Abstract
Persistent infection in the paranasal sinuses impairs sinus drainage and leads to bacterial chronic rhinosinusitis. Greater knowledge of the key molecules in the pathology will help to clarify the pathogenesis. Study of the gene expression profile and analysis of the associated pathway is important to identify key molecules. This study investigates the expression of different genes and analyzes the key pathway in the pathological process of bacterial chronic rhinosinusitis. Bacterial chronic rhinosinusitis was induced in mice using a Merocel nasal pack inoculated with Staphylococcus aureus. Three months of mucosa samples were collected for histological and ELISA analysis, and gene expression was tested using DNA microarray. Differentially expressed genes were selected and verified for pathway analysis. The nasal mucosa of mice with chronic rhinosinusitis showed epithelial damage and lamina propria edema in extra cellular matrix with obvious mucosal inflammation. A total of 6,018 genes in bacterial chronic rhinosinusitis group were differentially expressed compared with the control. Among them, plasma, coagulation factors, urokinase plasminogen activator and urokinase receptor plasminogen activator expression were increased. Following gene ontology analysis and reverse transcription-quantitative polymerase chain reaction, coagulation cascades associated cytokines were found to be upregulated in bacterial chronic rhinosinusitis. The present results suggest that, bacterial chronic rhinosinusitis showed severe mucosal inflammation and genes differential expression in the pathogenesis. In this process, the coagulation cascades pathways were upregulated.
Keywords: bacterial chronic rhinosinusitis, gene expression, murine model, nasal mucosa, coagulation cascade, coagulation factor XII, urokinase plasminogen activator, urokinase receptor plasminogen activator, collagen type I alpha 1, collagen type V alpha 1
Introduction
Chronic rhinosinusitis (CRS), a frequently seen disease in the rhinology department, involves inflammation of the nose and paranasal sinuses mucosa lasting >12 weeks (1). Chronic rhinosinusitis without nasal polyposis (CRSsNP) accounts for about 60% of chronic rhinosinusitis and is a common refractory disease in rhinology (2). CRS includes symptoms of nasal discharge, nasal obstruction, facial pain, and a decreased sense of smell. It severely affects the quality of life of patients.
CRSsNP often originates from bacterial infection and develops into chronic inflammation in uncorrected treatment. Gram-positive bacteria such as coagulase-negative staphylococci (such as Staphylococcus aureus and Streptococcus viridans) and Gram-negative enteric rods are known to exist in the sinuses of adults with CRS. Bacterial infection causes mucosal inflammation with infiltration of neutrophils and monocytes, reconstruction of the extracellular matrix, goblet cell hyperplasia, and tissue necrosis (3,4).
Several mediators and cytokines are involved in this pathologic process, with increased expression levels of interleukin (IL)-1 and 6, tumor necrosis factor (TNF-α), eosinophil cationic protein, collagen, and urokinase plasminogen activator (Plau). Increased expression levels of tissue remodeling genes such as matrix metalloproteinase (MMPs), fibroblast growth factor (FGF), bone morphogenetic proteins (BMP), and transforming growth factor (TGF-β1) have also been found in bacterial chronic rhinosinusitis (5). However, the genomic expression of coagulation cascade-related cytokines, which might serve a crucial role in host defense, inflammation and tissue remodeling, has not been found.
The present study was carried out to investigate the changes in the gene profile in bacterial CRS using a gene array test. Bacterial chronic rhinosinusitis, simulating CRSsNP, was induced in a mouse model, the pathway analyzed and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) used to verify the key pathway in this pathogenesis.
Materials and methods
Animals and experiment groups
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University. Adult male specific pathogen-free C57BL/6J mice (n=24) were obtained from Shanghai Laboratory Animal Research Center (Shanghai, China). Mice were aged between 10–12 weeks and weighed 24±3 g. All mice were housed at room temperature (20–23°C) in a 12 h light/ dark cycle with soft food and tap water provided continuously ad libitum. Two groups were created according to a randomization schedule (n=12 each): 1) bacterial chronic rhinosinusitis (CRS) group: The right nasal cavity was occluded for 3 months with Merocel nasal pack (Medtronic, Minneapolis, MN, USA) inoculated with 20 µl overnight cultured Staphylococcus aureus (cat. no. ATCC25293; American Type Culture Collection, Manassas, VA, USA), 2) Sham operation group (control group): The nasal cavity was opened but no Merocel was inserted.
