Abstract
Thromboembolism is a commonly observed condition in geriatrics that is caused by vascular endothelial injury, platelet activation, physiological coagulation processes, reduction of anticoagulant activity, decreased fibrinolytic activity and abnormal flow in the heart chamber, artery or vein. The protein C anticoagulant system serves a crucial role in anticoagulant therapy for the treatment of thromboembolism. Previous findings have suggested that edoxaban is an efficient oral anticoagulant in the acute treatment of venous thromboembolism. In the present study, the efficacy of edoxaban on thromboembolism induced by atrial fibrillation was investigated in a mouse model. Inflammatory factors interleukin (IL)-1, −4, −8 and tumor necrosis factor (TNF)-α were analyzed in the sera of mice with fibrillation induced by thromboembolism. Expression and activity of thymic stromal lymphopoietin (TSLP) and activated protein C resistance were investigated in platelets and vascular endothelial cells (VECs). TSLP-induced platelet viability, Wnt-β phosphorylation and integrin expression were analyzed in platelets. Furthermore, Wnt-β expression and the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway in VECs were analyzed. Results demonstrated that the expression levels of IL-1, −4, −8 and TNF-α were significantly downregulated in the sera of mice with fibrillation and thromboembolism following treatment with edoxaban (P<0.01). Furthermore, the expression levels of prostacyclin (PGI2), prostaglandin (PG)E2, PGD2 and PGF2α were significantly increased in the sera of experimental mice that received edoxaban therapy (P<0.01). Results also indicated that edoxaban significantly stimulated the protein expression of TSLP and activated Wnt-β phosphorylation and integrin expression in platelets (P<0.01). In addition, edoxaban therapy significantly upregulated the expression levels of PI3K and AKT, and subsequently increased the activity of protein C and S in VECs (P<0.01). Notably, edoxaban treatment improved atrial fibrillation and thromboembolism, as determined by pathological analysis. In conclusion, these results suggested that edoxaban elicited beneficial effects for mice with atrial fibrillation induced by thromboembolism through the regulation of the Wnt-β-induced PI3K/ATK-activated protein C system.
Keywords: thromboembolism, inflammatory factors, apoptosis, edoxaban, Wnt-β, thymic stromal lymphopoietin, phosphoinositide 3-kinase/protein kinase B
Introduction
Thromboembolism is a severe life-threatening disease that comprises deep vein thrombosis and deep artery thrombosis and is caused by vascular endothelial injury, platelet activation, physiological coagulation processes, reduction of anticoagulant activity, decreased fibrinolytic activity and abnormal flow in the heart chamber, artery or vein (1,2). Thromboembolism has been reported to significantly affect the quality of life in patients with venous thrombosis (3,4) and has also been indicated to lead to microcirculation dysfunction, embolism of skin mucous membrane necrosis, organ dysfunction and bleeding tendencies and pulmonary embolism (5,6). Clinical investigation has revealed that atrial fibrillation and thromboembolism significantly affects health (7). Furthermore, atrial fibrillation has been demonstrated to cause sudden fatality in patients with thromboembolism (7).
Previous studies have indicated that anticoagulation treatments are the most commonly used and efficient therapeutic agents for the prevention and treatment in adults and children who have undergone catheter-associated thrombosis in the clinical setting (8). Edoxabana, an inhibitor of coagulation factor Xa, is a clinically prescribed pharmacological agent for the treatment of non-atrial fibrillation that reduces stroke and systemic embolism by targeting coagulation factor Xa (9). A previous study indicated that systemic inflammation was observed and detected in patients with left atrial thrombus and non-rheumatic atrial fibrillation (10). In addition, previous findings indicated that the pathogenesis of chronic inflammation may be associated with the promotion of thrombus formation and dysfunction of platelet function, as determined by in vivo imaging (11). Furthermore, research has identified that apoptosis of vascular endothelial cells (VECs) is an important indicator for the severity of a venous thrombus (12).
Although the effects and safety profile of edoxaban have been investigated in patients with non-atrial fibrillation in previous reports, the application of edoxaban for patients with atrial fibrillation has not been fully elucidated and the molecular mechanism involved remains unclear (13). Previous results have indicated that the activity of the protein C system is correlated with thrombus formation and is involved in the inhibition of thrombin generation in the platelet microenvironment (14,15). In addition, Hadas et al (16) suggested that methylglyoxal induces platelet hyperaggregation and reduces thrombus stability through the upregulation of protein kinase C activity and downregulation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway. Furthermore, Yi et al (17) demonstrated that the PI3K inhibitor S14161 inhibits the modulation of platelet activation and thrombus formation, which suggests that PI3K may be a novel therapeutic target for the prevention of thrombotic disorders.
In the present study, PI3K and AKT expression levels in VECs from mice with atrial fibrillation and thromboembolism that received treatment with edoxaban were investigated. In addition, the efficacy of edoxaban on TSLP expression, Wnt-β phosphorylation and integrin expression in platelets was explored. In the present study, the effects of edoxabana on inflammation and apoptosis in a mouse model of atrial fibrillation and thromboembolism were investigated. The molecular mechanism of the edoxabana-mediated signaling pathway in VECs was explored and the association between edoxaban, the protein C system and atrial fibrillation and venous thrombosis was determined.
Materials and methods
Ethics statement
Animal experiments were implemented legitimately according to the Guide for the Care and Use of Laboratory Animals of Xinjiang Medical University (Urumchi, China) and approved by the Ethics Committee of The First Affiliated Hospital, Xinjiang Medical University.
Cells culture and regents
VECs and platelet cells were isolated from C57BL/6J mice (as described in the animal study section) with ferric chloride-induced vein thrombus (18). VECs and platelet cells were cultured in minimum essential medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 12% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA USA). All cells were cultured in a 37°C humidified atmosphere containing 5% CO2.
