Abstract
Disruption of the endothelial barrier is essential for vascular complications associated with diabetes mellitus, and damage to the endothelial glycocalyx has been demonstrated to participate in this process. Ginsenoside Rg1 (Rg1), the major active component isolated from Panax notoginseng, is widely applied for the protection against vascular injury. The present study aimed to analyze the effect of high glucose on endothelial barrier function and its association with endothelial glycocalyx in human umbilical vein endothelial cells (HUVECs), and explore the potential benefits of Rg1 in protecting endothelial barrier function from high glucose-induced injury. The results indicated that high glucose induced a disorder of the endothelial glycocalyx and increased heparanase mRNA expression in HUVECs, which was reversed by Rg1 treatment. In addition, Rg1 treatment reduced transendothelial electrical resistance and transendothelial albumin passage after high-glucose stimulation. The present study suggested that high glucose caused a disruption in the endothelial glycocalyx and increased heparanase expression, which finally resulted in endothelial barrier dysfunction in HUVECs. Of note, Rg1 has a protective effect on high glucose-induced endothelial barrier dysfunction by attenuating the associated increase in heparanase expression.
Keywords: endothelial barrier dysfunction, endothelial glycocalyx, heparanase, ginsenoside Rg1
Introduction
Diabetes, also known as diabetes mellitus (DM), has an increased morbidity and mortality and represents a serious public health issue worldwide (1,2). Vascular complications are involved in pathological changes of DM (3,4) and are the leading cause of mortality in this population. The endothelium, covering the luminal surface of all blood vessels, has a key role in the maintenance of vascular homeostasis (5,6). Endothelial dysfunction initiates vascular pathogenesis and may finally lead to diabetic vasculopathy (7). The luminal surface of endothelial cells is covered by a thick layer of glycocalyx, which is composed of proteoglycans (PGs) and glycoproteins (8,9). Therefore, dysfunction of glycocalyx may promote the development of diabetic vasculopathy.
It has been demonstrated that endothelial glycocalyx has a significant impact on factors including vascular permeability (10), inflammation (11), coagulation (12) and mechanotransduction (13). It was observed that the thickness of the glycocalyx was altered and its integrity was disrupted in a streptozocin-induced animal model of diabetes (14). Diabetic patients are characterized by endothelial glycocalyx damage, the severity of which is associated with vascular damage (15,16). An in vitro study also observed that hyperglycemia induced glycocalyx dysfunction in either microvascular or macrovascular endothelial cells (17,18). It has been reported that the loss of glycocalyx leads to a reduction in endothelial surface charge and accelerates atherosclerosis in patients with type 2 diabetes (19). Taken together, these studies indicate that glycocalyx dyfunction drives the development of vascular complications in diabetes.
Panax notoginseng is one of the most commonly used Chinese herbal medicines due to its efficacy in promoting blood circulation and removing blood stasis (20). It is frequently used in the management of diabetes in Asian countries (21) and also possesses cardiovascular protection effects by preserving endothelial cell function and inhibiting thermogenesis (22). Ginsenoside Rg1 (Rg1; Fig. 1), the major active component isolated from Panax notoginseng, appears to be accountable for its extensive pharmacological actions, including anti-oxidant, anti-inflammatory and anti-cancer effects (23). Rg1 has been demonstrated to have cardiovascular protection effects, and may be of potential preventive and therapeutic value for cardiovascular injury in diabetic patients (24,25). In addition, Rg1 has been demonstrated to be potent in improving renal function and attenuating diabetes-induced renal damage (26,27). However, the underlying mechanisms remain to be elucidated.
Figure 1.
