Abstract
Stem cells have the characteristics of long-term self-renewal and plasticity and the ability to differentiate into specialized cells. Stem cells are widely recognized as potential tools for use in the development of novel therapeutic strategies. The aim of the current study was to investigate the effect of demographic factors on adipogenic and chondrogenic differentiation in bone marrow-derived stem cell (BMSC) spheroids. Age- and gender-associated alterations in the adipogenic and chondrogenic differentiation potential of BMSCs were examined. Human BMSCs were isolated from male and female participants in their 20s, 30s and 50s. Cell morphology and relative values of adipogenesis and chondrogenesis were examined by measuring the relative intensity of oil red O and Alcian blue staining, respectively. Cell morphology alterations in BMSCs isolated from male and female participants in their 20s, 30s and 50s and grown in adipogenic media were very similar. In addition, there were no significant differences in the relative values of adipogenesis in BMSCs for the 20s, 30s and 50s age groups on day 8 and 16. Similarly, no significant differences were observed in the relative values of adipogenesis in BMSCs for the male and female groups on day 8 and 16. Cell morphology changes in BMSCs isolated from male and female participants in their 20s, 30s and 50s and grown in chondrogenic media were very similar. In addition, there were no significant differences in the relative values of chondrogenesis in BMSCs for the 20s, 30s and 50s age groups on day 8, however there was a significant difference observed in the relative values of chondrogenesis in BMSCs on day 16 for the 30s and 50s age groups, compared with the 20s age group. Furthermore, no significant differences were observed in the relative values of adipogenesis in BMSCs for the male and female groups on day 8 and 16. The current study demonstrated that there were no significant differences in the adipogenic and chondrogenic differentiation potential of BMSCs isolated from healthy male donors vs. healthy female donors. Similarly, no significant differences were observed in the adipogenic differentiation potential of BMSCs isolated from different age groups on day 8. However, there was a significant increase in the chondrogenic differentiation potential of BMSCs isolated from participants in their 30s and 50s, compared with BMSCs isolated from participants in their 20s on day 16.
Keywords: adipogenesis, cell differentiation, cellular spheroids, chondrogenesis, stem cells
Introduction
Stem cells are recognized as potential tools for use in the development of novel therapeutic strategies (1). All stem cells share two common characteristics, long-term self-renewal and plasticity and the ability to differentiate into specialized cells, which have therapeutic potential for the repair of different tissues and organs (2). Bone marrow-derived stem cells (BMSCs) are widely used for cell therapy in regenerative medicine (3). In a previous study, the age-associated osteogenic potential of BMSCs was investigated, and a decrease in osteogenic differentiation potential was observed during aging in humans (4). However, another study demonstrated that there were no age-associated changes in the osteoblastic differentiation potential or the steady state levels of messenger RNA (mRNA) of osteogenic gene markers (5). A previous study demonstrated that the capacity of BMSCs to form bone in vivo was maintained with age, which suggests that the observed senescence-associated decrease in bone formation may be due to a defect in bone microenvironment (6). A previous study demonstrated that the expression levels of bone-associated genes under osteogenic culture conditions were similar in BMSCs isolated from females compared with BMSCs isolated from males (7). However, the number of the colony-forming units which express alkaline phosphatase from bone marrow decreased significantly with age for women, but not for men (8). In addition, there was no significant difference observed in in vitro osteogenic activity in cultures of patient-derived mesenchymal stem cells compared with that in normal donor cultures (9). BMSCs have the ability to undergo adipogenic and chondrogenic differentiation (10). Few studies have investigated whether age- and gender-associated differences in the adipogenic and chondrogenic potential of BMSCs exist. The aim of the current study was to investigate the effect of demographic factors on adipogenic and chondrogenic differentiation in BMSC spheroids.
Materials and methods
Human bone marrow-derived stem cells
Human bone marrow-derived mesenchymal stem cells (Catholic MASTER Cells) were obtained from the Catholic Institute of Cell Therapy (Seoul, South Korea). BMSC isolation and characterization was performed, as previously reported (11). Tests performed by the Catholic Institute of Cell Therapy confirmed high expression levels of CD73 and CD90 (>90% positive; data not shown). The current study was approved by the Institutional Review Board of Seoul St. Mary's Hospital (approval no. KC17SNSI0606). Written informed consent was obtained from the participants as specified in the Declaration of Helsinki. The methods used in this study were performed in accordance with the relevant guidelines and regulations.
Cell culture
Human BMSCs were seeded in 24-well plates at a density of 2×104 cells/well and cultured in a-minimal essential medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 15% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 200 mM L-glutamine (Sigma-Aldrich; Merck KGaA) and 10 mM ascorbic acid 2-phosphate (Sigma-Aldrich; Merck KGaA) at 37°C.