The bacterial CRS mouse model was built according to the method described by Jacob et al (6), with certain modifications. A peritoneal injection of 1% pentobarbital sodium (0.6 ml/100 g; Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) was given to anesthetize the animals. An incision was then made over the right snout up to the nasal dorsum to expose the right bony anterior naris. A Merocel nasal pack of about 5 mm length soaked in 20 µl of a Staphylococcus aureus solution was inserted in the right nasal cavity and the incision was closed. Following the operation, the mice were returned to the mouse care facility where they were housed for the next 3 months.
Histological analysis
For histological studies, the mice were anesthetized with chloral hydrate (35 mg/100 g; Sinopharm Chemical Reagent Co., Ltd.) and sacrificed to harvest the nose. The tissue samples were fixed in 4% paraformaldehyde solution, decalcified in EDTA solution, embedded in paraffin blocks, sectioned, and then stained with hematoxylin staining solution for 5 min and eosin staining solution for 1 min at room temperature. Sections were imaged with a Leica light microscope system (Leica Microsystems GmbH, Wetzlar, Germany).
ELISA analysis
The mouse nasal mucosa was harvested and the total protein content quantified using Bicinchoninic Acid (BCA) kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The test was processed following the protocol on the IL-1β (Cat. no. MU30369, Bio-Swamp; www.bio-swamp.com/) and TNF-α ELISA (Cat. no. MU30030, Bio-Swamp) kit. The extracted protein samples of bacterial CRS and control nasal mucosa were added to the pre-coated 96-well standard strip plate. Measurements of the optical density were performed at 450 nm. The IL-1β and TNF-α expression levels were determined using the standard curve prepared for each assay.
cDNA microarray analysis
The nasal mucosal membrane was collected using angled tweezers and washed with PBS solution. The Takara RNA isolation kit (Takara Bio, Inc., Otsu, Japan) was used to extract the total RNA of each sample following the operating manual. The RNA integrity number of the inspected RNA samples was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). The sample was further purified using the RNeasy Micro Kit (Qiagen GmbH, Hilden, Germany). According to the manufacturer's protocol, the total RNA was amplified and labeled using a low input quick amp labeling kit after purification and hybridized using the gene expression hybridization kit (Agilent Technologies, Inc.). The microarrays were scanned using an Agilent microarray scanner system (G2565CA). Data were extracted from images using Feature Extraction software v10.7 (Agilent Technologies, Inc.) with default settings. Raw data were normalized by the quantile algorithm in Gene Spring v12.6.1 software (Agilent Technologies, Inc.).
Gene regulation in bacterial CRS
The significantly differential expressed genes (P<0.05) between the bacterial CRS and control groups were selected. David v6.7 online software (david.abcc.ncifcrf.gov/) was used to perform molecular function and pathway analysis.
Real time-quantitative polymerase chain reaction (RT-qPCR)
The selected genes were tested on the 7900 HT Sequenced Detection System (Applied Biosystems; Thermo Fisher Scientific, Inc.) using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) and ABI Power SYBR® Green PCR Master Mix Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The procedure was: 50°C for 2 min incubation; 95°C for 10 min; 95°C for 15 sec, and 60°C for 1 min for 40 cycles. The primers are presented in Table I. The relative quantification of the target genes were normalized to the GAPDH gene expression level. Relative quantification of the gene expression level was presented using the 2−ΔΔCq method (7).
Table I.
Reverse transcription-quantitative polymerase chain reaction primers used.
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| CF XII-F | ACAAGCCCGGAGTCTACACA |
| CF XII-R | GGGAAGGATAAAGCCTGGTT |
| Plau-F | CCTGAGCAAAAGCTGGTGTA |
| Plau-R | GGAGCATCACATGGAAGACC |
| Plaur-F | GTGCCCTCGTGTTGTCTTCT |
| Plaur-R | CAGCCCTTGTTCCAATTCTC |
| Col1a1-F | GCCAAGAAGACATCCCTGAA |
| Col1a1-R | TCAAGCATACCTCGGGTTTC |
| Col5a1-F | TGGCATCCGAGGTCTGAAG |
| Col5a1-R | CCCCCGGTCACCCTTTATT |
| GAPDH-F | CGTGTTCCTACCCCCAATGT |
| GAPDH-R | TGTCATCATACTTGGCAGGTTTCT |
CFXII, Coagulation factor XII; Plau, urokinase plasminogen activator; Plaur, urokinase receptor plasminogen activator; Plau, urokinase plasminogen activator; Plaur, urokinase receptor plasminogen activator; Col1a1, collagen type I alpha 1; Col5a1, collagen type V alpha 1 chain.
