 51 with T-cadherin-positive disease (>10% positive cancer cells), were followed for 2–60 months. The T-cadherin-negative group had a significantly worse prognosis compared with the T-cadherin-positive group (median survival: 18 months vs. 43 months, P<0.05).
Figure 1.
T-cadherin expression influences the prognosis of patients with gastric cancer. Kaplan-Meier survival curves of patients with gastric cancer. Five-year survival rates of T-cadherin-negative and positive patients are presented in blue and red, respectively (log-rank test, P=0.0028).
Effect of T-cadherin on cell growth
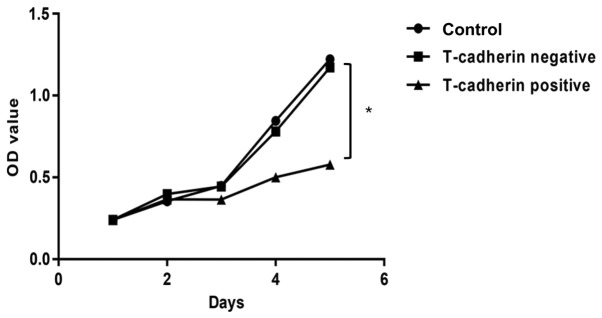
To assess roles of T-cadherin in GC cells, a stable T-cadherin-overexpressing HGC-27 cell line was established and T-cadherin expression was confirmed using RT-qPCR (data not shown). T-cadherin expression increased in cells transfected with pcDNA3.1-Tadherin but not in cells transfected with empty pcDNA3.1. An MTT cell proliferation assay was conducted to investigate the effect of T-cadherin on HGC-27 cell growth. Growth curves demonstrated that T-cadherin-overexpressing cells exhibited significant growth suppression compared with cells transfected with empty plasmid, with growth inhibition rates of 31.09% at 5 days post-transfection (Fig. 2).
Figure 2.
Growth inhibition in T-cadherin-overexpressing HGC-27 cells. T-cadherin-overexpressing cells were established by transfecting HGC-27 cells with pcDNA3.1-Tadherin plasmid. Cells transfected with empty pcDNA3.1 vector were used as T-cadherin-negative group. Untransfected HGC-27 cells served as blank controls. MTT assay-derived growth curve was plotted using absorbance determined at 570 nm. *P<0.05 vs. T-cadherin-negative group; n=5. OD, optical density.
Effect of T-cadherin on cell cycle
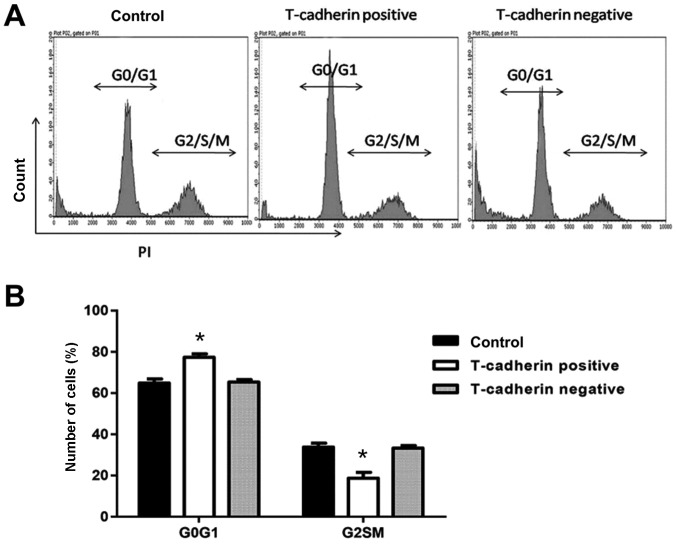
The effect of T-cadherin on the cell cycle of HGC-27 cells was determined using flow cytometry. Of HGC-27 cells transfected with pcDNA3.1-Tadherin, 77.4% remained in the G0/G1 phase, an increased percentage compared with cells transfected with empty vector (65.3%). Furthermore, the percentage of T-cadherin-overexpressing cells in the S/G2/M phase decreased significantly to 18.7%, compared with 33.2% for vector-transfected cells (P<0.05; Fig. 3), suggesting that T-cadherin overexpression induced cell cycle arrest in the G0/G1 phase of HGC-27 cells.