Adipogenic differentiation
To determine the adipocyte differentiation potential of BMSCs, isolated cells were cultured using a StemPro® Adipogenesis Differentiation kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Adipogenic induction medium and adipogenic maintenance medium were supplied. On day 8 and 16 respectively, cells were rinsed twice with phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde (Biosesang Inc., Seongnam, Korea) at 20°C for 5 min. Cells were subsequently washed with distilled water, rinsed with 60% isopropanol and covered with oil red O solution (Sigma-Aldrich; Merck KGaA) for 10 min (12). Following incubation, cells were rinsed in 60% isopropanol and washed with distilled water. Cell morphology was observed using an inverted microscope (Leica DM IRM; Leica Microsystems GmbH, Wetzlar, Germany) and relative values of adipogenesis were determined by measuring the intensity of oil red O staining using ImageJ (version 1.8.0, National Institutes of Health, Bethesda, MD, USA) analysis software (magnification, ×100 for day 8; ×200 for day 16).
Following induction of adipocyte differentiation, 1.5×105 cells were collected on day 8 and 16, respectively, and incubated with specific fluorescein isothiocyanate-conjugated mouse monoclonal human CD44 antibody (dilution 1:200, cat. no. 11-0441-81; Invitrogen; Thermo Fisher Scientific, Inc.) at 20°C for 20 min. Cells were analyzed using a flow cytometer (FACSCanto II; BD Biosciences, San Jose, CA, USA), and FACSDiva software (Version 8.0.1, BD Biosciences). Human BD Fc Block™ (564219, BD Biosciences) was used as a blocking reagent and 1% bovine serum albumin/PBS served as the washing reagent.
Chondrogenic differentiation
To determine the chondrogenic potential of BMSCs, isolated cells were cultured using a StemPro® Chondrogenesis Differentiation kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. On day 8 and 16 respectively, cells were rinsed twice with PBS and fixed with 4% paraformaldehyde at 20°C for 5 min. Cells were subsequently washed with distilled water, rinsed with 60% isopropanol and covered with Alcian blue solution (Sigma-Aldrich; Merck KGaA) for 10 min (13). Following incubation, cells were rinsed in 3% acetic acid and washed with distilled water. Cell morphology was observed using an inverted microscope and relative values of chondrogenesis were determined by measuring the intensity of Alcian blue staining using ImageJ analysis software (magnification, ×100 for day 8; ×200 for day 16).