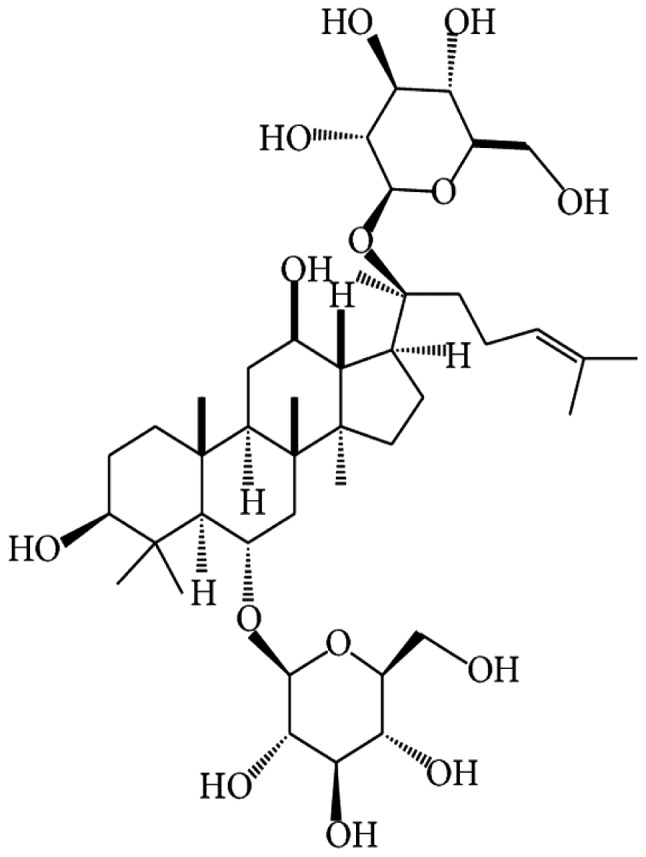
Chemical structure of ginsenoside Rg1.
Based on the above, the presents study hypothesized that Rg1 may attenuate diabetes-induced vascular dysfunction via restoring the loss of endothelial glycocalyx. To test this hypothesis, the effect of high glucose on the endothelial glycocalyx and endothelial barrier function, and the potential benefits of Rg1 in protecting endothelial barrier function from high glucose-induced endothelial cell injury were investigated.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 5.5 mmol/l D-glucose, supplemented with L-glutamine, 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in an incubator containing 5% CO2. Cells were seeded into cell culture dishes and cultured until confluent.
Treatment
For control and high-glucose treatment, HUVECs were cultured with DMEM containing 5.5 and 30 mmol/l D-glucose, respectively. For drug treatment, the cells were incubated with high-glucose DMEM, and at the same time, Rg1 (>98% pure; DiDa Kexiang Biological Co., Ltd., Guizhou, China) was added to the culture at concentrations ranging from 10−8 to 10−5 mol/l for 1 or 3 days.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HUVECs using TRIzol reagent (Thermo Fisher Scientific, Inc.). RNA was reverse-transcribed into complementary (c)DNA using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). The mRNA levels of heparanase (HPSE) were quantified with an RT-qPCR system (Mastercycler realplex2; Eppendorf, Hamburg, Germany), using SYBR Green SuperMix (Roche Diagnostics, Mannheim, Germany) with appropriate primers pairs. Sequences of primers used in the present study were as follows: HPSE forward, 5′-CCAAAGTTGCTGCTTGCATC-3′ and reverse, 5′-AGTGTCCCAGTGTCTCTCAA-3′; GAPDH forward, 5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAATG−3′. The reaction was started by pre-incubation at 95°C for 10 min, followed by 40 cycles of amplification (95°C for 15 sec, 65°C for 15 sec and 72°C for 20 sec). Gene expression levels of HPSE were normalized to those of the reference gene GAPDH measured in the same sample and the results were analyzed by the 2−∆∆Cq method (28).