Statistical analysis
The quantile algorithm in the Gene Spring v12.6.1 software (Agilent Technologies, Inc.) was used to normalize the gene expression microarray raw data. Differential expression >2-fold and a two-tailed probability value P<0.05 were considered statistically significant. The Student's t-test and SPSS v17.0 (SPSS, Inc., Chicago, IL, USA) were used for the statistical analysis. Data were presented as the mean ± standard deviation and P<0.05 was considered to indicate a statistically significant difference.
Results
Histological structure of the nasal mucosa in the bacterial CRS mouse model
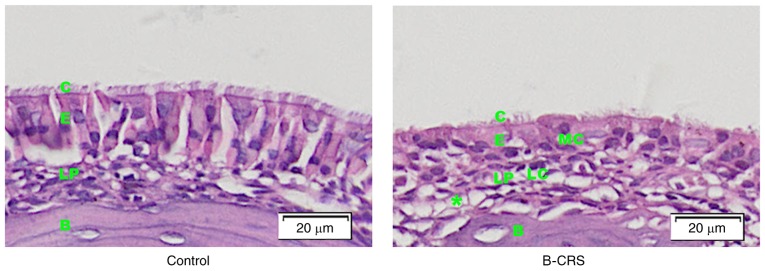
Histological structures of the nasal mucosa was observed in the bacterial CRS mouse model and control group. In the bacterial CRS group, hematoxylin and eosin staining showed damaged cilia of nasal epithelium, increased lymphocytes and macrophages infiltration, obvious lamina propria edema and gland hyperplasia (Fig. 1).
Figure 1.
H&E staining of the same position of middle turbinate nasal mucosa in control and bacterial CRS mouse model. B, bone; C, cilia; B-CRS, bacterial chronic rhinosinusitis; E, epithelium; LC, lymphocyte; LP, lamina propria; MC, macrophage; *, lamina propria edema.
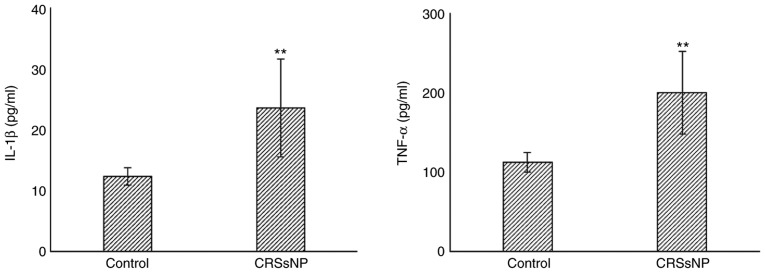
IL-1β and TNF-α in bacterial CRS murine model
The expression level of IL-1β and TNF-α in nasal mucosa of both bacterial CRS and control group were evaluated using ELISA. As shown in Fig. 2, the levels of IL-1β (P<0.01) and TNF-α (P<0.01) were significantly increased in bacterial CRS compared with the control.
Figure 2.
ELISA test of the level of IL-1β and TNF-α. Bacterial CRS group has increased IL-1β and TNF-α content compared to the control group. (**P<0.01). B-CRS, bacterial chronic rhinosinusitis; IL-1β, interleukin 1-beta; TNF-α, tumor necrosis factor alpha.
Gene expression in the nasal mucosa in bacterial CRS and control group
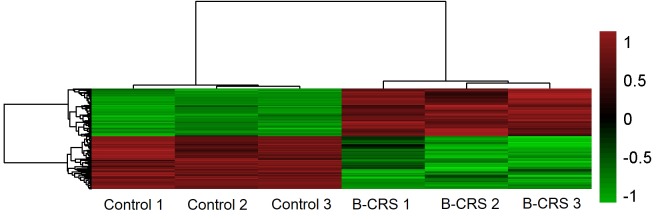
Gene microarray showed there were 6,018 genes expressed 2-fold differentially in the bacterial CRS group compared with the control. Among them, 2,865 genes were upregulated 2-fold, and 3,153 genes were downregulated 2-fold in bacterial CRS group as shown by the heat map (Fig. 3).
Figure 3.