Statistical analysis
Data are presented as the mean ± standard deviation. All analyses were performed using SPSS software (version 12.0; SPSS Inc., Chicago, IL, USA). A test for normality was performed, and all statistical comparisons between groups were determined using Student's t-test or one-way analysis of variance with Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphologic evaluation of adipogenic differentiation
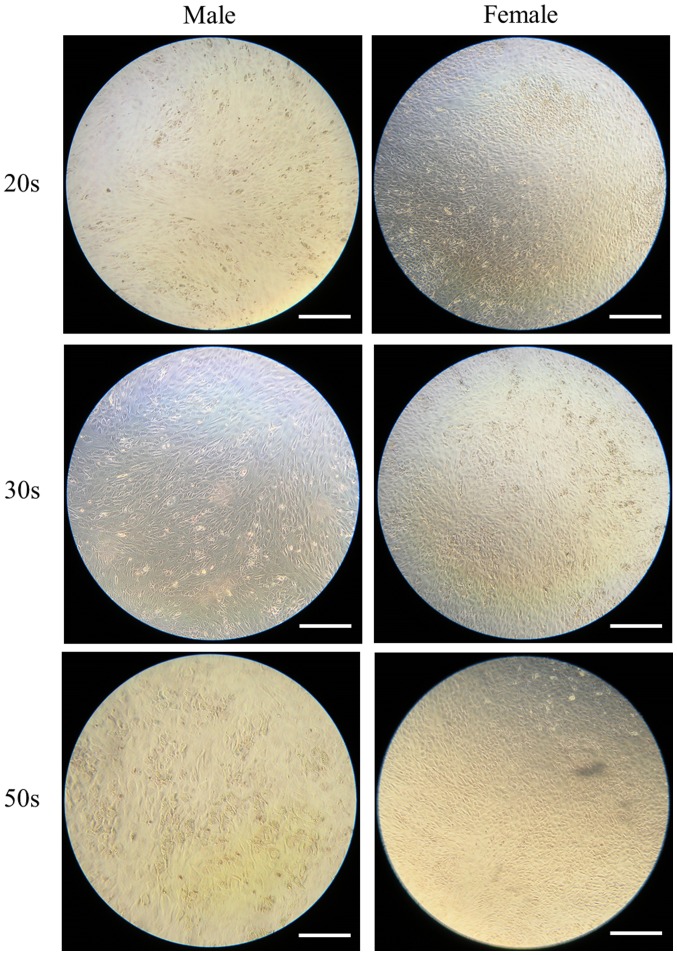
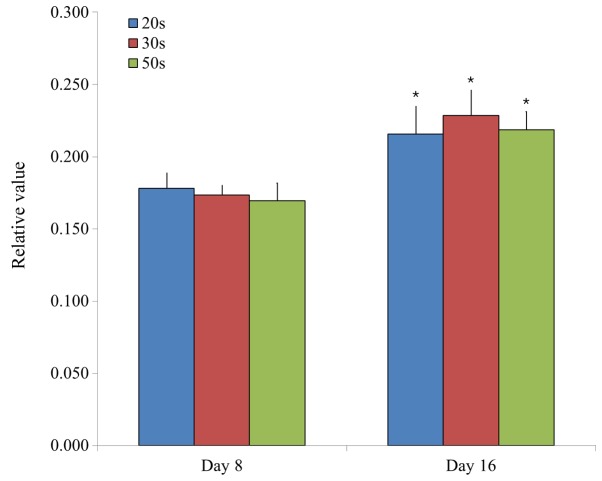
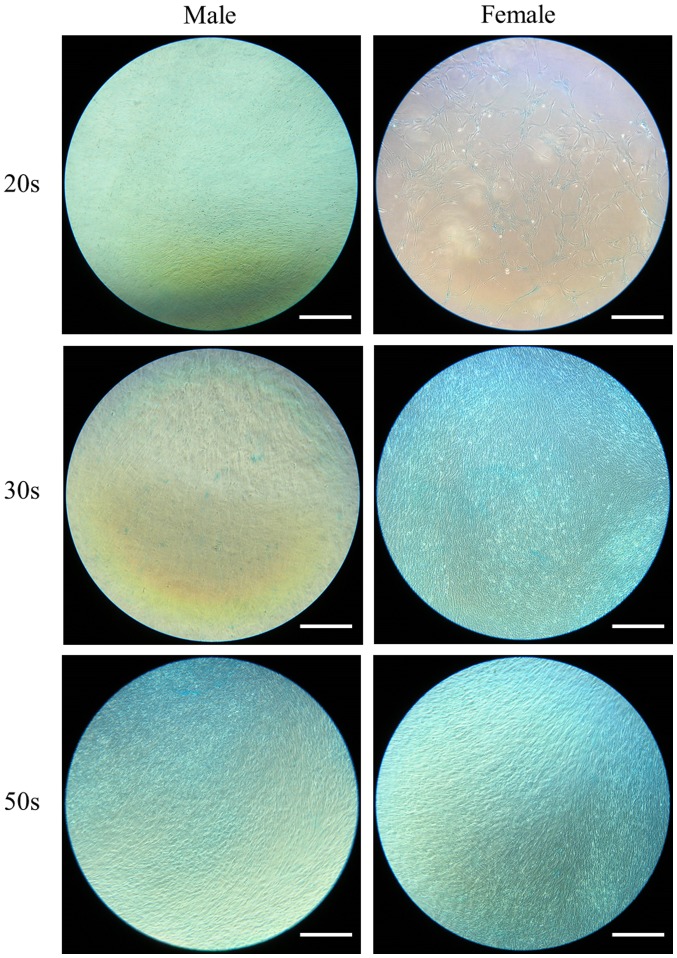
The ability of the isolated BMSCs to differentiate into adipocytes was examined in BMSCs isolated from male and female participants in their 20s, 30s and 50s and grown in adipogenic media. Following eight days of growth in adipogenic media, observed changes in cell morphology were very similar among the different age groups. In addition, similar changes in oil red O staining intensity were also observed (Fig. 1). Similarly, following 16 days of growth in adipogenic media, observed changes in cell morphology were very similar among the different age groups. In addition, similar changes in oil red O staining intensity were also observed (Fig. 2). In general, oil red O staining intensity was significantly increased at day 16 compared with day 8 in each group (P<0.05; Fig. 3).
Figure 1.
Evaluation of cell morphology on day 8 following adipogenic media-induced differentiation. Oil red O staining of bone marrow-derived stem cells isolated from male and female participants in their 20s, 30s and 50s (original magnification ×100; scale bar=400 µm).
Figure 2.
Evaluation of cell morphology on day 16 following adipogenic media-induced differentiation. Oil red O staining of bone marrow-derived stem cells isolated from male and female participants in their 20s, 30s and 50s (original magnification ×200; scale bar=200 µm).
Figure 3.
Relative values of adipogenesis in isolated bone marrow-derived stem cells from participants in their 20s, 30s and 50s, as determined by analysis of oil red O staining intensity. Data are presented as the mean ± standard deviation. *P<0.05 vs. day 8.
