Abstract
The current study aimed to investigate the inhibitory effect of tanshinone IIA (Tan IIA) on the inflammatory response in patients with rheumatoid arthritis (RA) and explore its mechanism. A total of 50 patients with RA were randomly separated into the control group (15 cases) and the research group (35 cases). The tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels in serum were determined, and peripheral blood mononuclear cells (PBMCs) were separated from patients with RA and cultured in vitro. The effects of the β-arrestin 2 small interfering (si)RNA incubation, lipopolysaccharide (LPS) stimulation or Tan IIA incubation on TNF-α and IL-6 levels, and the expression levels of β-arrestin 2, NAD-dependent protein deacetylase sirtuin-1 (SIRT1) and transcription factor p65 (p65) proteins were investigated. Prior to treatment, no significant differences in TNF-α and IL-6 levels in serum of patients with RA were identified between the research and control groups. Following treatment, the TNF-α and IL-6 levels in the serum of patients with RA in the research group were significantly lower compared with those in the research group prior to treatment and those in the control group following treatment (P<0.05). Tan IIA inhibited the LPS-induced secretion of TNF-α and IL-6, upregulated the LPS-inhibited expression of the β-arrestin 2 and SIRT1 proteins, and downregulated the LPS-induced expression of the p65 protein in the PBMCs of patients with RA. The β-arrestin 2 small interfering (si)RNA significantly upregulated the secretion of TNF-α and IL-6, inhibited the expression of the SIRT1 protein and upregulated the expression of the p65 protein in PBMCs of patients with RA. Tan II A effectively increased the weight of rats with rheumatoid arthritis, and reduced the circumference of the left posterior ankle, the posterior plantar metatarsal thickness, and the content of serum TNF-α and IL-6. Tan IIA did not significantly reverse these β-arrestin 2 siRNA-induced changes. Tan IIA inhibited the inflammatory response in PBMCs of patients with RA by upregulating β-arrestin 2 expression.
Keywords: tanshinone IIA, rheumatoid arthritis, inflammatory response, β-arrestin 2
Introduction
The key clinical features of rheumatoid arthritis (RA), an inflammatory autoimmune disease, are synovial inflammation and chronic corrosive damage to joints; however, its pathogenesis is unknown at present (1–3). In patients with RA, synovial hyperplasia and tissue inflammation may cause joint and surrounding tissue damage, resulting in joint deformity and dysfunction (4,5). Furthermore, lesions may also affect all joints with synovial membranes, with those of the hands and feet being the most common (4,5). Several studies have indicated that inflammatory cytokines and chemokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β serve key roles in the development and progression of autoimmune diseases, including systemic lupus erythematosus, systemic vasculitis, scleroderma and RA (1–3). Humanized anti-TNF-α (4) and anti-IL-6 receptor (5) monoclonal antibodies, and inflammatory cytokines or chemokines antagonist drugs are widely used to treat RA in clinic; these treatments demonstrate good clinical therapeutic effects on RA (5). In addition, certain therapeutic drugs that target immune cells have also been developed and applied clinically, including the CTLA4-IgG1 fusion protein, which was developed by Repligen and was approved for clinical use in the treatment of graft-versus-host responses (6). Furthermore, CD2 receptor antibodies and anti-T-cells are also at different stages of clinical research (7,8).
Therefore, it is feasible to treat RA by inhibiting the inflammatory response.
Tanshinone IIA (Tan IIA), the main active ingredient in tanshinone, can effectively inhibit the lipopolysaccharide (LPS)-induced expression of pro-inflammatory molecules in human peripheral blood mononuclear cells (PBMCs) (9), downregulate serum levels of inflammatory cytokine in coronary syndromes (10) and induces apoptosis in fibroblast-like synoviocytes in RA (11). However, the effect of Tan IIA on the inflammatory response in patients with RA and the corresponding mechanism of action remain unknown. Therefore, the aim of the current study was to investigate the inhibitory effect of Tan IIA on the inflammatory response in patients with RA and explore the corresponding mechanism by investigating the effect of Tan IIA on the LPS-induced expression of associated proteins [sirtuin-1 (SIRT-1) p65 and β-arrestin2] in PBMCs, and TNF-α and IL-6 levels. The current study also aimed to assess whether Tan IIA inhibits the inflammatory response in patients with RA by inhibiting the expression of various inflammatory factors (including TNF-α and IL-6) in PBMCs.