Representative heat map of the differentially expressed genes in bacterial CRS and control murine nasal mucosa (three arrays per group). The red to green regions indicate relative decreases in genes expression. A total of 2,865 genes were upregulated 2-fold and 3,153 genes were downregulated 2-fold in the bacterial CRS group compared to control group (P<0.05). B-CRS, bacterial chronic rhinosinusitis.
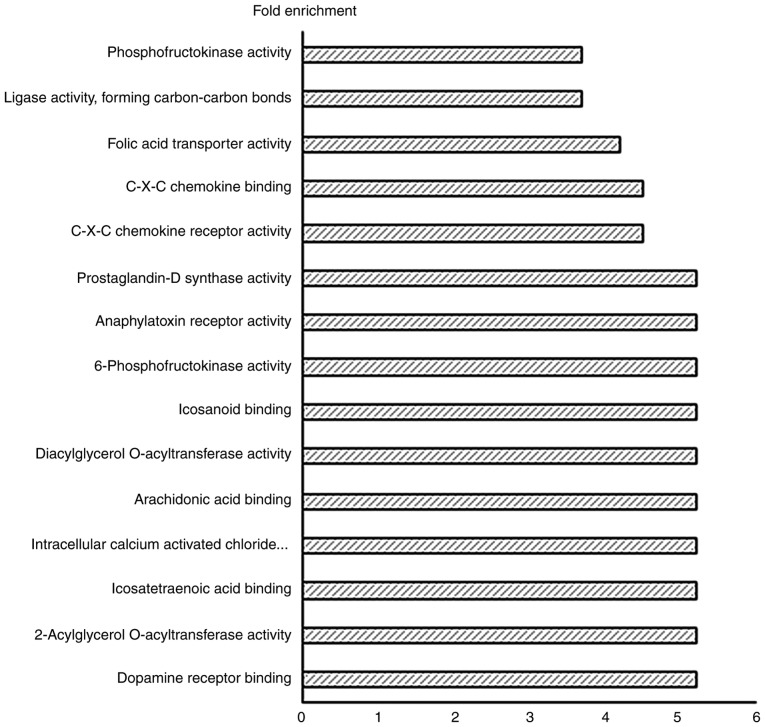
Gene Ontology (GO) analysis of differentially expressed genes
Out of the total number of 6,018 genes which were differentially expressed, 3,945 genes underwent GO analysis in DAVID v6.7 to determine molecular function. The important GO terms. demonstrated to have the higher fold enrichment GO socres, were: Dopamine receptor binding, 2-acylglycerol O-acyltransferase activity, eicosatetraenoic acid binding, intracellular calcium activated chloride channel activity, arachidonic acid binding, diacylglycerol O-acyltransferase activity, eicosanoid binding, 6-phosphofructokinase activity, anaphylatoxin receptor activity, prostaglandin-D synthase activity, C-X-C chemokine receptor activity and C-X-C chemokine binding, folic acid transporter activity, ligase activity, carbon-carbon bonding and phosphofructokinase activity (Fig. 4).
Figure 4.
Top 15 enhanced molecular functions of significant differentially expressed genes. X-axis represents the fold enrichment score.
Pathway analysis of the differentially expressed genes
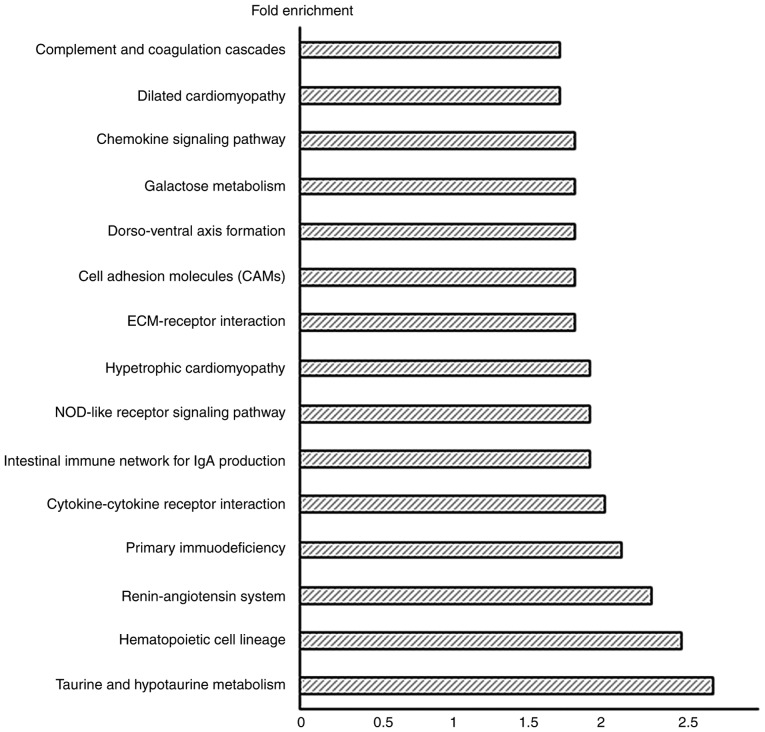
The differentially expressed genes in bacterial CRS underwent Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis using DAVID v6.7. The important pathways in the bacterial CRS mouse model were: Taurine and hypotaurine metabolism, hematopoietic cell lineage, renin-angiotensin system, primary immunodeficiency, cytokine-cytokine receptor interaction, intestinal immune network for Immunoglobulin (Ig)A production, nucleotide-binding oligomerization domain-like (NOD-like) receptor signaling pathway, hypertrophic cardiomyopathy, ECM-receptor interaction, cell adhesion molecules, dorso-ventral axis formation, galactose metabolism, chemokine signaling pathway, dilated cardiomyopathy, and complement and coagulation cascades (Fig. 5).