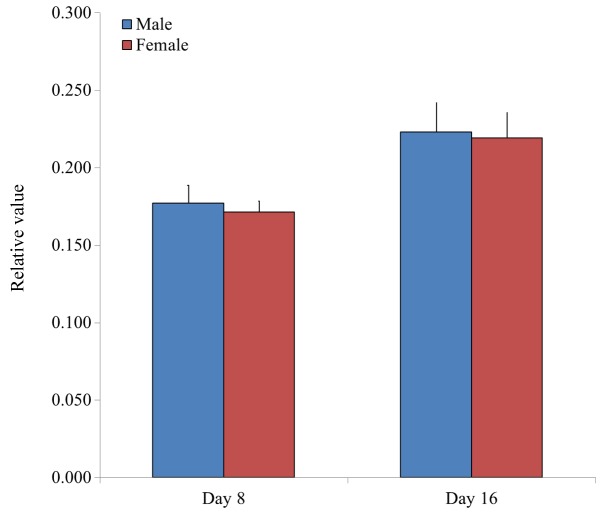
Adipogenesis was evaluated by measuring the relative intensity of oil red O staining in BMSCs. Relative values of adipogenesis were 0.178±0.010, 0.173±0.007 and 0.170±0.012 for the 20s, 30s and 50s age groups on day 8, respectively, whilst the relative values of adipogenesis were 0.216±0.019, 0.228±0.017 and 0.219±0.013 for the 20s, 30s, and 50s age groups on day 16, respectively (Fig. 3). Furthermore, the relative values of adipogenesis were 0.177±0.011 and 0.171±0.007 for male and female groups on day 8, respectively, whilst the relative values of adipogenesis were 0.223±0.019 and 0.219±0.016 for male and female groups on day 16, respectively (Fig. 4).
Figure 4.
Relative values of adipogenesis in isolated bone marrow-derived stem cells from male and female participants, as determined by analysis of oil red O staining intensity. Data are presented as the mean ± standard deviation.
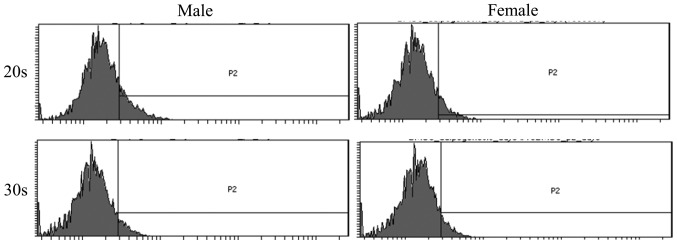
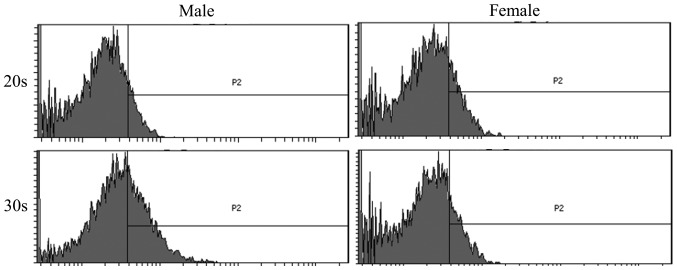
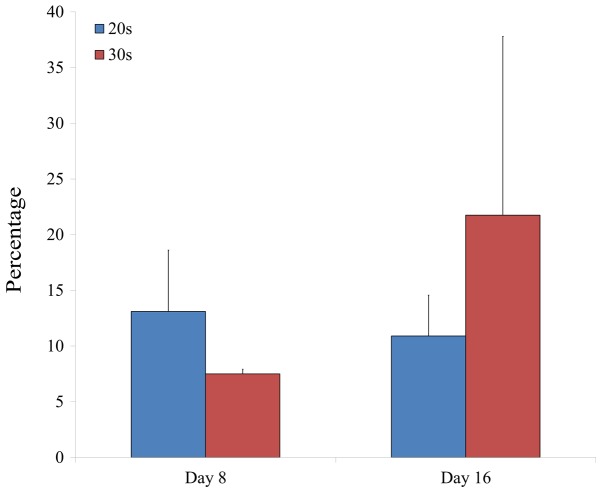
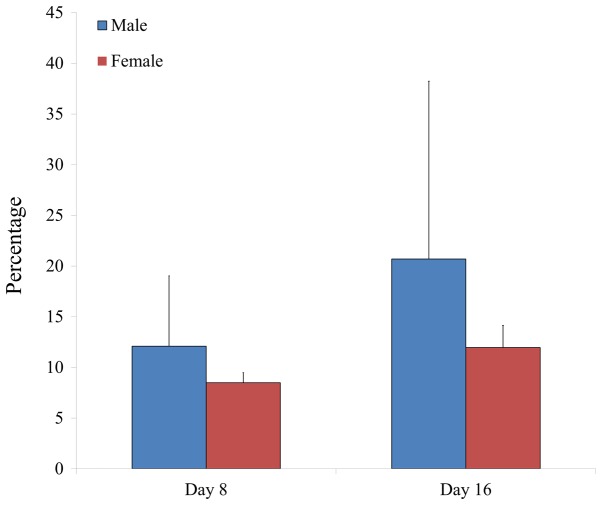
To determine the phenotype of isolated BMSCs following growth in adipogenic media, expression of the CD44 surface marker was examined in BMSCs isolated from male and female participants in their 20s and 30s. CD44 surface marker expression was analyzed on day 8 and 16 by flow cytometry (Figs. 5 and 6). The percentage of CD44 expression was 13.1±5.5 and 7.5±0.4% for the 20s and 30s age groups on day 8, respectively, whilst the percentage of CD44 expression was 10.9±3.7 and 21.8±16.1% for the 20s and 30s age groups on day 16, respectively (Fig. 7). Furthermore, the percentage of CD44 expression was 12.1±6.9 and 8.5±1.0% for male and female groups on day 8, respectively, whilst the percentage of CD44 expression was 20.7±17.5 and 12.0±2.2% for male and female groups on day 16, respectively (Fig. 8).