Materials and methods
Clinical data
The diagnosis of RA was performed according to the ACR/EULAR 2010 classification criteria (12). A total of 50 randomly selected patients with RA were recruited from the Department of Rheumatology, Third Affiliated Hospital of Zhejiang Chinese Medical University (Hangzhou, China) between January and June 2016. A total of 35 patients with RA, including 10 males and 25 females, with a mean age of 50.3±9.8 years and a mean progression time of 6.9±2.3 years were recruited as the research group. A total of 15 patients with RA, including 5 males and 10 females, with a mean age of 49.7±7.6 years and a mean progression time of 6.8±1.9 years were recruited as the control group. No significant differences in the age, gender, progression time and other general data were identified between the research and control groups. The current study was approved by the Ethics Committee of The Third Affiliated Hospital of Zhejiang Chinese Medical University. Written informed consent was obtained from each patient. The exclusion criteria were as follows: i) Patients with RA who also had other rheumatism diseases, immune system diseases, tumors and liver and kidney function deficiencies; ii) patients with RA who were pregnant, lactating or had mental diseases; and iii) patients with RA who used immunomodulatory drugs within half a year.
Clinical drugs and experimental materials
Methotrexate (product ID: H31020644) and meloxicam (product ID: H20030486) were purchased from Shanghai Sine Pharma Co., Ltd. (Shanghai, China) and Simcere Pharmaceutical Co., Ltd. (Nanjing, China), respectively. A Tan IIA sulfonate sodium injection (product ID: H31022558) and leflunomide (product ID: H20080420) were purchased from Shanghai No. 1 Biochemical & Pharmaceutical Co., Ltd. (Shanghai, China) and Jiangsu Yabang Aipusen Pharmaceutical Co., Ltd. (Yancheng, China), respectively. Tan IIA was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). TNF-α (cat. no. KLC103a.96) and IL-6 (cat. no. AF-200-06) ELISA kits were purchased from Shanghai Kang Lang Biological Technology Co., Ltd. (Shanghai, China) and Shanghai Institute of Biological Products Co., Ltd. (Shanghai, China), respectively. A rat TNF-α ELISA kit (cat. no. K1052-100) was purchased from Amyjet Scientific Inc. (Wuhan, China). A rat IL-6 ELISA kit (cat. no. KS12244) was purchased from Shanghai Keshun Biological Technology Co., Ltd. (Shanghai, China). An Animal tissue/cell total protein extraction kit (cat. no. SD-001/SN-002) was purchased from Amyjet Scientific, Inc.
Cell transfection
An siRNA negative control (F:5′-CCCAUUCAUUGUUGUCACUTT-3′, R:5′-AGUGACAACAAUGAAUGGGTT-3′), a β-arrestin 2 siRNA oligo sequence (oligo1, 5′-ACCUUUUCGUCUUUUGCUCCC-3′; oligo2, 5′-GAGCAAAAGACGAAAAGGUUG−3′) were synthesized by Shanghai Gene Pharma Co., Ltd. (Shanghai, China). These were directly transferred into cells via the Lipofectamine™ 2000 transfection reagent (cat. no. 11668019; Invitrogen; Thermo Fisher Scientific, Inc.). The final concentration of siRNA used was 50 nM.
Rheumatoid rat model
A total of 60 male Sprague Dawley rats (age, 5–6 weeks; weight, 200–220 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Animals were housed at a temperature of 20–24°C and a humidity of 60% with a 12 h light/dark cycle for one week. Rats were able to drink and eat freely prior to and during the experiment. A total of 10 rats were selected as the control group. The skin of left posterior foot on each rat was sterilized with 75% alcohol and iodophor disinfectant, and 0.1 ml saline was injected. The other 50 rats were selected as the model group. The procedure for the model group was as follows: Heat inactivated bacillus Calmette-Guérin and Freund's adjuvant (cat. no. YS-XQ0496; Shanghai Yansheng Biochemical Reagent Co., Ltd., Shanghai, China) in a l0 mg/ml oil and water emulsion was obtained from an ice bath, and then the skin of left posterior foot on each rat was sterilized with 75% alcohol and iodophor disinfectant and 0.1 ml prepared emulsion was injected. After 14 days, the rats in model group were again injected with 0.1 ml prepared emulsion.
Experimental animal administration and collection of specimens
Following the establishment of the RA model, the rats were fed for 1 week. Then the RA model rats were separated into the following three groups: Control model (RA), routine treatment (RT) and Tan IIA-treated (Tan IIA). The control group and RA groups were provided a normal diet. Saline (1 ml/l00 g) was given by gavage administration once daily for 3 weeks. The RT group was fed a normal diet; the rats were also administered with methotrexate tablets dissolved in physiological saline by gavage (0.51 mg/kg/day), once every 3 days for 3 weeks. The Tan IIA group was fed a normal diet; the rats were also administered with Tan IIA dissolved in physiological saline by gavage (0.50 mg/kg/d), once every 3 days for 3 weeks.