Small interfering (si)RNA transfection
VECs were cultured to 80% confluence and transfected with siRNA (0.12 µmol/l; cat. no. 6388; Cell Signaling Technology, Inc., Danvers, MA, USA) that targeted Wnt-β (Si-Wnt-β) or scrambled siRNA (Si-vector) using Lipofectamine RNAi MAX (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. siRNA-targeting Wnt-β and scrambled siRNA were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). The time interval between transfection and subsequent experimentation was 24 h.
Western blot analysis
VECs and platelet cells were isolated from experimental mice, homogenized using a radioimmunoprecipitation assay lysate buffer (Invitrogen; Thermo Fisher Scientific, Inc.) containing protease-inhibitor and centrifuged at 1,000 × g at 4°C for 10 min. The supernatant was used for protein analysis. Protein concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Western blotting was performed as described previously (19). Equal volumes of protein (50 µg) were loaded onto each well and separated with 10% SDS-PAGE. Polyvinylidene fluoride membranes were then used for immunoblotting. The membrane was blocked using 5% milk for 1 h at room temperature. Primary antibodies were then incubated at 4°C overnight. These were as follows: TSLP (1:1,000; cat. no. ab188166; Abcam, Cambridge, UK), Protein C (1:1,000; cat. no. GTX87071), Protein S (1:1,000; cat. no. GTX29027; both GeneTex, Inc., Irvine, CA, USA), APCI (1:2,000; cat. no. 250212; Abbiotec inc., San Diego, CA, USA), thrombomodulin (1:1,000; cat. no. ab230189; Abcam), αIlbβ3 (1:1,000; cat. no. 10139-R012; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), p-Wnt β (1:2,000; cat. no. 8655S; Cell Signaling Technology, Inc.), Thrombin (1:1,000; cat. no. ab92621), PI3K (1:1,000; cat. no. ab151549), AKT (1:1,000; cat. no. ab18785), integrin (1:1,000; cat. no. ab52971), β-actin (1:2,000; cat. no. ab8827; all Abcam). After washing using Tris buffered saline with Tween-20 three times for 8 min, secondary antibodies were incubated at room temperature for 1 h. GAPDH was used as an internal control. The secondary antibodies utilized were as follows: horseradish peroxidase conjugated Rabbit anti-mouse immunoglobulin G; 1:1,000; cat. no. ab6728; Abcam). Immunoreactivity was determined by enhanced chemiluminescent autoradiography using an ImageQuant Las4000 (Thermo Fisher Scientific, Inc.) Protein expression was analyzed using BandScan 5.0 software (Glyko, Inc.; BioMarin Pharmaceutical Inc., San Rafael, CA, USA).
Animal study
A total of 40 C57BL/6J female mice (6–8 weeks old, 20–25 g in body weight) were purchased from Vital River Laboratory Animal Technology Co. Ltd. Mice were housed in a temperature-controlled facility at 23±1°C with a relative humidity of 50±5% and 12 h light/dark cycle with free access to food and water. To develop venous thrombosis, ferric chloride was used to induce C57BL/6J mice according previous study (18). Mice with venous thrombosis and atrial fibrillation (as confirmed via ECGs) were divided into two groups (n=20) and received treatment with edoxaban (10 mg/kg, Simeiquan Biological Technology Co., Ltd., Shenzhen, China) or the same dose of phosphate-buffered saline (PBS) by gavage. The treatments were continued for 42 days and were administered once a day. On day 60, mice were sacrificed and the venous samples were obtained for further analysis.
Histological analysis
Cardiac veins were isolated from experimental mice, fixed with 4% paraformaldehyde for 2 h at 37°C and permeabilized by incubating with 80% absolute ethanol, then embedded in paraffin and cut into sections (5 µm). The sections then underwent microwave antigen retrieval. After cooling, they were washed with distilled water and blocked using normal goat serum (Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for 30 min. The cardiac vein sections were incubated with primary antibodies: anti Thrombin (1:1,000, cat. no. ab92621; Abcam) for 12 h at 4°C. Fluorescent-labeled AlexaFluor 488 horseradish peroxidase conjugated rabbit anti-mouse immunoglobulin G (1:1,000; cat. no. ab6728; Abcam) for 2 h at 37°C. The histologic sections were subsequently scanned using a confocal microscope (Carl Zeiss AG, Oberkochen, Germany; magnification, ×200).
ELISA
Following treatment for 60 days, the plasma concentration of interleukin (IL)-1 (cat. no. ab100704), IL-4 (cat. no. ab100710), IL-8 (cat. no. ab46032), tumor necrosis factor (TNF)-α (cat. no. ab208348), prostacyclin (PGI2; cat. no. ab120912), prostaglandin (PG)E2 (cat. no. ab133021; all Abcam), PGD2 (cat. no. RJ17502; Shanghai Renjie Biotechnology Co., Ltd., Shanghai, China), PGF2α (cat. no. YS3501B; Shanghai Yaji Biotechnology Co., Ltd., Shanghai, China) and Serotonin (cat. no. ab133053; Abcam) in mice with venous thrombosis and atrial fibrillation were analyzed using commercialized ELISA kits according to the manufacturer's protocol. The operational procedures were performed according to the manufacturer's instructions. The results were analyzed using an ELISA reader system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labelling (TUNEL) assay
Treated VECs were fixed with 4% neutral formaldehyde for 20 min at 4°C. After washing with PBS, cells were permeabilized on ice with 0.1% Triton X-100 in PBS for 2 min and mounted with neutral resins. The TUNEL assay kit (Biotool LLC, Houston, TX, USA) was used to determine the extent of apoptosis of VECs in experimental mice following treatment with 10 mg/kg edoxaban or the same dose of PBS. The procedures were performed as indicated in a previous study (20). Cellular nuclei were stained with DAPI (vector Laboratories, Inc., Burlingame, CA, USA). Images were captured with a ZEISS LSM 510 confocal microscope at 488 nm. Apoptotic cells were examined under a laser confocal microscope (Fluoview 1000; Olympus, Tokyo, Japan; magnification, ×200). The cellular nuclei and apoptotic cells were counted in three fields of view from each sample.