Western blot analysis
Total proteins were prepared using the lysis buffer (20 mmol/l Tris, pH 7.4, 150 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/lEGTA, 1% Triton X-100, 2.5 mmol/l deoxycholic acid, 1 mmol/l β-glycerophosphate and 1 mmol/l Na3VO4), supplemented with protease inhibitors. Protein concentration was determined using a BCA protein assay kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer's protocol. The protein of each sample (25 µg) was separated using 10% SDS-PAGE and then transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). After blocking with 5% fat-free milk for 1 h at room temperature, the membranes were incubated with the following respective primary antibodies: Syndecan-1 monoclonal antibody (1:1,000 dilution; cat. no. ab128936; Abcam, Cambridge, MA, USA), glypican-1 polyclonal antibody (1:1,000 dilution; cat. no. NBP1-33197; Novus Biologicals LLC, Littleton, CO, USA) and GAPDH monoclonal antibody (1:1,000 dilution; cat. no. sc32233; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. The membranes were then gently washed for three times (5 min each) and incubated with horseradish peroxide-conjugated goat anti-rabbit secondary antibody (1:3,000 dilution; cat. no. ZB2301; Zhongshan Goldenbridge Bio, Beijing, China) at room temperature for 1.5 h. After washing, the protein bands were visualized with chemiluminescent substrate (EMD Millipore) for 1 min, and capturing of images and densitometric analysis were performed using an imaging station (Bio-Rad Laboratoris, Inc., Hercules, CA, USA). The relative protein expression levels were normalized to GAPDH and the ratio was compared with that of the control group.
Detection of endothelial surface glycocalyx
Wheat germ agglutinin (WGA) from Triticum vulgaris binds to sugar moieties of glycocalyx present on the cell surface, the majority of which are likely to be PG constituents of glycocalyx. Therefore, endothelial surface glycocalyx was labeled by fluorescein isothiocyanate (FITC)-conjugated WGA lectin as previously reported (29). Cells were grown to confluence on glass coverslips and fixed in 4% paraformaldehyde for 10 min. After being washed for three times, the cells were incubated with FITC-WGA lectin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 2 µg/ml for 30 min. Coverslips were mounted and visualized using a fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Transendothelial electrical resistance (TEER) measurement
Cells were seeded onto microporous polyester membranes (0.4-µm pore size) of Transwell filter inserts (Corning Inc., Corning, NY, USA). The cells were grown onto the upper chamber of the Transwell until confluent. The HUVECs were stimulated with vehicle, 30 mmol/l high glucose or Rg1 for 24 h as indicated. The TEER of the monolayer of HUVECS was measured using a Millicell ERS-2 Volt-Ohm meter (EMD Millipore) according to the protocol of a previous study (30). After subtraction of the value determined using a blank, cell-free filter, the mean value of the TEER was expressed in common units (Ωcm2). TEER values of a vehicle-treated monolayer of endothelial cells were designated as baseline values. The percentage of TEER relative to baseline value was calculated via the following formula: TEER%=(TEER of experimental wells/baseline TEER of experimental wells) ×100%.
Transendothelial albumin passage
The transendothelial passage of albumin was analyzed by measuring the passage of FITC-labeled bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) across the monolayer as described previously (31). In brief, cells were seeded onto the upper chambers of Transwells and allowed to grow to confluence as described above. The medium in the insert was replaced with serum-free medium (SFM) containing 0.5 mg/ml FITC-labelled BSA and the medium in the well was replaced by SFM only. After 1, 2 and 3 h of incubation, the medium was collected from each well, and the fluorescence of the aliquots was measured on a fluorometer with excitation at 495 nm and emission at 520 nm. The amount of albumin passing the endothelial cell monolayer was calculated with a standard curve generated from a set of FITC-labeled BSA dilutions.