Figure 3.
T-cadherin-overexpressing HGC-27 cells arrest in the G0/G1 phase. HGC-27 cells transfected with pcDNA3.1-Tadherin and pcDNA3.1 were considered T-cadherin-positive and negative groups, respectively. Untransfected HGC-27 cells served as blank controls. (A) Representative flow cytometry plots of the cell cycle. (B) Percentages of cells in G0/G1 and G2/S/M phases. *P<0.05 vs. T-cadherin-negative group; n=5.
Effect of T-cadherin on cell invasion and migration
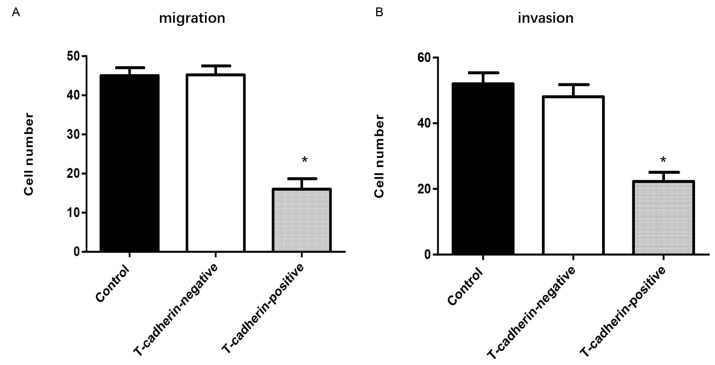
To examine whether T-cadherin overexpression may inhibit cell mobility, a Transwell migration assay was conducted. Significantly fewer T-cadherin-overexpressing HGC-27 cells migrated compared with empty vector-transfected cells (P<0.05; Fig. 4A). An invasion assay yielded a similar trend, with a 64.6% reduction in invasiveness among T-cadherin-overexpressing HGC-27 cells compared with control and vector-transfected cells (P<0.05; Fig. 4B). These findings suggest that T-cadherin ameliorated malignant phenotypes of HGC-27 cells by inhibiting cell migration and invasion.
Figure 4.
T-cadherin overexpression inhibits HGC-27 cell invasion and migration. HGC-27 cells transfected with pcDNA3.1-Tadherin and pcDNA3.1 were designated as T-cadherin-positive and negative groups, respectively. Untransfected HGC-27 cells served as blank controls. (A) Number of migrated cells in each high-power field (magnification, ×200). (B) Number of invaded cells in each high-power field (magnification, ×200). *P<0.05 vs. T-cadherin-negative group; n=5.
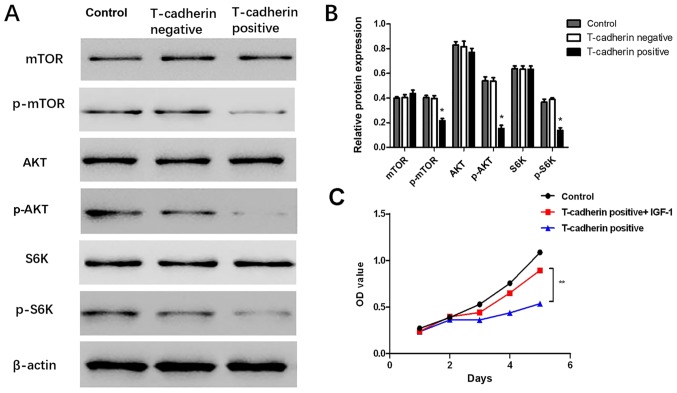
T-cadherin overexpression inhibits AKT/mTOR activity
To uncover potential mechanisms for T-cadherin-associated regulation of GC, western blot assays were performed to validate whether AKT and its downstream targets were altered in response to T-cadherin overexpression. The results demonstrated that levels of p-AKT, p-mTOR and p-S6K were significantly decreased in T-cadherin-overexpressing HGC-27 cells (P<0.05; Fig. 5A and B), suggesting that T-cadherin expression may regulate AKT/mTOR signaling pathway activities. The current study further investigated whether effects associated with T-cadherin overexpression may be reversed by administration of insulin-like growth factor-1 (IGF-1), an AKT-activator. It was observed that cell viability was partly restored when IGF-1 was added to the culture medium (Fig. 5C). These results suggested T-cadherin overexpression suppressed gastric tumorigenesis potentially through inhibition of the AKT/mTOR signaling pathway.