Patelet aggregation test and platelet release assay
Blood was collected and mixed with sodium citrate to produce a final concentration of 0.38%. Citrated blood was then mixed with an equal volume (5 ml) of PBS (pH 7.4). Platelet-rich plasma (PRP) was obtained via centrifugation at a speed of 50 × g for 10 min at 23°C with a refrigerated centrifuge (cat. no. TGL-16A; Hunan Xiangli Scientific Instrument Co., Ltd., Changsha, China). The PRP was then centrifuged a second time at 50 × g for 10 min at 23°C. The resultant suspension was further centrifuged at 750 × g for 10 min at 23°C to obtain the platelet pellet. The platelet pellet was washed once in HEPES/Tyrode's buffer (10 mM HEPES/NaOH, 5.56 mM glucose, 137 mM NaCl, 12 mM NaHCO3, 2.7 mM KCl, 0.36 mM NaH2PO4, 1 mM MgCl2, pH 7.4) in the presence of 1 mM EGTA 20. Following centrifugation at 750 × g for 10 min at room temperature the platelet pellet was gently suspended in HEPES/Tyrode's buffer and platelet concentrations were 2–3×108/ml. Washed platelets were then pre-incubated for 5 min at 37°C.
In vitro platelet aggregation was assessed in citrated platelet rich plasma using a previously described method (21). To minimize the loss of denser platelets, the platelet agonists were as follows: Collagen (4 µg/ml; Mascia Brunelli S.p.a., Milan, Italy), adenosine diphosphate (5 µM), and ristocetin (1.5 mg/ml; each from Sigma-Aldrich; Merck KGaA).
Platelet release was then induced for 10 min. by adding collagen to a final concentration of 50 µg/ml. Prior to the addition of collagen, CPsA (Shanghai Yaji Biotechnology Co., Ltd.) was added to the washed platelets to obtain a final concentration of 500 µg/ml. When reaction was completed, samples were centrifuged at 10,000 × g for 2 min at room temperature. The supernatants were then recovered and aliquoted. The levels of Serotonin were determined using the aforementioned ELISA kits.
Statistical analysis
Data are presented as mean ± standard error of the mean. Statistical differences between experimental groups were analyzed using a Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Edoxaban affects the protein expression levels of inflammatory factors and chemokines in the sera of mice with atrial fibrillation and venous thrombosis
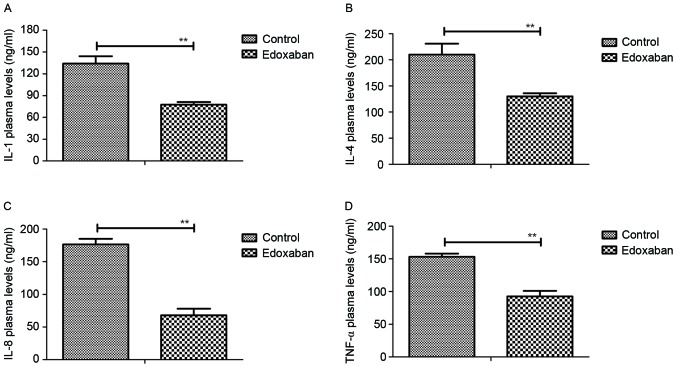
Inflammatory factor expression levels in the sera. As indicated in Fig. 1A-D, the expression levels of IL-1, −4, −8 and TNF-α were significantly decreased in the sera of mice with fibrillation and venous thrombosis that were treated with edoxaban when compared with mice that received PBS treatment (P<0.01). Furthermore, edoxaban therapy resulted in a significant increase in the expression levels of chemokines PGI2, PGE2, PGD2 and PGF2α compared with PBS treatment (P<0.01; Fig. 1E-H). These results suggest that edoxaban may inhibit the expression of inflammatory factors and promote the expression of chemokines in the sera of mice with atrial fibrillation and venous thrombosis.
Figure 1.
Effects of edoxaban on inflammatory factors and chemokines in the sera of mice with atrial fibrillation and vein thrombus. Plasma levels of (A) IL-1, (B) IL-4, (C) IL-8 and (D) TNF-α, (E) PGI2, (F) PGE2, (G) PGD2 and (H) PGF2α in mice with fibrillation and vein thrombus treated with edoxaban or phosphate-buffered saline. Data are presented as the mean ± standard deviation of the mean. **P<0.01 as indicated. IL, interleukin; TNF, tumor necrosis factor; PG, prostaglandin.
Edoxaban promotes the protein expression levels of TSLP and activates the protein C system in mice with atrial fibrillation and venous thrombosis
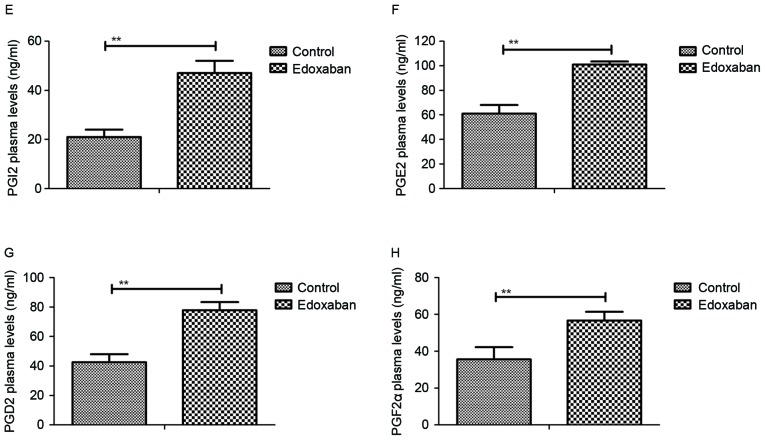
TSLP and activated protein C protein expression levels were examined in mice with atrial fibrillation and venous thrombosis using western blot analysis. As indicated in Fig. 2A, edoxaban significantly promoted TSLP protein expression levels in platelets (P<0.01). In addition, the expression levels of protein C system and APC components were detected. Results indicated that protein C and protein S expression levels were significantly upregulated in experimental mice treated with edoxaban compared with those treated with PBS (P<0.01; Fig. 2B). Furthermore, the protein expression levels of thrombomodulin (TM) were significantly upregulated, whereas the protein expression levels of activated protein C inhibitor (APCI) were significantly downregulated in edoxaban-treated mice with atrial fibrillation and venous thrombosis (P<0.01; Fig. 2C). Furthermore, it was demonstrated that P-selectin and integrin αIIbβ3 protein expression levels were significantly increased in mice that received edoxaban therapy (P<0.01; Fig. 2D). These results suggest that edoxaban may stimulate TSLP expression and activate the protein C system in mice with atrial fibrillation and venous thrombosis.