Statistical analyses
All data were analyzed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ± standard deviation. The independent Student's t-test and one-way analysis of variance (ANOVA) with a least significant difference (LSD) post hoc test, were used for comparison between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of Rg1 on the PG core proteins in high glucose-induced HUVECs
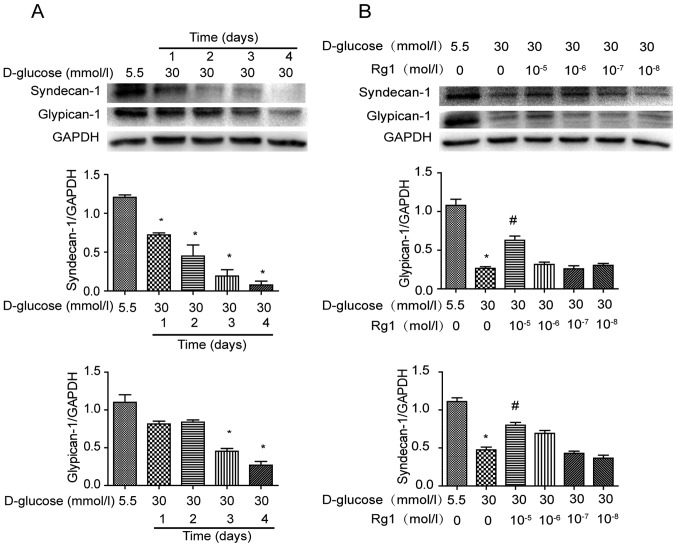
PG core proteins are important constituents of glycocalyx on the cell surface. Expression of PG core proteins syndecan-1 and glypican-1 was analyzed by western blot analysis after exposure to high glucose for different durations (Fig. 2). It was demonstrated that syndecan-1 was gradually decreased in HUVECs incubated with high glucose from 1–4 days. Over the same duration, the expression of glypican-1 was also decreased by high-glucose stimulation, with the changes being significant at 3 and 4 days (Fig. 2A). HUVECs under high-glucose stimulation were then treated with different concentrations of Rg1 for 3 days. It was observed that treatment with Rg1 increased PG core proteins in HUVECs at concentrations ranging from 10−8 to 10−5 mol/l and a significant difference was identified at 10−5 mol/l (Fig. 2B).
Figure 2.
Expression of proteoglycan core proteins in high glucose-induced HUVECs. (A) HUVECs were incubated with 30 mmol/l glucose for 1, 2, 3 and 4 days. (B) HUVECs were treated with 30 mmol/l glucose and various concentrations of Rg1 for 3 days. Specific antibody was used to detect syndecan-1, glypican-1 by western blotting. Quantified values are expressed as the mean ± standard deviation (n=3). *P<0.05 vs. normal glucose; #P<0.05 vs. high glucose group. HUVECS, human umbilical vein endothelial cells; Rg1, ginsenoside Rg1.
High glucose-induced HPSE expression is attenuated by Rg1 treatment in HUVECs
To assess the effects of high glucose and Rg1 treatment on HPSE expression, HUVECs were incubated with 30 mmol/l glucose and different concentrations of Rg1 as indicated. The expression of HPSE mRNA was detected by RT-qPCR. It was observed that HPSE mRNA expression in high glucose-treated cells was rapidly increased and reached a peak at 1 day, and then it returned to baseline levels at 3 and 4 days (Fig. 3A). To observe the effect of Rg1, HUVECs were first incubated with high glucose, followed by different concentrations of Rg1 for 1 day. It was indicated that HPSE expression in HUVECs was reduced by treatment with Rg1 at concentrations ranging from 10−8 to 10−5 mol/l, and a significant difference was observed at 10−7 mol (Fig. 3B).
Figure 3.
High glucose-induced heparanase expression in human umbilical vein endothelial cells. (A) Heparanase mRNA expression was increased by treatment with 30 mmol/l glucose for 1 and 2 days, and (B) reduced by treatment with Rg1. Values are expressed as the mean ± standard deviation (n=3). *P<0.05 vs. normal glucose; #P<0.05 vs. high glucose group. Rg1, ginsenoside Rg1.
Rg1 prevents the disruption of the glycocalyx induced by high glucose
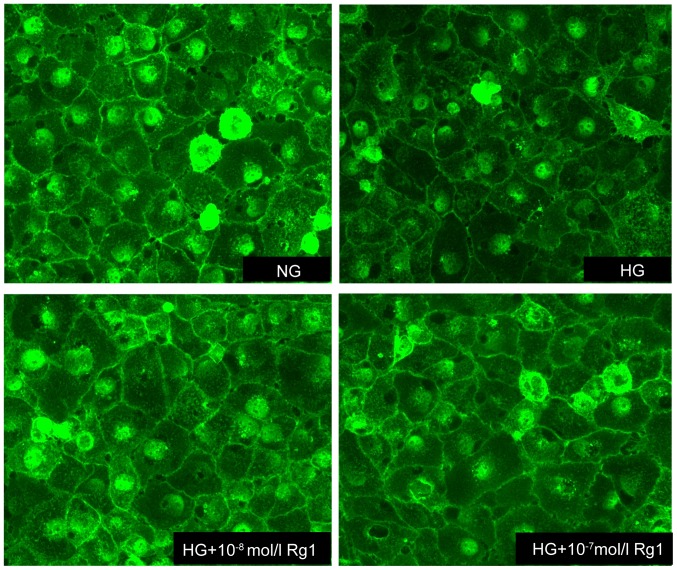
WGA-FITC, which binds to sugar residues on cell surface, was used to quantify the expression of glycocalyx in HUVECs. A marked reduction in WGA-FITC binding was observed in high glucose-induced cells compared with untreated controls, suggesting the disruption of endothelial glycocalyx. As expected, the expression of glycocalyx was increased by treatment with Rg1, indicating that Rg1 prevented the loss of glycocalyx and attenuated the disruption of the glycocalyx in HUVECs incubated with 30 mmol/l glucose (Fig. 4).