Figure 5.
T-cadherin overexpression inhibits the AKT/mTOR signaling pathway. HGC-27 cells transfected with pcDNA3.1-Tadherin and pcDNA3.1 were designated as T-cadherin-positive and negative groups, respectively. Untransfected HGC-27 cells served as blank controls. (A) Western blot analysis of AKT, mTOR and S6K and their phosphorylation products. (B) Relative protein expression determined vs. β-actin. (C) Effect of AKT pathway activator IGF-1 on cell proliferation phenotypes determined by MTT assay. *P<0.05 vs. T-cadherin-negative group, **P<0.05 vs. T-cadherin-positive group; n=5. AKT, protein kinase B; mTOR, mammalian target of rapamycin; S6K, ribosomal protein s6 kinase; p-, phosphorylated; IGF-1, insulin-like growth factor-1; OD, optical density.
Discussion
The current study focused on T-cadherin, the only cadherin known to be membrane-anchored via a GPI anchor rather than a transmembrane domain (23). Previous studies have described the human CDH13 gene to be an anti-tumor gene, as its expression is suppressed in several types of cancer (16,27,28). T-cadherin has been reported to inhibit bladder tumor cell proliferation, invasion and angiogenesis, whereas reduced T-cadherin was associated with a poor prognosis among patients with bladder cancer (29–31). However, few studies have reported associations between T-cadherin expression and clinicopathological features in GC.
In a previous study on the biological activity of T-cadherin in GC, it was reported that mRNA levels and T-cadherin protein expression were significantly downregulated in GC tissues compared with adjacent noncancerous tissues (24). Another study observed that downregulation of T-cadherin in tumor correlated with larger tumor size (diameter >4 cm), invasiveness, poor differentiation, lymph node metastasis and higher TNM stage (25). The current study revealed that T-cadherin expression was associated with overall survival in a follow-up study of 81 patients. Patients with high T-cadherin expression levels exhibited a significantly higher postoperative survival rate compared with patients with low T-cadherin levels, suggesting that T-cadherin may be useful as a therapeutic target and indicator of GC prognosis.
Previous studies on the effect of T-cadherin on cell growth reported cell type-dependent outcomes (32,33). Small interfering RNA-mediated silencing of T-cadherin expression had no significant effect on growth of Mahlavu hepatocellular carcinoma cells (34,35). However, Huang et al (36) demonstrated that T-cadherin inhibited growth of C6 glioma cells by increasing cell attachments to fibronectin and decreasing cell mobility. Similar to this, the current study revealed that T-cadherin overexpression inhibited growth of HGC-27 cells and induced G2 phase arrest during cell cycle, with a corresponding increase in the G0/G1 phase. In addition, T-cadherin overexpression significantly inhibited MGC8-03 and AGS GC cell growth, migration and invasion (24), suggesting that T-cadherin exerts antiproliferation activity in different GC cell lines. HGC-27 was established through culturing of metastatic lymph node cells from a patient with GC diagnosed histologically as undifferentiated carcinoma (37). The current study suggested T-cadherin downregulation may be a risk factor for lymph node metastasis in GC.