After 3 weeks, the weights of the rats was recorded prior to and following the 12-h fasting state. Rats were anesthetized with chloral hydrate (400 mg/kg, intraperitoneal; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and blood was extracted from the heart of each rat. Rats were subsequently sacrificed by cervical dislocation. Blood (5 ml) was extracted using a vacuum chamber without anticoagulants for 30 min in a 37°C constant temperature water bath. The blood was then centrifuged at a speed of 500 × g for 10 min at room temperature to separate the serum. The serum was then divided into 100 µl tubes and preserved at 4°C.
General examination of different groups
Three variables were measured 0, 14, 21, 28, 35 and 42 days after the establishment of the RA model. The variables were as follows: Weight, perimeter of the left posterior ankle and posterior plantar metatarsal thickness.
Clinical treatment methods and detection indices
Patients in the research and control groups orally received conventional drug treatment, including 5 mg methotrexate once every 2 weeks and 7.5 mg meloxicam and 10 mg leflunomide once daily. Patients in the research group received a Tan IIA injection of 40 mg via an intravenous drip. Prior to treatment and following 1 week of continuous treatment, 10 ml venous blood was obtained from all patients; the blood was centrifuged at 500 × g for 10 min at room temperature to obtain serum. TNF-α and IL-6 serum levels were determined using ELISA kits following the manufacturer's protocol.
Separation and culture of PBMCs
Prior to treatment, venous blood was obtained from patients with RA in the control group and used to obtain PBMCs by density gradient centrifugation (1,000 × g for 25 min at room temperature). Two PBMCs samples (3–5×106 cells) were obtained from each patient and cultured at 37°C in RPMI-1640 (cat. no. 12491–15; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; cat. no. 10100-147; Thermo Fisher Scientific, Inc.). Subsequently, one cultured PBMC sample was treated with 1 µg/ml LPS (cat. no. 00-4976-93; Thermo Fisher Scientific, Inc.) for 48 h at 37°C. The other sample was treated with 1 µg/ml LPS for 24 h at 37°C, then 0.75 µg/ml Tan IIA was added for a further 24 h of induction at 37°C. Subsequently, both PBMCs samples were centrifuged (at 500 × g for 5 min at room temperature) to obtain the supernatant and PBMCs. TNF-α and IL-6 serum levels were determined using ELISA kits in accordance with the manufacturer's protocol. The total proteins of PBMCs were extracted using total cell protein extraction kit and stored at −80°C prior to analysis.
siRNA interference assay
A total of 5 ml PBMCs (1.0–1.5×106/ml) were conventionally cultured and centrifuged (500 × g for 5 min at room temperature) to remove medium. Following one wash of the cells in PBS, PBMCs were treated with Lipofectamine 2000 transfection reagent kit (cat. no. 11668019; Thermo Fisher Scientific, Inc.) to transfect β-arrestin 2 siRNA (100 nM). PBMCs treated with β-arrestin 2 siRNA were regarded as the control group. After transfecting the cells for 4 h, the medium (RPMI 1640 and 10% FBS) was refreshed. After incubating the cells for 24 h, 1 µg/ml LPS was added to the medium for another 24 h. Then 0.75 µg/ml Tan IIA was or was not added to the PBMCs for another 24 h. Subsequently, the PBMCs samples were centrifuged (at 500 × g for 5 min at room temperature) to obtain supernatant and PBMCs. TNF-α and IL-6 serum levels were determined using ELISA kits. The total proteins of PBMCs were extracted using total cell protein extraction kit and stored at −80°C until analysis.