Figure 2.
Analysis of TSLP expression and protein C system activity in mice with atrial fibrillation and vein thrombus. (A-D) Protein expression levels of TSLP, PC, PS, TM, APCI, P-selectin and integrin αIIbβ3 in experimental mice treated with edoxaban or phosphate-buffered saline. Data are presented as mean ± standard deviation of the mean. **P<0.01 as indicated. TSLP, thymic stromal lymphopoietin; PC, protein C; PS, protein S; TM, thrombomodulin; APCI, activated protein C inhibitor.
Edoxaban activates Wnt-β phosphorylation and inhibits platelet aggregation and secretion in mice with atrial fibrillation and venous thrombosis
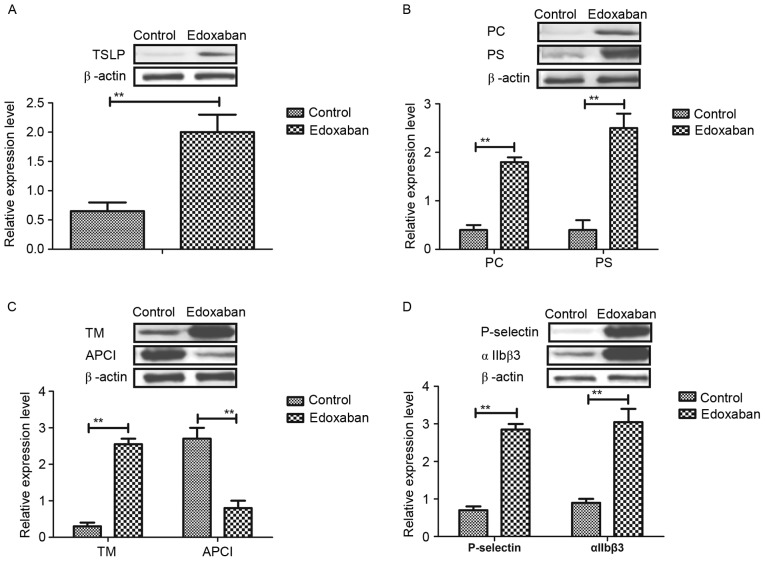
To investigate the mechanism of edoxaban-induced upregulation of TSLP expression levels, Wnt-β phosphorylation and integrin expression was detected in platelets in mice with atrial fibrillation and venous thrombosis. The results in Fig. 3A indicated that edoxaban significantly promoted Wnt-β phosphorylation in platelets (P<0.01). In addition, it was observed that edoxaban treatment significantly inhibited the aggregation of platelet cells and significantly downregulated the protein expression levels of thrombin compared with the control (P<0.01; Fig. 3B and C). Furthermore, results indicated that platelet serotonin release was significantly decreased in experimental mice treated with edoxaban compared with the control (P<0.01; Fig. 3D). Taken together, the results suggest that edoxaban may activate Wnt-β phosphorylation and inhibit platelet aggregation and secretion in mice with atrial fibrillation and venous thrombosis.
Figure 3.
Effects of edoxaban on Wnt-β phosphorylation, and platelet aggregation and secretion in mice with atrial fibrillation and vein thrombus. (A) Wnt-β phosphorylation in platelets in edoxaban-treated mice with atrial fibrillation and vein thrombus. (B) Aggregation of platelet cells in experimental mice. (C) Protein expression levels of thrombin and (D) platelet serotonin release in mice with atrial fibrillation and vein thrombus. All data are presented as the mean ± standard deviation of the mean. **P<0.01 as indicated.
Edoxaban regulates protein C system through Wnt-β-mediated PI3K/AKT signal pathway in VECs
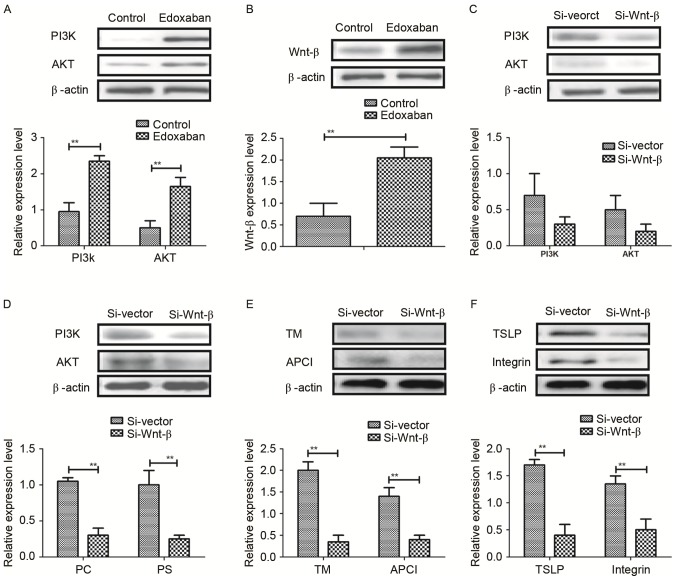
The edoxaban-mediated regulation of the protein C system in VECs from experimental mice with atrial fibrillation and venous thrombosis was further investigated. As indicated in Fig. 4A, the protein expression levels of PI3K and AKT were significantly upregulated following edoxaban treatment (P<0.01). In addition, edoxaban significantly upregulated Wnt-β protein expression levels in VECs (P<0.01; Fig. 4B). Furthermore, results indicated that inhibition of Wnt-β by siRNA (Si-Wnt-β) inhibited the protein expression levels of PI3K and AKT in VECs (Fig. 4C). Additionally, it was observed that Si-Wnt-β treatment significantly decreased the protein expression levels of protein C and protein S (P<0.01; Fig. 4D). Expression levels of TM and APCI were also significantly downregulated by Si-Wnt-β (P<0.01; Fig. 4E). Furthermore, TSLP and integrin protein expression levels were significantly suppressed by Si-Wnt-β (P<0.01; Fig. 4F). Taken together, these results suggest that edoxaban may regulate the protein C system through the Wnt-β-mediated PI3K/AKT signaling pathway in VECs.