Figure 4.
Expression of WGA in HG-induced HUVECs. Fluorescence microscopy after labeling the glycocalyx of HUVECs with WGA-FITC lectin (magnification, ×200). The binding of WGA-FITC lectin was reduced by treatment with HG, while this effect was attenuated by Rg1. WGA, wheat germ agglutinin; FITC, fluorescein isothiocyanate; HUVECS, human umbilical vein endothelial cells; Rg1, ginsenoside Rg1; HG, high glucose; NG, normal glucose.
Rg1 treatment increases TEER in the presence of high glucose
TEER is associated with the integrity of cell monolayers. Its decline represents an increase in the passage of water and small molecules across the cell monolayer and an impaired cell barrier function. To study the effects of Rg1 treatment on TEER, HUVECs were stimulated with high glucose in the presence or absence of Rg1. High glucose caused a modest reduction in the mean TEER by up to 20% relative to that in the controls, which was inhibited in the presence of Rg1 (Fig. 5A).
Figure 5.
Influence of HG and Rg1 on endothelial barrier function in HUVECs. (A) HG decreased the TEER of HUVECs, which was inhibited by Rg1. (B) HG increased transendothelial albumin passage of HUVECs. Cumulative passage of fluorescein isothiocyanate-labeled albumin across the HUVECs monolayer was determined over time. Values are expressed as the mean ± standard deviation (n=3). *P<0.05 HG vs. NG; #P<0.05 HG+Rg1(10−7 mol/l) vs. HG group. Rg1, ginsenoside Rg1; HUVECS, human umbilical vein endothelial cells; TEER, transendothelial electrical resistance; HG, high glucose; NG, normal glucose.
High glucose leads to increased transendothelial albumin passage that is attenuated by treatment with Rg1
Since 30 mmol/l high glucose leads to decreased TEER, it was examined whether Rg1 was able to preserve the permeability of cultured HUVEC monolayers. The permeability was assessed by measuring the passage of FITC-labeled BSA across the monolayer, i.e., the transendothelial albumin passage. As presented in Fig. 5B, stimulation with 30 mmol/l high glucose significantly increased transendothelial albumin passage across HUVECs, while this effect was inhibited in the presence of Rg1.
Discussion
A pivotal role of the endothelium is to serve as a regulated barrier to partially separate the contents of the blood from the extravascular space (32). The endothelial glycocalyx covers the luminal surface of the vascular endothelium (33,34), and is responsible for endothelial barrier function. Widespread loss of the endothelial surface glycocalyx leads to damage of vascular function and an elevation in the microvascular permeability to water as well as albumin, and results in systemic vascular dysfunction in proteinuric kidney disease (35). The results of the present study indicated a marked decrease in the PG core proteins syndecan-1 and glypican-1 on HUVECs after exposure to high glucose, while treatment with Rg1 increased the formation of PG core proteins. This was further confirmed in an assay using WGA-FITC lectin, in which WGA-FITC lectin binding was markedly reduced after high-glucose treatment, while this reduction was inhibited by Rg1 treatment. These results suggested that treatment with Rg1 increased the formation of PG and enhanced the integrity of the endothelial cell monolayer, which is beneficial for preserving endothelial barrier function under a high-glucose conditions.