T-cadherin negatively regulated squamous cell carcinoma growth by regulating cell adhesion to the extracellular matrix and β1 integrins and inhibiting epidermal growth factor receptor phosphorylation to reduce invasiveness (17). Invasiveness and metastasis are important biological characteristics of malignancies that affect disease recurrence and influence the prognosis of cancer patients (38). In the present study, the results of Transwell assays on migration and invasion revealed that T-cadherin overexpression significantly decreased both characteristics in HGC-27 cells. In other words, T-cadherin may promote overall survival in patients with GC by partial inhibition of tumor cell invasion and metastasis. These results were consistent with findings of previous studies. Yan et al (35) indicated that cell proliferation decreased in HepG2 cells expressing high levels of T-cadherin. Philippova et al (17) observed an increase in squamous cell carcinoma invasion and metastasis in the absence of T-cadherin and Hebbard et al (39) reported that loss of T-cadherin promoted tumor angiogenesis and metastasis in breast cancer.
It is well known that AKT/mTOR signaling serves a critical role in tumor development and progression (40,41). The current study determined effects of T-cadherin on AKT/mTOR signaling in HGC-27 cells. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 (9). The current study confirmed that T-cadherin overexpression decreased p-AKT, p-mTOR and p-S6K expression in HGC-27 cells, when compared with blank and negative control cells, but did not affect AKT, mTOR and S6K. Additionally, AKT-activator IGF-1 significantly inhibited the suppressive role of T-cadherin overexpression in HGC-27 cells, suggesting that AKT/mTOR may act as downstream signaling mediator of T-cadherin. A previous study reported that T-cadherin overexpression suppressed GC cell migration and invasion by upregulating E-cadherin expression and downregulation of vimentin and matrix metalloproteinase-2 expression (10). The current study investigated effects of AKT/mTOR signaling in HGC-27 cells regulated by T-cadherin, however, the mechanisms by which T-cadherin influences the AKT/mTOR signaling pathway require further investigation. Luciferase and pull down assays may be performed to demonstrate whether T-cadherin directly or indirectly regulates downstream markers.
In conclusion, the present study provided evidence for the role of T-cadherin in GC tumorigenesis. It demonstrated that overall survival was associated with T-cadherin overexpression. Furthermore, T-cadherin overexpression significantly inhibited HGC-27 cell proliferation and led to cell cycle arrest in the G0/G1 phase. It was further demonstrated that T-cadherin-overexpressing HGC-27 cells exhibited reduced invasiveness and metastatic potential. Studies of the molecular mechanism suggested that T-cadherin regulated AKT/mTOR signaling pathway proteins and their downstream mediators. Administration of AKT-activator IGF-1 in T-cadherin-overexpressing HGC-27 cells restored the proliferation phenotype. Based on these results, it is suggested that T-cadherin may be a novel target for therapeutic intervention of GC.
Acknowledgements
Not applicable.
Funding
The study was supported by the Fujian Natural Science Foundation (grant no. 2015J01439).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL conceived, designed and performed experiments, analyzed data and prepared the manuscript. ZC conceived and designed experiments, analyzed data and prepared the manuscript. ZH, FC, ZY, SL and WW performed experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University (Quanzhou, Fujian, China) and all patients agreed to participate in the study.
Patient consent for publication
All patients provided their informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, Inoue M. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17:4421–4428. doi: 10.3748/wjg.v17.i39.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Yang J, Wang J, Guo H, Jing N. LMO1 is a novel oncogene in lung cancer, and its overexpression is a new predictive marker for anti-EGFR therapy. Med Oncol. 2014;31:99. doi: 10.1007/s12032-014-0099-0. [DOI] [PubMed] [Google Scholar]