Figure 5.
Top 15 enhanced pathways analysis of significant differentially expressed target genes. X-axis represents the fold enrichment score.
Differentially expressed gene analysis in the coagulation cascade pathway
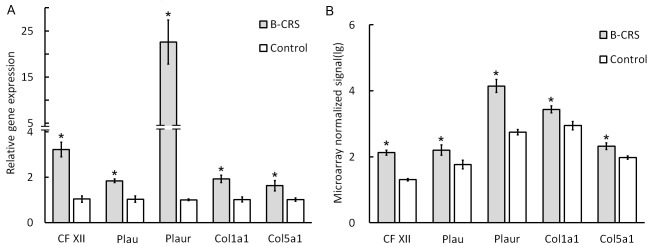
Twenty-eight genes were expressed differentially (Table II) as detected in the KEGG coagulation cascade pathway; of these, 24 were upregulated and four genes were downregulated: Alpha-2-macroglobulin, coagulation factor VIII, complement component 9 and Mannan-binding lectin (protein C); RT-qPCR verified Coagulation factor XII (CF XII), urokinase plasminogen activator (Plau), collagen type V alpha 1 chain (Col5a1), urokinase receptor plasminogen activator (Plaur), and collagen type I alpha 1 (Col1a1) genes which may serve an important role in the activation of the coagulation cascade pathway. All verified genes showed similar alterations in the gene microarray test (P<0.05; Fig. 6).
Table II.
Genes with fold change >2 or <0.5 (differential expression of genes between bacterial CRS group and control group; P<0.05).
| NM_No | Gene Symbol | Fold change | P-values |
|---|---|---|---|
| NM_010776 | Mbl2 | 0.061 | 0.041 |
| NM_013485 | C9 | 0.124 | 0.008 |
| NM_007977 | F8 | 0.248 | 0.001 |
| NM_181858 | Cd59b | 0.448 | 0.001 |
| NM_028066 | F11 | 2.056 | 0.030 |
| NM_019775 | Cpb2 | 2.109 | 0.031 |
| NM_009779 | C3ar1 | 2.166 | 0.016 |
| NM_009776 | Serping1 | 2.272 | 0.002 |
| NM_010406 | Hc | 2.458 | 0.019 |
| NM_008873 | Plau | 2.773 | 0.020 |
| NM_144938 | C1s1 | 2.804 | 0.005 |
| NM_011576 | Tfpi | 2.893 | 0.001 |
| NM_023143 | C1ra | 3.217 | 0.001 |
| NM_028784 | F13a1 | 3.637 | 0.007 |
| NM_175628 | A2m | 4.251 | 0.016 |
| D16492 | Masp1 | 4.373 | 0.003 |
| NM_008223 | Serpind1 | 4.634 | 0.001 |
| NM_010172 | F7 | 4.875 | 0.001 |
| NM_021489 | F12 | 6.707 | <0.001 |
| NM_009778 | C3 | 12.681 | <0.001 |
| NM_008198 | Cfb | 19.482 | <0.001 |
| NM_011113 | Plaur | 20.906 | 0.006 |
| NM_007972 | F10 | 25.379 | 0.001 |
| NM_007577 | C5ar1 | 30.771 | 0.010 |
| NM_009245 | Serpina1c | 35.437 | <0.001 |
| BC037008 | Serpina1b | 78.520 | <0.001 |
| NM_009246 | Serpina1d | 83.682 | <0.001 |
Figure 6.