Figure 5.
Evaluation of CD44 surface marker expression on day 8 following adipogenic media-induced differentiation. Flow cytometric profile of CD44 expression in bone marrow-derived stem cells isolated from male and female participants in their 20s and 30s.
Figure 6.
Evaluation of CD44 surface marker expression on day 16 following adipogenic media-induced differentiation. Flow cytometric profile of CD44 expression in bone marrow-derived stem cells isolated from male and female participants in their 20s and 30s.
Figure 7.
Relative percentage of cells expressing CD44 surface marker in isolated bone marrow-derived stem cells from participants in their 20s and 30s.
Figure 8.
Relative percentage of cells expressing CD44 surface marker in isolated bone marrow-derived stem cells from male and female participants.
Morphologic evaluation of chondrogenic differentiation
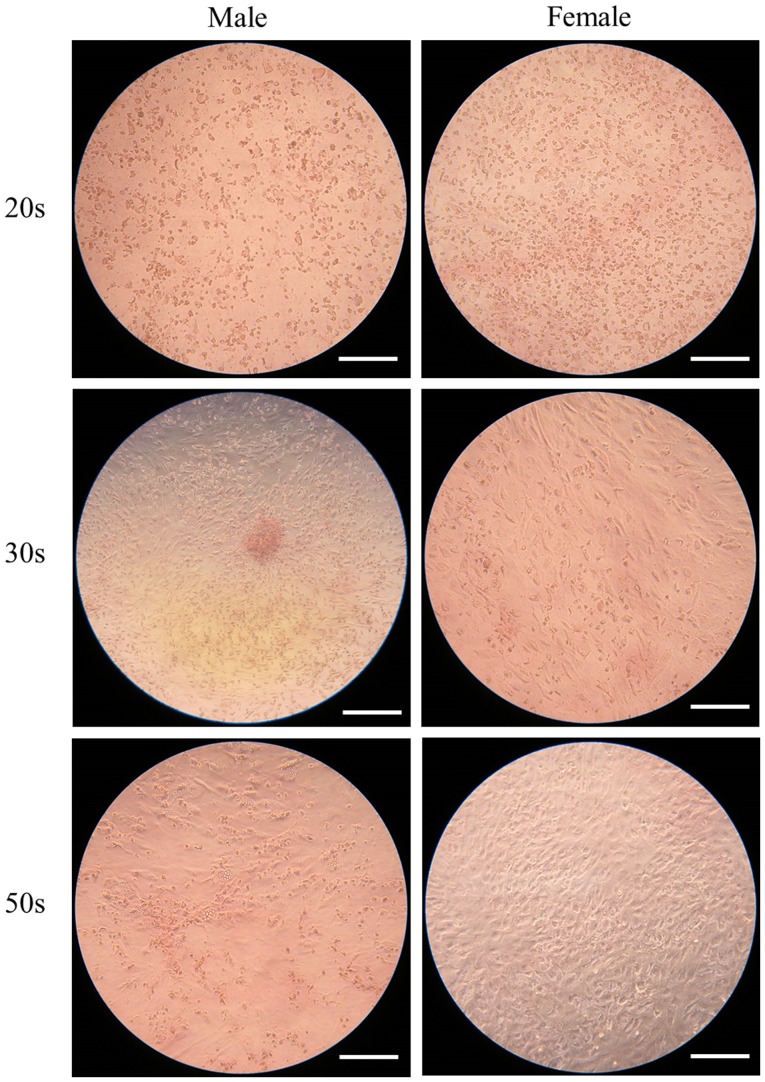
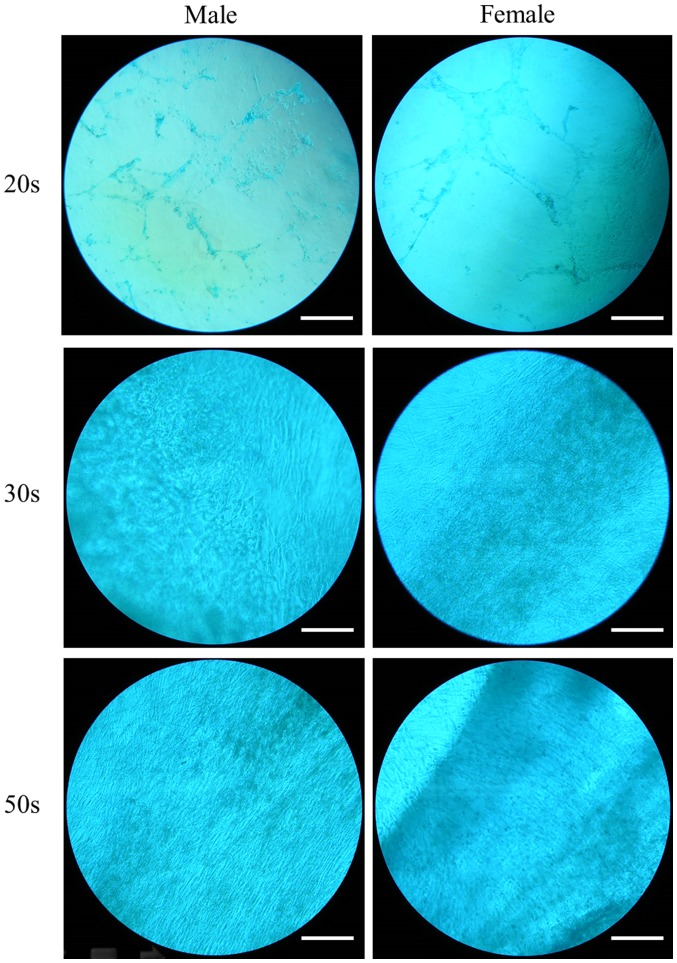
The ability of the isolated BMSCs to differentiate into chondrocytes was examined in BMSCs isolated from male and female participants in their 20s, 30s and 50s and grown in chondrogenic media. Following eight days of growth in chondrogenic media, observed changes in cell morphology were very similar among the different age groups. In addition, similar changes in Alcian blue staining intensity were also observed (Fig. 9). Following 16 days of growth in chondrogenic media, observed changes in cell morphology were very similar among the different age groups. In addition, similar changes in Alcian blue staining intensity were also observed (Fig. 10). In general, Alcian blue staining intensity was increased at day 16 compared with day 8 (P<0.05; Fig. 11).