Figure 4.
Edoxaban regulates the protein C system through the Wnt-β-mediated PI3K/AKT signal pathway in VECs. (A) Expression levels of PI3K and AKT in VEC in experimental mice. (B) Wnt-β expression levels in VEC in Edoxaban-treated mice with atrial fibrillation and vein thrombus. (C) Effects of Si-Wnt-β on Expression levels of PI3K and AKT in VEC in Edoxaban-treated mice with atrial fibrillation and vein thrombus. (D) Effects of Si-Wnt-β on Expression levels of PC and PS in VEC in Edoxaban-treated mice with atrial fibrillation and vein thrombus. (E) Expression levels of TM and APCI were downregulated by Si-Wnt-β. (F) Effects of Si-Wnt-β on Expression levels of TSLP and integrin in VEC in Edoxaban-treated mice with atrial fibrillation and vein thrombus. All data are presented as the mean ± standard deviation of the mean. **P<0.01 as indicated. VEC, vascular endothelial cells; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; TM, thrombomodulin; APCI, activated protein C inhibitor; TSLP, thymic stromal lymphopoietin; PS, protein S; PC, protein C.
Edoxaban improves atrial fibrillation and venous thrombosis in a mouse model
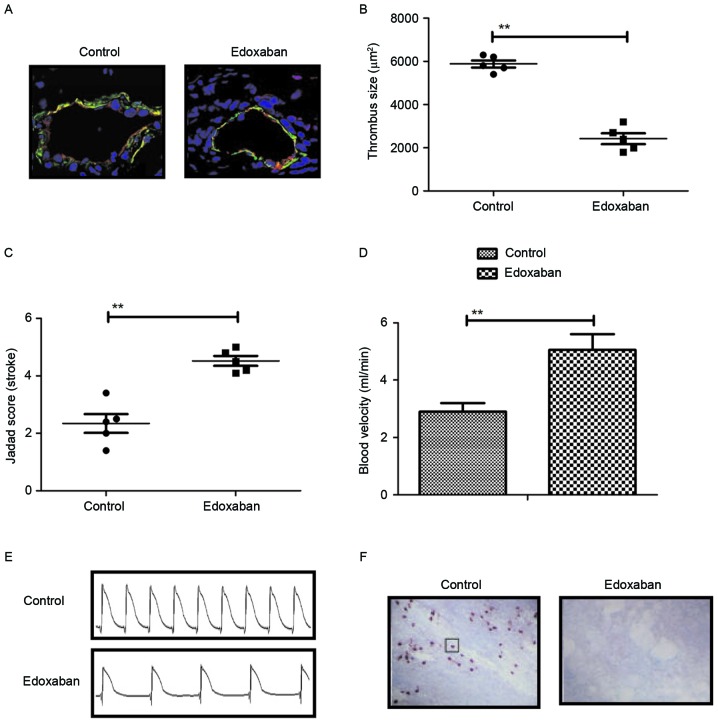
In vivo therapeutic effects of edoxaban were investigated in mice with atrial fibrillation and venous thrombosis. As indicated in Fig. 5A, data indicated that edoxaban improved apoptosis (as determined by reduced staining) of VECs compared with control mice. Furthermore, defective thrombus formation was significantly improved by edoxaban compared with the control according to shear rates data (P<0.01; Fig. 5B). In addition, results indicated that stroke and blood velocity were significantly improved in experimental mice treated with edoxaban (P<0.01; Fig. 5C and D). Furthermore, data indicated that atrial fibrillation was improved as the area of venous thrombosis was markedly decreased in edoxaban-treated mice with atrial fibrillation and vein thrombus (Fig. 5E and F). Taken together, the results suggest that edoxaban may improve apoptosis, stroke, atrial fibrillation and venous thrombosis in a mouse model.
Figure 5.
Effects of edoxaban on stroke, atrial fibrillation and vein thrombus in mice with atrial fibrillation and vein thrombus. (A) Apoptosis of vascular endothelial cells in mice treated with edoxaban or phosphate-buffered saline (magnification, ×200). (B) Effects of edoxaban on thrombus formation in the mice model of atrial fibrillation and vein thrombus. Efficacy of edoxaban was analyzed on (C) stroke and (D) blood velocity in experimental mice treated with edoxaban or phosphate-buffered saline. Effects of (E) atrial fibrillation and (F) area of vein thrombus in mice with atrial fibrillation and vein thrombus treated with edoxaban or phosphate-buffered saline (magnification, ×200). The black box indicates vein thrombus. All data are presented as the mean ± standard deviation of the mean. **P<0.01 as indicated.
Discussion
Venous thrombosis is a common disorder that is associated with various molecular mechanisms and diverse processes (22). Multiple factors may induce the pathology of venous thromboembolism, including VEC injury, blood flow state changes and increased blood clotting (22). Notably, venous thromboembolism may induce a large number of other diseases, including pulmonary embolism, neurologic diseases and skin disease (23–25). Recently, atrial fibrillation and venous thrombosis has been associated with significantly increased morbidity and mortality rates in patients with cardiovascular disease in the clinical setting (26). Anticoagulant therapy is prescribed for millions of patients worldwide for the prevention and treatment of venous thrombosis (27,28).