Western blot analysis
Protein was extracted using RIPA (cat. no. P0013C; Beyotime Institute of Biotechnology, Haimen, China) and the concentration of total PBMC protein was determined using a BCA protein assay kit. Then total protein (70 µg/lane) was separated using SDS-PAGE (5% gel for concentration and 12% gel for separation) and transferred to a polyvinylidene difluoride membrane (cat. no. LC2002; Thermo Fisher Scientific, Inc.). Following blocking at room temperature with 5% bovine serum albumin for 1 h (cat. no. 30036727; Thermo Fisher Scientific, Inc.), the membrane was incubated with primary and second antibodies. Following incubation with BeyoECL plus (cat. no. p0018M; Beyotime Institute of Biotechnology), the protein bands were analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). The primary antibody included anti-β-arrestin 2 (cat. no. ab54790), anti-NAD-dependent protein deacetylase SIRT1 (19A7AB4; cat. no. ab110304; both 1:2,000), anti-transcription factor p65 (p65; cat. no. ab16502; 1:1,000) and anti-β-actin (cat. no. ab8227; 1:2,000; Abcam, Cambridge, UK) antibodies. The secondary antibodies included horseradish-peroxidase-conjugated goat anti-mouse (cat. no. ab6789) and anti-rabbit (cat. no. ab6721; both 1:2,000; Abcam) antibodies.
Detection of mRNA expression by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from PBMCs using cell RNA kit (cat. no. DP419; Tiangen Biotech, Co., Ltd., Beijing, China). cDNA was synthesized from RNA using a One Step PrimeScript miRNA cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan). The RT protocol was as follows: 37°C for 60 min and 85°C for 5 sec. PCR was subsequently performed using SYBR Premix Ex Taq™ II (Takara Bio, Inc.) and ABI 7500 Fluorescence Quantitative PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a 20 µl volume. The thermocycling conditions were a follows: 95°C for 30 sec, 40 cycles of 90°C for 5 sec and 65°C for 30 sec. cDNA (10 µl/lane) was separated in 1% nucleic acid electrophoresis. 1.2% agarose (cat. no. A9414; Sigma-Aldrich; Merck KGaA) and 5 µl Goodview nucleic acid dye (cat. no. HGV-2; Beijing SBS Genetech Co., Ltd., Beijing, China) were used to separate PCR products, the results of which were observed using a Bio-Rad Gel Imaging Analyzer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Image J software v1 (National Institutes of Health, Bethesda, MD, USA) was used to analyze gray values. The relative expression of each gene was normalized to β-actin. Primer sequences were as follows: β-arrestin 2 forward (F), 5′-ACCCATCACCCTCTGATCCT-3′ and reverse (R), 5′-CTGCCCTCTCCTAGTCAGGT-3′; SIRT1 F, 5′-TGCAGGATTTTAGCCCTGGAG-3′ and R, 5′-AAAGGATTTTGAGGCAAAAGAAGA-3′; p65 F, 5′-CTGTCCCCAAGCCAGGTAAG and R, 5′-AGAGGTGATTTTTGTTCCCCCA-3′; and β-actin F, 5′-AAGTACTCCGTGTGGATCGG-3′ and R, 5′-TCAAGTTGGGGGACAAAAAG-3′.
Statistical analysis
All data are represented as mean ± standard deviation. The differences among two groups were analyzed by independent-samples t-tests and multiple groups were compared using one-way analysis of variance followed by Duncan's post-hoc test. Statistical analyses were conducted using SPSS 19.0 (IBM Corp., Armonk, NY, USA). P<0.05 indicated that the difference between groups was statistically significant.
Results
Tan IIA alleviates RA in rats with the condition
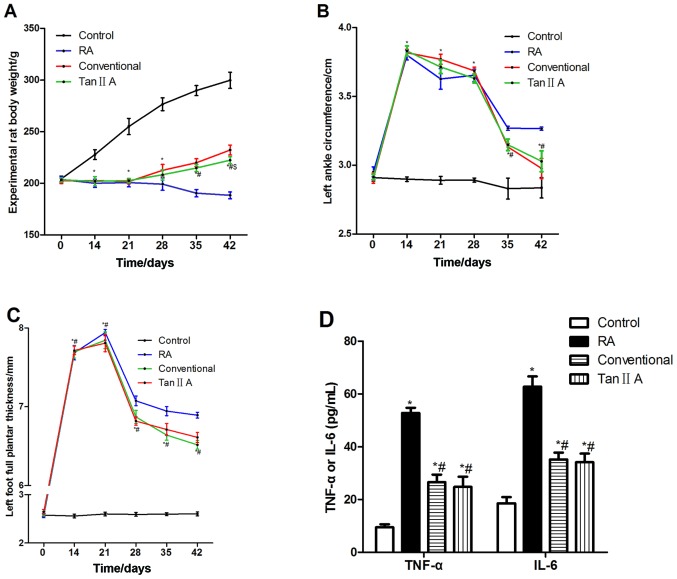
No significant difference was identified in the weight of rats in each group prior to the establishment of the RA model (Fig. 1A). On days 14 and 21, rats in the control group were significantly heavier compared with those in the Tan IIA group (P<0.05). After 1, 2 and 3 weeks of treatment (28, 35 and 42 days after model establishment), the Tan IIA group was significantly lighter compared with the control group (P<0.05). After 2 and 3 weeks of treatment, the Tan IIA group was significantly lighter compared with the RT group (P<0.05). After 3 weeks of treatment, the Tan IIA group was significantly heavier compared with the RA group (P<0.05). The weights of the treated groups were higher compared with those in the RA group.