Figure 9.
Evaluation of cell morphology on day 8 following chondrogenic media-induced differentiation. Alcian blue staining of bone marrow-derived stem cells isolated from male and female participants in their 20s, 30s and 50s (original magnification ×100; scale bar=400 µm).
Figure 10.
Evaluation of cell morphology on day 16 following chondrogenic media-induced differentiation. Alcian blue staining of bone marrow-derived stem cells isolated from male and female participants in their 20s, 30s and 50s (original magnification ×200; scale bar=200 µm).
Figure 11.
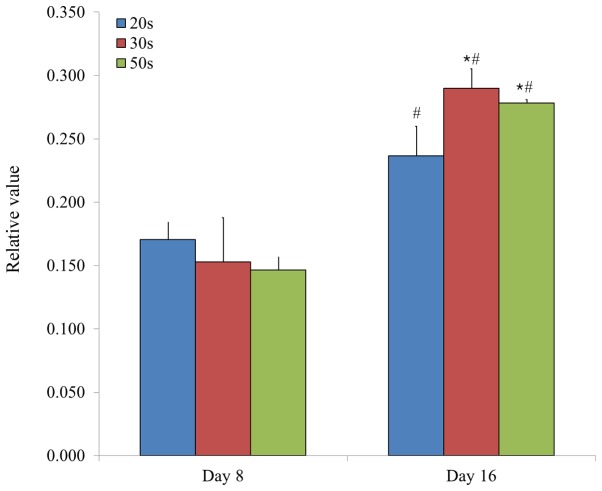
Relative values of chondrogenesis in isolated bone marrow-derived stem cells from participants in their 20s, 30s and 50s, as determined by analysis of Alcian blue staining intensity. Data are presented as the mean ± standard deviation. *P<0.05 vs. 20s group at day 16; #P<0.05 vs. day 8.
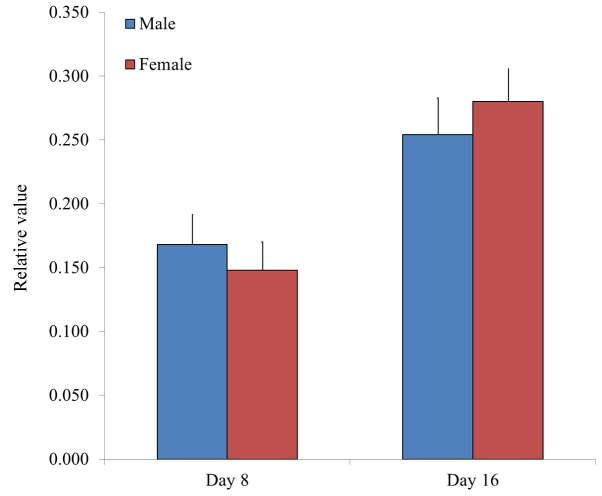
Chondrogenesis was evaluated by measuring the relative intensity of Alcian blue staining in BMSCs. Relative values of chondrogenesis were 0.171±0.013, 0.153±0.035 and 0.147±0.010 for the 20s, 30s and 50s age groups on day 8, respectively, whilst the relative values of chondrogenesis were 0.237±0.023, 0.290±0.016 and 0.278±0.003, for the 20s, 30s and 50s age groups on day 16, respectively (P<0.05, 50s and 30s vs. 20s group at day 16; Fig. 11). Furthermore, the relative values of chondrogenesis were 0.168±0.023 and 0.148±0.022 for male and female groups on day 8, respectively, whilst the relative values of chondrogenesis were 0.254±0.029 and 0.280±0.026 for male and female groups on day 16, respectively (Fig. 12).