In the present study, edoxaban was administered for the treatment of atrial fibrillation and venous thrombosis and the efficacy and molecular mechanism of edoxaban was investigated. Inflammatory factors and chemokine serum levels were studied in experimental mice treated with edoxaban. In addition, the association between edoxaban and the protein C system in VECs was assessed in experimental mice. Furthermore, the Wnt-β-mediated PI3K/AKT signaling pathway in VECs was also investigated. The present results suggested that edoxaban inhibited the serum levels of inflammatory factors, but also improved apoptosis, stroke, atrial fibrillation and venous thrombosis through regulation of the Wnt-β-mediated PI3K/AKT signaling pathway in a mouse model of atrial fibrillation and venous thrombosis.
Serum levels of inflammatory factors are an important measurement index in patients with cardiovascular disease in the clinical setting (29). Previous results identified that increased levels of inflammatory markers were associated with disease severity of patients with rheumatic mitral stenosis predisposed to left atrial thrombus formation (30). In addition, Empana et al (31) revealed that inflammation contributed to thrombotic diseases based on microparticles and sudden cardiac death due to coronary occlusion. Furthermore, Sadowski et al (32) indicated that coronary thrombus composition was associated with inflammation, and platelet and endothelial markers. Notably, the expression levels of the chemokines have been demonstrated to serve an essential role in portal vein thrombosis (33). The present results suggested that edoxaban significantly inhibited the serum levels of inflammatory factors and upregulated the levels of chemokines in mice with atrial fibrillation and venous thrombosis. These regulations are beneficial for the treatment of atrial fibrillation and venous thrombosis.
Protein C is important regulatory protein in the progression of venous thrombosis (14,15). The present study investigated the role of edoxaban on the protein C system in a mouse model of atrial fibrillation and venous thrombosis. It is well acknowledged that TSLP expression is associated with the activity of the protein C system and may predict the severity of patients with atrial fibrillation and venous thrombosis (34,35). In addition, a previous report has suggested that the levels of TM and APCI was also correlated with venous thrombosis (36). The present results indicated that edoxaban promoted TM expression and inhibited APCI in the serum of mice with venous thrombosis. Furthermore, previous reports have indicated that P-selectin and integrin may inhibit thrombus resolution and prevent vein wall fibrosis (37,38). In the present study, it was indicated that P-selectin and integrin αIIbβ3 expression levels were increased in mice following edoxaban therapy. These results suggest that edoxaban may stimulate TSLP expression and activate the protein C system in mice with atrial fibrillation and venous thrombosis.
The PI3K/AKT signaling pathway is associated with the progression of vein thrombus (16). However, to the best of our knowledge, the effect of edoxaban on the PI3K/AKT signaling pathway has not been previously studied. Guidetti et al (39) suggested that the PI3K/AKT signaling pathway is stimulated by integrin engagement and further inhibits activation of platelet in thrombus formation and stabilization. These findings highlighted the possibility of novel venous thrombosis and anti-thrombotic therapeutic strategies. In addition, Su et al (40) also indicated that human cathelicidin LL-37 inhibits the aggregation of platelets and further lead to the inhibition of thrombosis via Src/PI3K/Akt signaling. Notably, blood activity of platelets serve a crucial role in hemostasis and formation of thrombosis (41). In the present analysis, it was revealed that PI3K and AKT expression levels were significantly upregulated by edoxaban through the Wnt-β-mediated PI3K/AKT signaling pathway in a mouse model of atrial fibrillation and venous thrombosis. However, inhibition of Wnt-β by its inhibitor (Si-wnt-β) suppressed the protein expression levels of TM, TSLP and integrin, which were also downregulated by Si-Wnt-β in VECs. Taken together, these results suggest that edoxaban may regulate the protein C system through the Wnt-β-mediated PI3K/AKT signal pathway in VECs.
In conclusion, the present study identified that edoxaban may be a potential oral anticoagulant in the acute treatment of atrial fibrillation and venous thrombosis. The novel findings suggest a potential insight to explore more efficient preclinical mechanisms and therapeutic strategies of edoxaban for treating atrial fibrillation and venous thrombosis. Notably, the present findings demonstrated preclinical and experimental evidence to support the efficacy of edoxaban, but also suggested the molecular mechanism of edoxaban-mediated changes of Wnt-β, P-selectin and integrin αIIbβ3 expression through the PI3K/AT signaling pathway in VECs obtained from mice with atrial fibrillation and venous thrombosis. Results identified that edoxaban activated the protein C system by stimulation of the PI3K/AT signaling pathway in VECs obtained from mice with atrial fibrillation and venous thrombosis. Taken together, the present outcomes suggest that edoxaban may improve the venous lesions by regulation of apoptosis, stroke, atrial fibrillation and venous thrombosis through the Wnt-β-mediated protein C system via the PI3K/AKT signaling pathway, suggesting eoxaban may be an efficient anti-thrombotic therapeutic agent for the treatment of atrial fibrillation and venous thrombosis in the clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Xinjiang Natural Science Fund (Efficacy and mechanism of protein C system in progression of atrial fibrillation and vein thrombus, grant no. 2016D01C304).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XS and SM designed the present study and analyzed the data; XS and ZL performed the experiments. MW analyzed the data.