Figure 1.
Tan IIA alleviates RA in rats. The (A) body weight, (B) circumference of the left posterior ankle and (C) posterior plantar metatarsal thickness were measured over 42 weeks. RT and Tan IIA treatment were administered 21 days after the establishment of the RA models. (D) TNF-α and IL-6 protein concentrations after 3 weeks of treatment. *P<0.05 vs. the Control group; $P<0.05 vs. the RT group. #P<0.05 Tan IIA vs. RA group RA, rheumatoid arthritis; RT, routine treatment (conventional group); Tan IIA, tanshinone II A; TNF, tumor necrosis factor; IL, interleukin.
No significant difference was identified in the circumference of the left posterior ankle of the rats prior to the establishment of the RA model (Fig. 1B). On days 14 and 21, the circumference in the control group was significantly decreased compared with those in the Tan IIA group (P<0.05). After 1 week of treatment, the circumference was significantly greater in the Tan IIA group compared with the control group (P<0.05). After 2 and 3 weeks of treatment, the Tan IIA group was significantly increased and decreased compared with the control and RA groups, respectively (P<0.05).
No significant difference was identified in the posterior plantar metatarsal thickness of the rats in each group prior to the establishment of the RA model (Fig. 1C). On days 14 and 21, the thickness of the Tan IIA group was significantly greater compared with the control group (P<0.05). After 1, 2 and 3 weeks of treatment, the posterior plantar metatarsal thickness in the Tan IIA group was significantly increased and decreased compared with the control and RA groups, respectively (P<0.05). No significant difference was identified in the posterior plantar metatarsal thickness of the Tan IIA group compared with the RT group.
After 3 weeks of treatment, the concentration of TNF-α and IL-6 in the Tan IIA group was significantly lower compared with those in the RA group (P<0.05; Fig. 1D). No significant difference in protein expression was identified between the Tan IIA and RT groups.
Tan IIA injections decrease TNF-α and IL-6 levels in the serum of patients with RA
Prior to treatment, no significant differences in TNF-α and IL-6 levels in the serum of patients with RA were identified between the research and control group (Table I). Following treatment, the TNF-α and IL-6 levels in research group were significantly lower compared with those in the research group prior to treatment and those in the control group following treatment (all P<0.05).
Table I.
TNF-α and IL-6 levels in the serum of patients with rheumatoid arthritis prior to and following treatment.
| TNF-α (pg/ml) | IL-6 (pg/ml) | ||||
|---|---|---|---|---|---|
| Group | Number | Prior to treatment | Following treatment | Prior to treatment | Following treatment |
| Research | 35 | 45.37±8.15 | 38.14±6.76a,b | 8.72±3.45 | 4.46±1.38a,b |
| Control | 15 | 44.82±6.97 | 42.67±5.42 | 8.67±2.87 | 8.23±1.22 |
P<0.05 vs. control group
P<0.05 vs. research group prior to treatment. TNF, tumor necrosis factor; IL, interleukin.
Tan IIA treatment decreases TNF-α and IL-6 levels in PBMCs of patients with RA
Tan IIA significantly inhibited the LPS-induced secretion of TNF-α and IL-6 in PBMCs of patients with RA (both P<0.01; Fig. 2).
Figure 2.
Tan IIA treatment decreases TNF-α and IL-6 levels in peripheral blood mononuclear cells of patients with rheumatoid arthritis. TNF-α and IL-6 protein concentrations after 48 h of treatment. **P<0.01 vs. LPS group. Tan IIA, tanshinone II A; TNF, tumor necrosis factor; IL, interleukin; LPS, lipopolysaccharides.