In response to persistent activators of the endothelium, i.e., in diabetes mellitus, hypertension and systemic inflammation, endothelial cells undergo pathological changes (36,37), including the induction of the expression of certain enzymes, including HPSE. HPSE, a degrading enzyme of the endothelial cell glycocalyx, is the only known mammalian enzyme to cleave PGs (38). It has been reported that increased HPSE expression causes damage to the glycocalyx of mouse glomerular endothelial cells and increases the trans-endothelial albumin passage of the cell monolayer (39). The present study indicated that high glucose increased HPSE mRNA expression on HUVECs at the early stage of high-glucose treatment, whereas Rg1 reduced the increase of HPSE induced by high glucose. The results indicated that Rg1 inhibited the production of HPSE to ameliorate endothelial glycocalyx disorders.
Endothelial glycocalyx is located between the blood stream and the endothelium, provides a barrier for certain molecules, and has an important role in endothelial permeability and endothelial functions (40,41). A significant degradation of the glomerular glycocalyx has been reported in the setting of diabetes in that loss of glycocalyx increases vascular permeability (16). In, TEER is used as an indicator of the passage of water and small molecules across a cell layer. TEER is regarded as a measure of the resistance to the passage of ions across a confluent cell monolayer. In the present study, the passage of albumin across cell monolayers was tested to evaluate the permeability of the endothelial cell monolayer, and the results indicated that Rg1 attenuated the damage of endothelial barrier function by enhancing TEER and decreasing the cell transendothelial albumin passage.
In conclusion, the present study demonstrated that Rg1 inhibited the loss of endothelial glycocalyx and HPSE mRNA expression, and increased TEER, while decreasing endothelial cell monolayer permeability and protecting endothelial barrier function. The present results may provide a novel mechanism of action of Rg1 in the treatment of diabetic vasculopathy.
Acknowledgements
This study was supported by the Science and Technology Development Fund of Macau (grant no. 071/2014/A), the Education Department Program of Sichuan Province (grant no. 17TD0046), the Key Project of the Health Department of Sichuan Province (grant no. 16ZD034) and the Innovative Research Team of Luzhou (grant no. 2016LZXNYD-T05).
Competing interests
The authors declare that they have no competing interests.
References
- 1.García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Potenza MA, Gagliardi S, Nacci C, Carratu' MR, Montagnani M. Endothelial dysfunction in diabetes: From mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 4.Santi D, Giannetta E, Isidori AM, Vitale C, Aversa A, Simoni M. Therapy of endocrine disease: Effects of chronic use of phosphodiesterase inhibitors on endothelial markers in type 2 diabetes mellitus: A meta-analysis. Eur J Endocrinol. 2015;172:R103–R114. doi: 10.1530/EJE-14-0700. [DOI] [PubMed] [Google Scholar]
- 5.Bertoluci MC, Cé GV, da Silva AM, Wainstein MV, Boff W, Punales M. Endothelial dysfunction as a predictor of cardiovascular disease in type 1 diabetes. World J Diabetes. 2015;6:679–692. doi: 10.4239/wjd.v6.i5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 7.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 9.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: Albuminuria and increased microvascular permeability. J Pathol. 2012;226:562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 11.Mulivor AW, Lipowsky HH. Inflammation-and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672–H1680. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 12.Pearson MJ, Lipowsky HH. Effect of fibrinogen on leukocyte margination and adhesion in postcapillary venules. Microcirculation. 2004;11:295–306. doi: 10.1080/10739680490425994. [DOI] [PubMed] [Google Scholar]