- 4.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: Diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 5.Dasen B, Vlajnic T, Mengus C, Ruiz C, Bubendorf L, Spagnoli G, Wyler S, Erne P, Resink TJ, Philippova M. T-cadherin in prostate cancer: Relationship with cancer progression, differentiation and drug resistance. J Pathol Clin Res. 2016;3:44–57. doi: 10.1002/cjp2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philippova M, Joshi M, Kyriakakis E, Pfaff D, Erne P, Resink T. A guide and guard: The many faces of T-cadherin. Cell Signal. 2009;21:1035–1044. doi: 10.1016/j.cellsig.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 8.Fedor-Chaiken M, Hein PW, Stewart JC, Brackenbury R, Kinch MS. E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes. 2003;10:105–118. doi: 10.1080/cac.10.2.105.118. [DOI] [PubMed] [Google Scholar]
- 9.Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–314. doi: 10.1016/S1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 10.Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 11.Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov D, Philippova M, Allenspach R, Erne P, Resink T. T-cadherin upregulation correlates with cell-cycle progression and promotes proliferation of vascular cells. Cardiovasc Res. 2004;64:132–143. doi: 10.1016/j.cardiores.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Dai Y, Huo J. Decreased expression of T-cadherin is associated with gastric cancer prognosis. Hepatogastroenterology. 2012;59:1294–1298. doi: 10.5754/hge12016. [DOI] [PubMed] [Google Scholar]
- 14.Kong DD, Yang J, Li L, Wang W, Chen YN, Wang SB, Zhou YZ. T-cadherin association with clinicopathological features and prognosis in axillary lymph node-positive breast cancer. Breast Cancer Res Treat. 2015;150:119–126. doi: 10.1007/s10549-015-3302-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Wang B, Guo H, Shi G, Hong X. Clinicopathological significance and potential drug target of T-cadherin in NSCLC. Drug Des Devel Ther. 2014;9:207–216. doi: 10.2147/DDDT.S74259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren JZ, Huo JR. Correlation between T-cadherin gene expression and aberrant methylation of T-cadherin promoter in human colon carcinoma cells. J Med Oncol. 2012;29:915–918. doi: 10.1007/s12032-011-9836-9. [DOI] [PubMed] [Google Scholar]
- 17.Philippova M, Pfaff D, Kyriakakis E, Buechner SA, Iezzi G, Spagnoli GC, Schoenenberger AW, Erne P, Resink TJ. T-cadherin loss promotes experimental metastasis of squamous cell carcinoma. Eur J Cancer. 2013;49:2048–2058. doi: 10.1016/j.ejca.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Bosserhoff AK, Ellmann L, Quast AS, Eberle J, Boyle GM, Kuphal S. Loss of T-cadherin (CDH-13) regulates AKT signaling and desensitizes cells to apoptosis in melanoma. Mol Carcinog. 2014;53:635–647. doi: 10.1002/mc.22018. [DOI] [PubMed] [Google Scholar]
- 19.Toyooka KO, Toyooka S, Virmani AK, Sathyanarayana UG, Euhus DM, Gilcrease M, Minna JD, Gazdar AF. Loss of expression and aberrant methylation of the CDH13 (H-cadherin) gene in breast and lung carcinomas. Cancer Res. 2001;61:4556–4560. [PubMed] [Google Scholar]
- 20.Wang XD, Wang BE, Soriano R, Zha J, Zhang Z, Modrusan Z, Cunha GR, Gao WQ. Expression profiling of the mouse prostate after castration and hormone replacement: Implication of H-cadherin in prostate tumorigenesis. Differentiation. 2007;75:219–234. doi: 10.1111/j.1432-0436.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi T, Misaki A, Liang SB, Tachibana A, Hayashi N, Sonobe H, Ohtsuki Y. Expression of T-cadherin (CDH13, H-Cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J Neurochem. 2000;74:1489–1497. doi: 10.1046/j.1471-4159.2000.0741489.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW. H-cadherin, a novel cadherin with growth inhibitory functions and diminished expression in human breast cancer. Nat Med. 1996;2:776–782. doi: 10.1038/nm0796-776. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi T, Misaki A, Chen BK, Ohtsuki Y. H-cadherin expression in breast cancer. Histopathology. 1999;35:87–88. doi: 10.1046/j.1365-2559.1999.0728c.