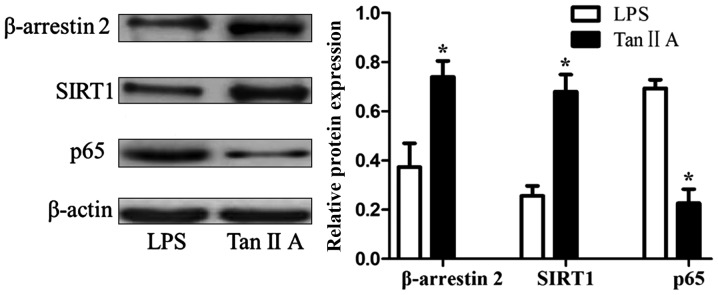
Tan IIA decreases β-arrestin 2 and SIRT1, and increases p65 proteins in PBMCs of patients with RA
Tan IIA significantly upregulated the LPS-inhibited expression of β-arrestin 2 and SIRT1 proteins, and downregulated the LPS-induced expression of the p65 protein in PBMCs of patients with RA (all P<0.05; Fig. 3).
Figure 3.
Tan IIA decreases β-arrestin 2 and SIRT1, and increases p65 proteins in peripheral blood mononuclear cells of patients with rheumatoid arthritis. β-arrestin 2, SIRT1 and p65 protein expression after 48 h of treatment. *P<0.05 vs. the LPS group. Tan IIA, tanshinone II A; LPS, lipopolysaccharides; SIRT1, NAD-dependent protein deacetylase sirtuin-1; p65, transcription factor p65.
Tan IIA decreases β-arrestin 2 and SIRT1, and increases p65 mRNA in PBMCs of patients with RA
Tan IIA significantly upregulated the expression of the β-arrestin 2 and SIRT1 mRNAs in PBMC stimulated by LPS, and inhibited the expression of p65 mRNA compared with cells only stimulated with LPS (all P<0.05; Fig. 4).
Figure 4.
Tan IIA decreases β-arrestin 2 and SIRT1, and increases p65 mRNA in peripheral blood mononuclear cells of patients with rheumatoid arthritis. β-arrestin 2, SIRT1 and p65 mRNA expression after 48 h of treatment. *P<0.05 vs. the LPS group. Tan IIA, tanshinone II A; LPS, lipopolysaccharides; SIRT1, NAD-dependent protein deacetylase sirtuin-1; p65, transcription factor p65.
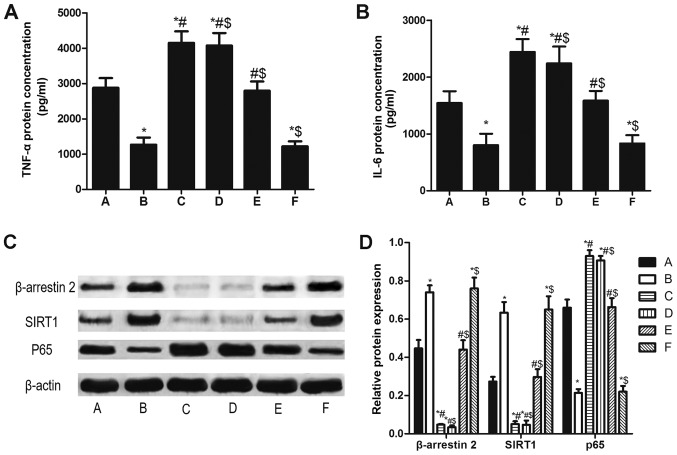
β-arrestin 2 siRNA increases TNF-α, IL-6 and p65, and decreases SIRT1 in PBMCs of patients with RA
Compared LPS stimulation for 48 h alone (A group), TNF-α and IL-6 in PBMCs pretreated with β-arrestin 2 siRNA for 4 h (C group) increased significantly. Furthermore, SIRT1 protein expression decreased and p65 protein expression increased (all P<0.05; Fig. 5). Tan IIA did not significantly reverse the β-arrestin 2 siRNA-induced changes to expression.
Figure 5.
β-arrestin 2 siRNA increases TNF-α, IL-6 and p65, and decreases SIRT1 in peripheral blood mononuclear cells of patients with rheumatoid arthritis. The concentration of (A) TNF-α and (B) IL-6. (C) Qualitative and (D) quantitative analysis of western blotting results for β-arrestin 2, SIRT1 and P65. The groups were as follows: A, LPS stimulation for 48 h; B, LPS stimulation for 48 h and Tan IIA treatment for another 24 h; C, β-arrestin 2 siRNA incubation for 4 h and LPS stimulation for 48 h; D, β-arrestin 2 siRNA incubation for 4 h, LPS stimulation for 48 h and Tan IIA treatment for another 24 h; E, negative control siRNA incubation and LPS stimulation for 48 h; E, negative control siRNA incubation, LPS stimulation for 48 h and Tan IIA treatment for another 24 h. *P<0.05 vs. A group; #P<0.05 vs. B group; $P<0.05 vs. C group. si, small interfering; SIRT1, NAD-dependent protein deacetylase sirtuin-1; p65, transcription factor p65; TNF, tumor necrosis factor; IL, interleukin; Tan IIA, tanshinone II A; LPS, lipopolysaccharides.