- 13.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 14.Dogné S, Rath G, Jouret F, Caron N, Dessy C, Flamion B. Hyaluronidase 1 deficiency preserves endothelial function and glycocalyx integrity in early streptozotocin-induced diabetes. Diabetes. 2016;65:2742–2753. doi: 10.2337/db15-1662. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 16.Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahwa R, Nallasamy P, Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J Diabetes Complications. 2016;30:563–572. doi: 10.1016/j.jdiacomp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Fridén V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, Haraldsson B, Mathieson PW, Satchell SC. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol. 2011;300:F40–F48. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nassimizadeh M, Ashrafian H, Drury NE, Howell NJ, Digby J, Pagano D, Frenneaux MP, Born GV. Reduced negative surface charge on arterial endothelium explains accelerated atherosclerosis in type 2 diabetic patients. Diab Vasc Dis Res. 2010;7:213–215. doi: 10.1177/1479164110376207. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Xiong X, Wang H, Wang J. Protective effects of panax notoginseng saponins on cardiovascular diseases: A comprehensive overview of experimental studies. Evid Based Complement Alternat Med. 2014;2014:204840. doi: 10.1155/2014/204840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): A review. Phytother Res. 2014;28:510–516. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- 22.Ling S, Nheu L, Dai A, Guo Z, Komesaroff P. Effects of four medicinal herbs on human vascular endothelial cells in culture. Int J Cardiol. 2008;128:350–358. doi: 10.1016/j.ijcard.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 23.Lü JM, Yao Q, Chen C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu HT, Zhen J, Pang B, Gu JN, Wu SS. Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. J Zhejiang Univ Sci B. 2015;16:344–354. doi: 10.1631/jzus.B1400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H, Zhen J, Yang Y, Gu J, Wu S, Liu Q. Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J Cell Mol Med. 2016;20:623–631. doi: 10.1111/jcmm.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LN, Xie XS, Zuo C, Fan JM. Effect of ginsenoside Rgl on the expression of TNF-alpha and MCP-1 in rats with diabetic nephropathy. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:466–471. (In Chinese) [PubMed] [Google Scholar]
- 27.Ma X, Xie X, Zuo C, Fan J. Effects of ginsenoside Rg1 on streptozocin-induced diabetic nephropathy in rats. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2010;27:342–347. (In Chinese) [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Xu Q, Wu J, Zhou X, Weng J, Xu J, Wang W, Huang Q, Guo X. Role of src in vascular hyperpermeability induced by advanced glycation end products. Sci Rep. 2015;5:14090. doi: 10.1038/srep14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garsen M, Sonneveld R, Rops AL, Huntink S, van Kuppevelt TH, Rabelink TJ, Hoenderop JG, Berden JH, Nijenhuis T, van der Vlag J. Vitamin D attenuates proteinuria by inhibition of heparanase expression in the podocyte. J Pathol. 2015;237:472–481. doi: 10.1002/path.4593. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, Wu X, Hack BK, Bao L, Cunningham PN. TNF causes changes in glomerular endothelial permeability and morphology through a Rho and myosin light chain kinase-dependent mechanism. Physiol Rep. 2015;3:e12636. doi: 10.14814/phy2.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haraldsson B, Nyström J. The glomerular endothelium: New insights on function and structure. Curr Opin Nephrol Hypertens. 2012;21:258–263. doi: 10.1097/MNH.0b013e3283522e7a. [DOI] [PubMed] [Google Scholar]
- 34.Fridén V, Oveland E, Tenstad O, Ebefors K, Nyström J, Nilsson UA, Haraldsson B. The glomerular endothelial cell coat is essential for glomerular filtration. Kidney Int. 2011;79:1322–1330. doi: 10.1038/ki.2011.58. [DOI] [PubMed] [Google Scholar]
- 35.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol. 2010;6:404–414. doi: 10.1038/nrneph.2010.65. [DOI] [PubMed] [Google Scholar]
- 37.Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein AM, van Kuppevelt T, Meirovitz A, Pisano C, Li JP, et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky I, van der Vlag J, Berden JH. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 2006;70:2100–2108. doi: 10.1038/sj.ki.5001985. [DOI] [PubMed] [Google Scholar]
- 39.Garsen M, Lenoir O, Rops AL, Dijkman HB, Willemsen B, van Kuppevelt TH, Rabelink TJ, Berden JH, Tharaux PL, van der Vlag J. Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol. 2016;27:3545–3551. doi: 10.1681/ASN.2015091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goligorsky MS. Vascular endothelium in diabetes. Am J Physiol Renal Physiol. 2017;312:F266–F275. doi: 10.1152/ajprenal.00473.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sieve I, Münster-Kühnel AK, Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul Pharmacol. 2018;100:26–33. doi: 10.1016/j.vph.2017.09.002. [DOI] [PubMed] [Google Scholar]