Figure 12.
Relative values of adipogenesis in isolated bone marrow-derived stem cells from male and female participants, as determined by analysis of Alcian blue staining intensity. Data are presented as the mean ± standard deviation.
Discussion
The current study investigated the effect of demographic factors on adipogenic and chondrogenic differentiation potential in BMSC spheroids. A previous study demonstrated that the expression levels of adipocyte-associated genes significantly increased in BMSCs isolated from female mice compared with BMSCs isolated from male mice (7). Similarly, gene expression analysis by reverse transcription-quantitative polymerase chain reaction examining aging and gender-associated effects on BMSC differentiation, identified an increase in adipogenesis with aging (14). In addition, a previous study demonstrated that adipogenesis is enhanced in BMSCs isolated from older female mice (15). Furthermore, a reduced chondrogenic and adipogenic differentiation potential was observed in BMSCs isolated from patients with advanced osteoarthritis (9). However, a previous study demonstrated that there were no age-associated changes in the adipocytic colony formation or the steady-state level mRNA expression of adipogenic gene markers in marrow stromal cells isolated from patients with osteoporosis compared with age-matched healthy controls (5). In the current study, no significant differences in the androgenic differentiation potential of BMSCs isolated from participants in the 20s, 30s and 50s age groups were observed. Similarly, no gender-associated effects were observed in the androgenic differentiation potential of isolated BMSCs.
A previous study demonstrated histological, immunohistochemical and molecular evidence for the in vitro chondrogenic differentiation of bone marrow-derived mesenchymal progenitor cells (16). In addition, when bone marrow-derived cells were passaged in a monolayer culture as many as 20 times, cells maintained the chondrogenic differentiation potential after each passage (17). There was a significant reduction in in vitro chondrogenic activity in cultures of patient-derived mesenchymal stem cells (MSCs) from patients with advanced osteoarthritis compared with normal cultures (9). A previous study demonstrated that the chondrogenic potential of human adult MSCs is independent of age or osteoarthritis etiology (18). However, the chondrogenic potential of BMSCs decreased with age in rat models (19). In the current study, no significant differences in the chondrogenic differentiation potential of BMSCs isolated from participants in the 20s, 30s and 50s age groups were observed on day 8, however on day 16 there was a significant difference in the chondrogenic differentiation potential of BMSCs isolated from participants in the 30s and 50s age groups, compared with those in the 20s age group. In addition, no gender-associated effects were observed in the chondrogenic differentiation potential of isolated BMSCs.
The effects of aging and gender on adipogenic and chondrogenic differentiation potential is varied, and this may be due to changes in the model system used, the passage and stage of differentiation, and the culture period (20,21). Investigating the effects of aging have proved contradictory due to the relative narrow age range of participants in a number of the studies (22).
The differentiation potential of MSCs varies depending on their origin. In a previous study, adipose tissue-derived MSCs isolated from young female participants were revealed to be more resistant to senescence under in vitro culture conditions when compared with those isolated from older patients (23). The effect of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells was examined, which revealed that male-derived cells differentiated faster and more efficiently compared with female-derived cells (24). In addition, the influence of gender on the chondrogenic potential of muscle-derived stem cells (MDSCs) was examined, which revealed that male MDSCs displayed a higher chondrogenic differentiation with increased cartilage regeneration potential (25). Furthermore, a decrease in the chondrogenic potential of periosteum with aging was observed in a rabbit model (26). However, the chondrogenic potential of periosteum-derived stem cells varied between donor sites, an effect potentially caused by differences in total cell count (21).
The current study demonstrated no significant differences in the adipogenic and chondrogenic differentiation potential of BMSCs derived from healthy male donors compared with healthy female donors. Similarly, there were no significant differences in the adipogenic and chondrogenic differentiation among the different age groups.
Acknowledgements
Not applicable.
Funding
The current study was partially supported by the Research Fund of Seoul St. Mary's Hospital, The Catholic University of Korea. The current study was also supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by a grant from the Ministry of Science, Information and Communication Technology & Future Planning (grant no. NRF-2017R1A1A1A05001307).
Availability of data and materials
All datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
HL, SM and JP designed the study. HL, SM and JP collected and analyzed data. HL, SM and JP performed the experiments. HL, SM and JP wrote the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
The Catholic MASTER cells supplied by the Catholic Institute of Cell Therapy (Seoul, Korea) were derived from human bone marrow donated by healthy donors after informed consent. The current study was approved by the Institutional Review Board of Seoul St. Mary's Hospital (Seoul, Republic of Korea). Written informed consent was obtained from the participants as specified in the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing interests.