Reverse transcription-quantitative polymerase chain reaction test on selectively upregulated genes (A) CFXII, Plau, Plaur, Col1a1 and Col5a1 (n=3 for each group). (B) Gene array test of CFXII, Plau, Plaur, Col1a1 and Col5a1, in bacterial CRS and control groups (n=3 for each group). *P<0.05 vs. control. CFXII, coagulation factor XII; Col1a1, collagen type I alpha 1; Col5a1, collagen type V alpha 1 chain; CRS, chronic rhinosinusitis; Plau, urokinase plasminogen activator; Plaur, urokinase receptor plasminogen activator; B-CRS, bacterial chronic rhinosinusitis.
Discussion
Chronic rhinosinusitis (CRS) has a prevalence of 8% in China and 10.9% in Europe (1,8). CRSsNP is the most common type of CRS (2). Previous studies suggested that genes responsible for antigen presentation, innate and adaptive immune responses, tissue remodeling, and arachidonic acid metabolism are involved in the pathological process of CRS (9). However, no reports have shown comprehensive gene expression patterns in bacterial CRS.
The present study utilized a murine model to profile gene expression in bacterial CRS to prove that bacterial CRS causes significant tissue inflammation by the alteration of multiple genes. Some of these are the T-cell receptor complex, immunoglobulin complex, integrin complex, ciliary rootlet and collagen cell component, which may be relevant to cytokine-cytokine receptor interaction, chemokine signaling, cell adhesion molecules, and the complement and coagulation cascade pathway in bacterial CRS.
To the best of our knowledge, this finding of gene expression profile being changed in bacterial CRS has not been reported before. This provides a basis for further identification of pivotal cell pathways and key target molecules in bacterial CRS, and has potential importance for the diagnosis and treatment of bacterial CRS.
Activation of the coagulation cascade and deposition of fibrin as a consequence of inflammation is well known, and is thought to serve a critical role in host defense and in containing microbial or toxic agents (10). Previous studies have proved that in CRSsNP, excessive extracellular collagen deposition occurs in the sinus cavities and pseudocysts are absent in the thickened submucosa (11). In addition, dysregulation of the coagulation cascade may serve an etiological role in many other diseases, including rheumatoid arthritis, severe asthma glomerulonephritis, and delayed-type hypersensitivity through excessive fibrin deposition (12–14).
Infiltration of chronic inflammatory cells such as neutrophils and mononuclear cells occurs in the nasal mucosa (15). In this pathological process, genes involved in antigen presentation, the innate and adaptive immune response and tissue remodeling are regulated (9). Many studies have focused on the gene expression in chronic rhinosinusitis with nasal polyposis using DNA microarray (16,17) but none have studied the gene expression profile in CRSsNP. In the present study, bacterial CRS in the mice were studied using gene expression profile.
Consistent with previous studies in CRSsNP patients using nasal membrane samples (18), ciliary damage of the nasal epithelium, lamina propria edema, and infiltration of inflammatory cells were found in the sinus membranes of mice used in this study. A total of 6,018 genes were found to have a 2-fold differential expression in bacterial CRS. Among them, immunoglobulin-associated genes such as the immunoglobulin (Ig) heavy chain variable, Ig light chain, and Ig kappa chain variable were increased.
As reported in previous studies (19,20), MMP-7, MMP-9, TGF-β1, Plau (uPA) and Plaur (uPAR) also showed increased expression in this study. In the study by Sejima et al uPA/uPAR expression was found to be upregulated in bacterial CRS (20). Both Plau and Plaur are believed to activate inflammatory cells such as neutrophils and monocytes, releasing many kinds of inflammatory and chemotactic mediators, with direct cytolytic effects on several bacterial species (21,22). Additionally, uPA/uPAR could modulate the extracellular matrix by activating plasmin and MMPs and affect leukocyte migration (23). There are many other unreported genes associated with bacterial CRS, further bioinformatics and experimental studies are required to identify the crucial genes.