Ethics approval and consent to participate
All animal procedures were reviewed and approved by the Ethical Committee of The First Affiliated Hospital, Xinjiang Medical University and the China Experimental Animal Protection Association. All efforts were made to minimize the suffering of the experimental mice.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lim MS, Enjeti AK. Safety of anticoagulation in the treatment of venous thromboembolism in patients with haematological malignancies and thrombocytopenia: Report of 5 cases and literature review. Crit Rev Oncol Hematol. 2016;105:92–99. doi: 10.1016/j.critrevonc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Nagler M, Kremer Hovinga JA, Alberio L, Peter-Salonen K, von Tengg-Kobligk H, Lottaz D, Neerman-Arbez M, Lämmle B. Thromboembolism in patients with congenital afibrinogenaemia. Long-term observational data and systematic review. Thromb Haemost. 2016;116:722–732. doi: 10.1160/TH16-02-0082. [DOI] [PubMed] [Google Scholar]
- 3.Shlebak A. Antiphospholipid syndrome presenting as cerebral venous sinus thrombosis: A case series and a review. J Clin Pathol. 2016;69:337–343. doi: 10.1136/jclinpath-2015-203077. [DOI] [PubMed] [Google Scholar]
- 4.Samoš M, Bolek T, Ivanková J, Stančiaková L, Kovář F, Galajda P, Kubisz P, Staško J, Mokáň M. Heparin induced thrombocytopenia presenting with deep venous thrombosis and pulmonary embolism successfully treated with rivaroxaban: Clinical case report and review of current experiences. J Cardiovasc Pharmacol. 2016;68:391–394. doi: 10.1097/FJC.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 5.McKeage K, Keating GM. Parnaparin: A review of its use in the management of venous thromboembolism, chronic venous disease and other vascular disorders. Drugs. 2008;68:105–122. doi: 10.2165/00003495-200868010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini VD., Jr Prophylaxis against venous thromboembolism after total hip and knee arthroplasty: A critical analysis review. JBJS Rev. 2015;3 doi: 10.2106/JBJS.RVW.N.00111. 01874474-201509000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Devlin R, Bonanno L, Badeaux J. The incidence of thromboembolism formation following the use of recombinant factor VIIa in patients suffering from blunt force trauma compared with penetrating trauma: A systematic review. JBI Database System Rev Implement Rep. 2016;14:116–138. doi: 10.11124/JBISRIR-2016-2063. [DOI] [PubMed] [Google Scholar]
- 8.Barco S, Atema JJ, Coppens M, Serlie MJ, Middeldorp S. Anticoagulants for the prevention and treatment of catheter-related thrombosis in adults and children on parenteral nutrition: A systematic review and critical appraisal. Blood. 2017;15:369–377. doi: 10.2450/2016.0031-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piazza G, Mani V, Goldhaber SZ, Grosso MA, Mercuri M, Lanz HJ, Schussler S, Hsu C, Chinigo A, Ritchie B, et al. Magnetic resonance venography to assess thrombus resolution with edoxaban monotherapy versus parenteral anticoagulation/warfarin for symptomatic deep vein thrombosis: A multicenter feasibility study. Vasc Med. 2016;21:361–368. doi: 10.1177/1358863X16645853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maehama T, Okura H, Imai K, Saito K, Yamada R, Koyama T, Hayashida A, Neishi Y, Kawamoto T, Yoshida K. Systemic inflammation and left atrial thrombus in patients with non-rheumatic atrial fibrillation. J Cardiol. 2010;56:118–124. doi: 10.1016/j.jjcc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura S. Pathogenesis of chronic inflammation revealed by in vivo imaging: Thrombus formation and platelet function. Rinsho Ketsueki. 2010;51:620–624. (In Japanese) [PubMed] [Google Scholar]
- 12.Mo XR, Luo XJ, Li CP, Pan XF, Zhou LL. Effect of mannitol injection by intravenous catheter on ear vein endothelial cell apoptosis and venous thrombus in rabbits. Eur Rev Med Pharmacol Sci. 2015;19:491–497. [PubMed] [Google Scholar]
- 13.Shirai Y, Noguchi T, Kita S, Nakamura A, Tanimoto H, Ishigaki N, Aoyama K, Yoshida T. A study of usefulness for portal vein thrombus with edoxaban. Nihon Shokakibyo Gakkai Zasshi. 2016;113:439–440. doi: 10.11405/nisshoshi.113.439. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 14.Fei D, Meng X, Zhao M, Kang K, Tan G, Pan S, Luo Y, Liu W, Nan C, Jiang H, et al. Enhanced induction of heme oxygenase-1 suppresses thrombus formation and affects the protein C system in sepsis. Transl Res. 2012;159:99–109. doi: 10.1016/j.trsl.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Lozano M, Escolar G, Schwarz HP, Hernández R, Bozzo J, Ordinas A. Activated protein C inhibits thrombus formation in a system with flowing blood. Br J Haematol. 1996;95:179–183. doi: 10.1046/j.1365-2141.1996.d01-1870.x. [DOI] [PubMed] [Google Scholar]
- 16.Hadas K, Randriamboavonjy V, Elgheznawy A, Mann A, Fleming I. Methylglyoxal induces platelet hyperaggregation and reduces thrombus stability by activating PKC and inhibiting PI3K/Akt pathway. PLoS One. 2013;8:e74401. doi: 10.1371/journal.pone.0074401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi W, Li Q, Shen J, Ren L, Liu X, Wang Q, He S, Wu Q, Hu H, Mao X, Zhu L. Modulation of platelet activation and thrombus formation using a pan-PI3K inhibitor S14161. PLoS One. 2014;9:e102394. doi: 10.1371/journal.pone.0102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava AK, Kalita J, Haris M, Gupta RK, Misra UK. Radiological and histological changes following cerebral venous sinus thrombosis in a rat model. Neurosci Res. 2009;65:343–346. doi: 10.1016/j.neures.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Wai-Hoe L, Wing-Seng L, Ismail Z, Lay-Harn G. SDS-PAGE-based quantitative assay for screening of kidney stone disease. Biol Proced Online. 2009;11:145–160. doi: 10.1007/s12575-009-9007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naganuma Y, Ichii O, Otsuka S, Hashimoto Y, Kon Y. Analysis of TdT-mediated dUTP nick end labeling (TUNEL)-positive cells associated with cardiac myogenesis in mouse embryo. J Vet Med Sci. 2013;75:283–290. doi: 10.1292/jvms.12-0339. [DOI] [PubMed] [Google Scholar]