References
- 1.Tögel F, Westenfelder C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev Dyn. 2007;236:3321–3331. doi: 10.1002/dvdy.21258. [DOI] [PubMed] [Google Scholar]
- 2.Totey S, Totey S, Pal R, Pal R. Adult stem cells: A clinical update. J Stem Cells. 2009;4:105–121. [PubMed] [Google Scholar]
- 3.Maria OM, Khosravi R, Mezey E, Tran SD. Cells from bone marrow that evolve into oral tissues and their clinical applications. Oral Dis. 2007;13:11–16. doi: 10.1111/j.1601-0825.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 4.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 5.Justesen J, Stenderup K, Eriksen EF, Kassem M. Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int. 2002;71:36–44. doi: 10.1007/s00223-001-2059-x. [DOI] [PubMed] [Google Scholar]
- 6.Stenderup K, Rosada C, Justesen J, Al-Soubky T, Dagnaes-Hansen F, Kassem M. Aged human bone marrow stromal cells maintaining bone forming capacity in vivo evaluated using an improved method of visualization. Biogerontology. 2004;5:107–118. doi: 10.1023/B:BGEN.0000025074.88476.e2. [DOI] [PubMed] [Google Scholar]
- 7.Bragdon B, Burns R, Baker AH, Belkina AC, Morgan EF, Denis GV, Gerstenfeld LC, Schlezinger JJ. Intrinsic Sex-linked variations in osteogenic and adipogenic differentiation potential of bone marrow multipotent stromal cells. J Cell Physiol. 2015;230:296–307. doi: 10.1002/jcp.24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 9.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 10.Zheng YH, Xiong W, Su K, Kuang SJ, Zhang ZG. Multilineage differentiation of human bone marrow mesenchymal stem cells in vitro and in vivo. Exp Ther Med. 2013;5:1576–1580. doi: 10.3892/etm.2013.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA, Jeun SS. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int. 2014;2014:129145. doi: 10.1155/2014/129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JE, Kim BB, Ko Y, Jeong SH, Park JB. Effects of Cimicifugae Rhizoma on the osteogenic and adipogenic differentiation of stem cells. Exp Ther Med. 2017;13:443–448. doi: 10.3892/etm.2016.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JB, Bae SS, Lee PW, Lee W, Park YH, Kim H, Lee K, Kim I. Comparison of stem cells derived from periosteum and bone marrow of jaw bone and long bone in rabbit models. Tissue Eng Regen Med. 2012;9:224–230. doi: 10.1007/s13770-012-0343-7. [DOI] [Google Scholar]
- 14.Jiang Y, Mishima H, Sakai S, Liu YK, Ohyabu Y, Uemura T. Gene expression analysis of major lineage-defining factors in human bone marrow cells: Effect of aging, gender and age-related disorders. J Orthop Res. 2008;26:910–917. doi: 10.1002/jor.20623. [DOI] [PubMed] [Google Scholar]
- 15.Bolt AM, Grant MP, Wu TH, Flores Molina M, Plourde D, Kelly AD, Negro Silva LF, Lemaire M, Schlezinger JJ, Mwale F, Mann KK. Tungsten promotes sex-specific adipogenesis in the bone by altering differentiation of bone marrow-resident mesenchymal stromal cells. Toxicol Sci. 2016;150:333–346. doi: 10.1093/toxsci/kfw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 17.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Buhring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25:3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- 19.Zheng H, Martin JA, Duwayri Y, Falcon G, Buckwalter JA. Impact of aging on rat bone marrow-derived stem cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 2007;62:136–148. doi: 10.1093/gerona/62.2.136. [DOI] [PubMed] [Google Scholar]
- 20.Barbero A, Grogan S, Schäfer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Gallay SH, Miura Y, Commisso CN, Fitzsimmons JS, O'Driscoll SW. Relationship of donor site to chondrogenic potential of periosteum in vitro. J Orthop Res. 1994;12:515–525. doi: 10.1002/jor.1100120408. [DOI] [PubMed] [Google Scholar]
- 22.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Ock SA, Lee YM, Park JS, Shivakumar SB, Moon SW, Sung NJ, Lee WJ, Jang SJ, Park JM, Lee SC, et al. Evaluation of phenotypic, functional and molecular characteristics of porcine mesenchymal stromal/stem cells depending on donor age, gender and tissue source. J Vet Med Sci. 2016;78:987–995. doi: 10.1292/jvms.15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T, Kubo S, Meszaros LB, Corsi KA, Cooper GM, Li G, Usas A, Osawa A, Fu FH, Huard J. The influence of sex on the chondrogenic potential of muscle-derived stem cells: Implications for cartilage regeneration and repair. Arthritis Rheum. 2008;58:3809–3819. doi: 10.1002/art.24125. [DOI] [PubMed] [Google Scholar]
- 26.O'Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19:95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.