Coagulation and inflammation are considered two distinct pathologies, but they closely interact with each other at multiple levels, for example, coagulation factor Xa, coagulation factor XIa and plasmin activate both complement component 5 and complement component 3 to complement component 5a and complement component 3a, respectively, which are involved in the inflammatory response (24). In the present study, GO term molecular function analysis and pathway analysis were used to study the differentially expressed genes. Apart from the immune-related pathways, 28 genes involved in the complement and coagulation cascade pathways were expressed differentially. RT-qPCR revealed that the levels of coagulation factor XII (CF XII), Plau, Col5a1, Plaur, and Col1a1, which serve a key role in the complement and coagulation pathways (22,25), were found to be upregulated in bacterial CRS. Of these, Col1a1 (important components of collagen I) is significantly elevated in chronic sinusitis (26), and the expression of Plau and Plaur are elevated in sinusitis (27).
The CFXII-driven plasma contact system provided a link between procoagulant and proinflammatory reactions and contributes to host defense mechanisms as a component of the innate immune system. It was also found to promote inflammatory reactions by activating the kallikrein-kinin system (28), increasing the formation of bradykinin which caused vascular permeability and vasodilation (29) and enhanced the inflammatory reaction.
The present study demonstrated that CFXII is an important factor in the coagulation system and inflammatory reaction, which requires further study. The major coagulation factors CFXII, Plau and Plaur serve a vital role in inflammation and immunity, they may also be crucial to the pathogenesis of bacterial CRS (Fig. 7).
Figure 7.
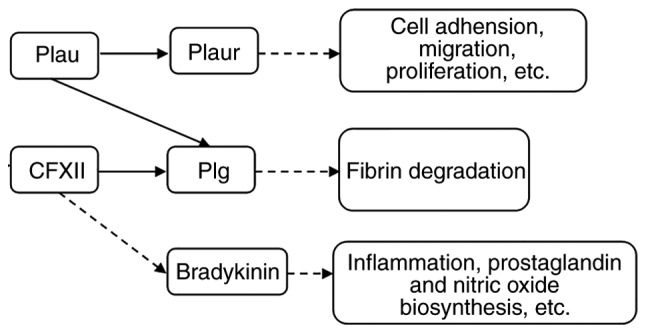
Molecular network and possible functions of CFXII, Plau, Plaur, plg and bradykinin. CFXII, Coagulation factor XII; Plau, urokinase plasminogen activator; Plaur, urokinase receptor plasminogen activator; plg, plasminogen.
In summary, the gene expression profile in the nasal mucosa of mice in bacterial CRS is presented in the present study, to the best of our knowledge, for the first time. This study demonstrates that genes responsible for the complement and coagulation cascade pathways have an important immunomodulatory function. Further studies are required to better understand the pathogenesis of bacterial CRS.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science Foundation of China (grant no. 81400445).
Availability of the data and materials
All data generated or analyzed during the present study is included in this published article.
Authors' contributions
LG and SW performed the bacterial chronic rhinosinusitis animal model, mucosa tissue collection, and the histological and ELISA assays. YZ and HH designed the present study, including analysis and interpretation of the cDNA microarray data. All authors read and approved the final manuscript.
Ethical approval and consent to participate
The animal experiments and procedures were performed in accordance with the guidelines for the use and care of laboratory animals in Shanghai Jiao Tong University, the present study was approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23 3, p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 2.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125(Suppl 2):S103–S115. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(Suppl 6):S155–S212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malekzadeh S, Hamburger MD, Whelan PJ, Biedlingmaier JF, Baraniuk JN. Density of middle turbinate subepithelial mucous glands in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2002;127:190–195. doi: 10.1067/mhn.2002.126800. [DOI] [PubMed] [Google Scholar]
- 5.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, Norton J, Grammer LC, Tan BK, Chandra RK, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132:584–592.e4. doi: 10.1016/j.jaci.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob A, Faddis BT, Chole RA. Chronic bacterial rhinosinusitis: Description of a mouse model. Arch Otolaryngol Head Neck Surg. 2001;127:657–664. doi: 10.1001/archotol.127.6.657. [DOI] [PubMed] [Google Scholar]