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Chen Z, Huang Z, Chen F, Ye Z, Lin S, Wang W. Upregulation of T-cadherin suppresses cell proliferation, migration and invasion of gastric cancer in vitro. Exp Ther Med. 2017;14:4194–4200. doi: 10.3892/etm.2017.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei B, Shi H, Lu X, Shi A, Cheng Y, Dong L. Association between the expression of T-cadherin and vascular endothelial growth factor and the prognosis of patients with gastric cancer. Mol Med Rep. 2015;12:2075–2081. doi: 10.3892/mmr.2015.3592. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Chan DW, Lee JMF, Chan PC, Ng IO. Genetic and epigenetic inactivation of T-cadherin in human hepatocellular carcinoma cells. Int J Cancer. 2008;123:1043–1052. doi: 10.1002/ijc.23634. [DOI] [PubMed] [Google Scholar]
- 28.Adachi Y, Takeuchi T, Nagayama T, Furihata M. T-cadherin modulates tumor-associated molecules in gallbladder cancer cells. Cancer Invest. 2010;28:120–126. doi: 10.3109/07357900903124472. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Sun G, Liu X, Chen Y, Zhang C. Clinical significance of T-cadherin tissue expression in patients with bladder transitional cell carcinoma. Urol Int. 2011;86:340–345. doi: 10.1159/000322962. [DOI] [PubMed] [Google Scholar]
- 30.Lin YL, Xie PG, Ma JG. Aberrant methylation of CDH13 is a potential biomarker for predicting the recurrence and progression of non-muscle-invasive bladder cancer. Med Sci Monit. 2014;20:1572–1577. doi: 10.12659/MSM.892130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin YL, He ZK, Li ZG, Guan TY. Downregulation of CDH13 expression promotes invasiveness of bladder transitional cell carcinoma. Urol Int. 2012;90:225–232. doi: 10.1159/000345054. [DOI] [PubMed] [Google Scholar]
- 32.Fujishima Y, Maeda N, Matsuda K, Masuda S, Mori T, Fukuda S, Sekimoto R, Yamaoka M, Obata Y, Kita S, et al. Adiponectin association with T-cadherin protects against neointima proliferation and atherosclerosis. FASEB J. 2017;31:1571–1583. doi: 10.1096/fj.201601064R. [DOI] [PubMed] [Google Scholar]
- 33.Kong DD, Wang MH, Yang J, Li L, Wang W, Wang SB, Zhou YZ. T-cadherin is associated with prognosis in triple-negative breast cancer. Oncology letters. 2017;14:2975–2981. doi: 10.3892/ol.2017.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riou P, Saffroy R, Chenailler C, Franc B, Gentile C, Rubinstein E, Resink T, Debuire B, Piatier-Tonneau D, Lemoine A. Expression of T-cadherin in tumor cells influences invasive potential of human hepatocellular carcinoma. FASEB J. 2006;20:2291–2301. doi: 10.1096/fj.06-6085com. [DOI] [PubMed] [Google Scholar]
- 35.Yan Q, Zhang ZF, Chen XP, Gutmann DH, Xiong M, Xiao ZY, Huang ZY. Reduced T-cadherin expression and promoter methylation are associated with the development and progression of hepatocellular carcinoma. Int J Oncol. 2008;32:1057–1063. [PubMed] [Google Scholar]
- 36.Huang ZY, Wu Y, Hedrick N, Gutmann DH. T-cadherin-mediated cell growth regulation involves G2 phase arrest and requires p21(CIP1/WAF1) expression. Mol Cell Biol. 2003;23:566–578. doi: 10.1128/MCB.23.2.566-578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akagi T, Kimoto T. Human cell line (HGC-27) derived from the metastatic lymph node of gastric cancer. Acta Medica Okayama. 1976;30:215–219. [PubMed] [Google Scholar]
- 38.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237:227–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 39.Hebbard LW, Garlatti M, Young LJ, Cardiff RD, Oshima RG, Ranscht B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res. 2008;68:1407–1416. doi: 10.1158/0008-5472.CAN-07-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claudio F. Targeting the PI3K/AKT/mTOR pathway in prostate cancer development and progression: Insight to therapy. Clin Cancer Drugs. 2016;20:R83–R99. [Google Scholar]
- 41.Ewald F, Nörz D, Grottke A, Bach J, Herzberger C, Hofmann BT, Nashan B, Jücker M. Vertical targeting of AKT and mTOR as well as dual targeting of AKT and MEK signaling is synergistic in hepatocellular carcinoma. J Cancer. 2015;6:1195–1205. doi: 10.7150/jca.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.