Discussion
TNF-α has been demonstrated to promote the occurrence of inflammation by inducing T cells to produce a variety of inflammatory factors (1–3). IL-6, a cytokine produced in monocytes, macrophages and lymphocytes, can stimulate and be associated with the immune response in the body by inducing the differentiation of mononuclear cells and B cells, and enhancing the activity of NK cells (1–3). A previous study demonstrated that TNF-α and IL-6 levels in the serum and synovial fluid of patients with RA were high, indicating that they serve key roles in the pathogenesis of RA by promoting the inflammatory response of patients with RA (2). The current study revealed that TNF-α and IL-6 levels in the serum of patients with RA in the research group following treatment were significantly lower than those in the research group prior to treatment and the control group following treatment, indicating that Tan IIA sulfonate sodium injections could inhibit the inflammatory response in patients with RA. Nizamutdinova et al (13) demonstrated that Tan IIA could inhibit the expression of TNF-α by regulating the phosphatidylinositol 4,5-bisphosphate 3-kinase/RAC-α serine/threonine-protein kinase, protein kinase C and tyrosine-protein kinase JAK/signal transducer and activator of transcription (STAT)3 signaling pathways. The aforementioned study also revealed that Tan IIA could inhibit the TNF-α-induced ICAM-1 expression. Lin et al (14) revealed that Tan IIA inhibited the in vitro and in vivo growth of breast cancer stem cells by downregulating the IL-6/STAT3/NF-κB signaling pathway, suggesting that Tan IIA regulated the expression of TNF-α and IL-6 through regulating multiple signaling pathways.
To further reveal the mechanism behind the Tan IIA-induced downregulation of TNF-α and IL-6 levels, the effect of Tan IIA on the expression of associated proteins (including SIRT1, β-arrestin 2, TNF-α and IL-6) in LPS-stimulated PBMCs was investigated. The current study demonstrated that Tan IIA inhibited the LPS-induced secretion of TNF-α and IL-6, upregulated the LPS-inhibited expression of β-arrestin 2 and SIRT1 proteins, and downregulated the LPS-induced expression of p65 protein in PBMCs of patients with RA. However, Tan IIA could not inhibit the β-arrestin 2 siRNA-induced secretion of TNF-α and IL-6 in PBMCs of patients with RA. These results indicated that Tan IIA inhibited the expression of TNF-α and IL-6 in patients with RA through upregulating β-arrestin 2 expression, thus the inflammatory response in patients with RA was inhibited.
β-arrestin 2 can regulate human immunological functions by inhibiting activation of the NF-κB signaling pathway, and regulating the chemotaxis of immune cells and multiple signaling pathways (1–3). As β-arrestin 2 serves a key role in regulating human immunological functions, it may be associated with the development and progression of certain autoimmune associated diseases (1–3). Li et al (15) demonstrated that β-arrestin 2 inhibited RA progression by inhibiting the inflammatory response in RA rats; the authors hypothesized that the inhibitory response may be associated with inhibiting the NF-κB signaling pathway. The NF-κB signaling pathway is one of the most important signaling pathways in mammalian cells and a node in multiple cell signaling pathways (16). Following its activation, the NF-κB signaling pathway was revealed to regulate the expression of a variety of downstream inflammatory cytokines, which can regulate the inflammatory response (17).