- 7.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 8.Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ, Zhu DD, Lv W, Liu SX, Li PZ, Ou CQ, Xu G. Epidemiology of chronic rhinosinusitis: Results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70:533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu J, Avila PC, Kern RC, Hayes MG, Schleimer RP, Pinto JM. Genetics of chronic rhinosinusitis: State of the field and directions forward. J Allergy Clin Immunol. 2013;131:977–993. doi: 10.1016/j.jaci.2013.01.028. 993.e1-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med. 2011;17:568–573. doi: 10.2119/molmed.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, Cuvelier C, Van Cauwenberge P, Bachert C. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–259.e1-e2. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 12.de Boer JD, Majoor CJ, van't Veer C, Bel EH, van der Poll T. Asthma and coagulation. Blood. 2012;119:3236–3244. doi: 10.1182/blood-2011-11-391532. [DOI] [PubMed] [Google Scholar]
- 13.Gabazza EC, Osamu T, Yamakami T, Ibata H, Sato T, Sato Y, Shima T. Correlation between clotting and collagen metabolism markers in rheumatoid arthritis. Thromb Haemost. 1994;71:199–202. [PubMed] [Google Scholar]
- 14.Neale TJ, Tipping PG, Carson SD, Holdsworth SR. Participation of cell-mediated immunity in deposition of fibrin in glomerulonephritis. Lancet. 1988;2:421–424. doi: 10.1016/S0140-6736(88)90413-8. [DOI] [PubMed] [Google Scholar]
- 15.Van Bruaene N, Bachert C. Tissue remodeling in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2011;11:8–11. doi: 10.1097/ACI.0b013e32834233ef. [DOI] [PubMed] [Google Scholar]
- 16.Platt M, Metson R, Stankovic K. Gene-expression signatures of nasal polyps associated with chronic rhinosinusitis and aspirin-sensitive asthma. Curr Opin Allergy Clin Immunol. 2009;9:23–28. doi: 10.1097/ACI.0b013e32831d8170. [DOI] [PubMed] [Google Scholar]
- 17.Payne SC, Han JK, Huyett P, Negri J, Kropf EZ, Borish L, Steinke JW. Microarray analysis of distinct gene transcription profiles in non-eosinophilic chronic sinusitis with nasal polyps. Am J Rhinol. 2008;22:568–581. doi: 10.2500/ajr.2008.22.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Bruaene N, C PN, Van Crombruggen K, De Ruyck N, Holtappels G, Van Cauwenberge P, Gevaert P, Bachert C. Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy. 2012;42:883–890. doi: 10.1111/j.1365-2222.2011.03898.x. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, Zhang J, Holtappels G, Luo B, Zhou P, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:1061–1068. doi: 10.1016/j.jaci.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Sejima T, Holtappels G, Bachert C. The expression of fibrinolytic components in chronic paranasal sinus disease. Am J Rhinol Allergy. 2011;25:1–6. doi: 10.2500/ajra.2011.25.3537. [DOI] [PubMed] [Google Scholar]
- 21.Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25:450–455. doi: 10.1016/j.it.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Del Rosso M, Margheri F, Serratì S, Chillà A, Laurenzana A, Fibbi G. The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation. Curr Pharm Des. 2011;17:1924–1943. doi: 10.2174/138161211796718189. [DOI] [PubMed] [Google Scholar]
- 23.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahlbäck B. Coagulation and inflammation-close allies in health and disease. Semin Immunopathol. 2012;34:1–3. doi: 10.1007/s00281-011-0298-0. [DOI] [PubMed] [Google Scholar]
- 25.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25:5681–5703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Kim DK, Kang SI, Kong IG, Cho YH, Song SK, Hyun SJ, Cho SD, Han SY, Cho SH, Kim DW. Two-track medical treatment strategy according to the clinical scoring system for chronic rhinosinusitis. Allergy Asthma Immunol Res. 2018;10:490–502. doi: 10.4168/aair.2018.10.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Zhan Z, Sun J, Zeng B, Xue Y, Wang S. Nasal mucosa remodeling in chronic rhinosinusitis without nasal polyps. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 27:1110–1113. 1117, 2013 (In Chinese) [PubMed] [Google Scholar]
- 28.Kenne E, Nickel KF, Long AT, Fuchs TA, Stavrou EX, Stahl FR, Renné T. Factor XII: A novel target for safe prevention of thrombosis and inflammation. J Intern Med. 2015;278:571–585. doi: 10.1111/joim.12430. [DOI] [PubMed] [Google Scholar]
- 29.Björkqvist J, Jämsä A, Renné T. Plasma kallikrein: The bradykinin-producing enzyme. Thromb Haemost. 2013;110:399–407. doi: 10.1160/TH13-03-0258. [DOI] [PubMed] [Google Scholar]