- 21.González-Manchón C, Larrucea S, Pastor AL, Butta N, Arias-Salgado EG, Ayuso MS, Parrilla R. Compound heterozygosity of the GPIbalpha gene associated with Bernard-Soulier syndrome. Thromb Haemost. 2001;86:1385–1391. doi: 10.1055/s-0037-1616740. [DOI] [PubMed] [Google Scholar]
- 22.Hawbaker S. Venous thromboembolism in the cancer population: Pathology, risk, and prevention. J Adv Pract Oncol. 2012;3:23–33. [PMC free article] [PubMed] [Google Scholar]
- 23.Vitale C, D'Amato M, Calabro P, Stanziola AA, Mormile M, Molino A. Venous thromboembolism and lung cancer: A review. Multidiscip Respir Med. 2015;10:28. doi: 10.1186/s40248-015-0021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ungprasert P, Tanratana P, Srivali N. Autoimmune hemolytic anemia and venous thromboembolism: A systematic review and meta-analysis. Thromb Res. 2015;136:1013–1017. doi: 10.1016/j.thromres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez MM, Hogue S, Preblick R, Kwong WJ. Review of the cost of venous thromboembolism. Clinicoecon Outcomes Res. 2015;7:451–462. doi: 10.2147/CEOR.S85635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santin BJ, Lohr JM, Panke TW, Neville PM, Felinski MM, Kuhn BA, Recht MH, Muck PE. Venous duplex and pathologic differences in thrombus characteristics between de novo deep vein thrombi and endovenous heat-induced thrombi. J Vasc Surg Venous Lymphat Disord. 2015;3:184–189. doi: 10.1016/j.jvsv.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Corral J, Huntington JA, González-Conejero R, Mushunje A, Navarro M, Marco P, Vicente V, Carrell RW. Mutations in the shutter region of antithrombin result in formation of disulfide-linked dimers and severe venous thrombosis. J Thromb Haemost. 2004;2:931–939. doi: 10.1111/j.1538-7836.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 28.Vayá A, Gómez I, Mira Y, Ferrando F, Corella D. Homocysteine levels in patients with deep vein thrombosis lacking thrombophilic defects. Thromb Haemost. 2008;99:1132–1134. doi: 10.1160/TH07-12-0748. [DOI] [PubMed] [Google Scholar]
- 29.Dittmeier M, Wassmuth K, Schuhmann MK, Kraft P, Kleinschnitz C, Fluri F. Dabigatran etexilate reduces thrombin-induced inflammation and thrombus formation in experimental ischemic stroke. Curr Neurovasc Res. 2016;13:199–206. doi: 10.2174/1567202613666160517122605. [DOI] [PubMed] [Google Scholar]
- 30.Pulimamidi VK, Murugesan V, Rajappa M, Satheesh S, Harichandrakumar KT. Increased levels of markers of oxidative stress and inflammation in patients with rheumatic mitral stenosis predispose to left atrial thrombus formation. J Clin Diagn Res. 2013;7:2445–2448. doi: 10.7860/JCDR/2013/7251.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Empana JP, Boulanger CM, Tafflet M, Renard JM, Leroyer AS, Varenne O, Prugger C, Silvain J, Tedgui A, Cariou A, et al. Microparticles and sudden cardiac death due to coronary occlusion. The TIDE (Thrombus and Inflammation in sudden DEath) study. Eur Heart J Acute Cardiovasc Care. 2015;4:28–36. doi: 10.1177/2048872614538404. [DOI] [PubMed] [Google Scholar]
- 32.Sadowski M, Zabczyk M, Undas A. Coronary thrombus composition: Links with inflammation, platelet and endothelial markers. Atherosclerosis. 2014;237:555–561. doi: 10.1016/j.atherosclerosis.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Guo W, Shi J, Xue J, Hu H, Xie D, Wu M, Cheng S. Expression of the chemokine receptor CXCR4 in human hepatocellular carcinoma and its role in portal vein tumor thrombus. J Exp Clin Cancer Res. 2010;29:156. doi: 10.1186/1756-9966-29-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadava K, Sichelstiel A, Luescher IF, Nicod LP, Harris NL, Marsland BJ. TSLP promotes influenza-specific CD8+ T-cell responses by augmenting local inflammatory dendritic cell function. Mucosal Immunol. 2013;6:83–92. doi: 10.1038/mi.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janda J, Plattet P, Torsteinsdottir S, Jonsdottir S, Zurbriggen A, Marti E. Generation of equine TSLP-specific antibodies and their use for detection of TSLP produced by equine keratinocytes and leukocytes. Vet Immunol Immunopathol. 2012;147:180–186. doi: 10.1016/j.vetimm.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Tate CM, Blosser W, Wyss L, Evans G, Xue Q, Pan Y, Stancato L. LY2228820 dimesylate, a selective inhibitor of p38 mitogen-activated protein kinase, reduces angiogenic endothelial cord formation in vitro and in vivo. J Biol Chem. 2013;288:6743–6753. doi: 10.1074/jbc.M112.425553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz JA, Wrobleski SK, Pechota AR, Hawley AE, Roelofs KJ, Doornbos NK, Gabriel JE, Reynolds G, Lester P, Londy F, et al. P-selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von Willebrand factor. J Vasc Surg Venous Lymphat Disord. 2014;2:114. doi: 10.1016/j.jvsv.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 38.Rossaint J, Vestweber D, Zarbock A. GDF-15 prevents platelet integrin activation and thrombus formation. J Thromb Haemost. 2013;11:335–344. doi: 10.1111/jth.12100. [DOI] [PubMed] [Google Scholar]
- 39.Guidetti GF, Canobbio I, Torti M. PI3K/Akt in platelet integrin signaling and implications in thrombosis. Adv Biol Regul. 2015;59:36–52. doi: 10.1016/j.jbior.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Su W, Chen Y, Wang C, Ding X, Rwibasira G, Kong Y. Human cathelicidin LL-37 inhibits platelet aggregation and thrombosis via Src/PI3K/Akt signaling. Biochem Biophys Res Commun. 2016;473:283–289. doi: 10.1016/j.bbrc.2016.03.095. [DOI] [PubMed] [Google Scholar]
- 41.McFadyen J, Peter K. Forget about thrombosis: Platelets and Alzheimer's disease, yet another sticky situation. Sci Signal. 2016;9:fs9. doi: 10.1126/scisignal.aaf8702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.