The current study revealed that β-arrestin 2 expression in PBMCs of patients with RA was positively associated with SIRT1 expression and was negatively associated with p65. SIRT1 is a histone deacetylase that is widely expressed in human cells (18–20). SIRT1 can deacetylate p53, UCP2, NF-κB or other transcription factors to exert biological functions (14–20). p65, a key protein in the NF-κB signaling pathway, is acetylated to exert its biological functions. SIRT1 can downregulate the acetylation level of the p65 protein in the inflammatory response, which can inhibit the level of transcription of downstream inflammatory genes, including TNF-α and IL-6 (17). TNF-α and IL-6, as two important inflammatory factors, are not only associated with regulating the body's inflammatory response (21,22), but also serve an important role in the development of rheumatoid diseases (23,24). In summary, the present findings suggested that Tan IIA inhibited NF-κB activity through upregulating β-arrestin 2 expression to inhibit the inflammatory response in PBMCs of patients with RA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XW conceived, designed and revised the current study. JT and SZ analyzed the data and wrote the manuscript. FZ analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study was approved by the Ethics Committee of The Third Affiliated Hospital of Zhejiang Chinese Medical University (Hangzhou, China). A written informed consent form was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res Official. 2011;31:927–940. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mcinnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 3.Kunz M, Ibrahim SM. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediators Inflamm. 2009;2009:979258. doi: 10.1155/2009/979258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simsek I. TNF inhibitors for rheumatoid arthritis-a year in review. Bull NYU Hosp Jt Dis. 2011;69:220–224. [PubMed] [Google Scholar]
- 5.Woodrick RS, Ruderman EM. Interleukin 6 inhibition-RA and beyond. Bull NYU Hosp Jt Dis. 2011;69:225–229. [PubMed] [Google Scholar]
- 6.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 7.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, Donlin LT, Henderson LA, Wei K, Mizoguchi F, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542:110–114. doi: 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Wang D, Lu S, Xu Q, Zhao L, Zhao J, Song Y, Wang H. Increased circulating follicular treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol. 2018;70:711–721. doi: 10.1002/art.40430. [DOI] [PubMed] [Google Scholar]
- 9.Yu Q, Chen H, Sheng L, Liang Y, Li Q. Sodium tanshinone IIA sulfonate prolongs the survival of skin allografts by inhibiting inflammatory cell infiltration and T cell proliferation. Int immunopharmacol. 2014;22:277–284. doi: 10.1016/j.intimp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Shang Q, Wang H, Li S, Xu H. The effect of sodium tanshinone IIA sulfate and simvastatin on elevated serum levels of inflammatory markers in patients with coronary heart disease: A study protocol for a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:756519. doi: 10.1155/2013/756519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jie L, Du H, Huang Q, Wei S, Huang R, Sun W. Tanshinone IIA induces apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis via blockade of the cell cycle in the G2/M phase and a mitochondrial pathway. Biol Pharm Bull. 2014;37:1366–1372. doi: 10.1248/bpb.b14-00301. [DOI] [PubMed] [Google Scholar]
- 12.Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford): 2012;51(Suppl 6):vi5–vi9. doi: 10.1093/rheumatology/kes279. [DOI] [PubMed] [Google Scholar]
- 13.Nizamutdinova IT, Kim YM, Jin H, Son KH, Lee JH, Chang KC, Kim HJ. Tanshinone IIA inhibits TNF-α-mediated induction of VCAM-1 but not ICAM-1 through the regulation of GATA-6 and IRF-1. Int Immunopharmacol. 2012;14:650–657. doi: 10.1016/j.intimp.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Lin C, Wang L, Wang H, Yang L, Guo H, Wang X. Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-κB signaling pathways. J Cell Biochem. 2013;114:2061–2070. doi: 10.1002/jcb.24553. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Cook JA, Gilkeson GS, Luttrell LM, Wang L, Borg KT, Halushka PV, Fan H. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: Isoform specific regulation of inflammation. Mol Immunol. 2011;49:64–74. doi: 10.1016/j.molimm.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan W, Petznick A, Heryati S, Rifada M, Tong L. Nuclear factor-κB: Central regulator in ocular surface inflammation and diseases. Ocul Surf. 2012;10:137–148. doi: 10.1016/j.jtos.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Zheng C, Yin Q, Wu H. Structural studies of NF-κB signaling. Cell Res. 2011;21:183–195. doi: 10.1038/cr.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakur BK, Chandra A, Dittrich T, Welte K, Chandra P. Inhibition of SIRT1 by HIV-1 viral protein Tat results in activation of p53 pathway. Biochem Biophys Res Commun. 2012;424:245–250. doi: 10.1016/j.bbrc.2012.06.084. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuno A, Hori YS, Hosoda R, Tanno M, Miura T, Shimamoto K, Horio Y. Resveratrol improves cardiomyopathy in dystrophin-deficient mice through SIRT1 protein-mediated modulation of p300 protein. J Biol Chem. 2013;288:5963–5972. doi: 10.1074/jbc.M112.392050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelová H, Hošek J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 22.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Seriolo B, Paolino S, Sulli A, Fasciolo D, Cutolo M. Effects of anti-TNF-alpha treatment on lipid profile in patients with active rheumatoid arthritis. Ann NY Acad Sci. 2006;1069:414–419. doi: 10.1196/annals.1351.039. [DOI] [PubMed] [Google Scholar]
- 24.Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Murata N, van der Heijde D, Kishimoto T. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): Evidence of clinical and radiographic benefit from an × ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.