Abstract
The prognostic role of CD44v9, a variant isoform of CD44 and a new cell surface marker of cancer stem cells, remains unclear in bladder cancer (BC) patients. Furthermore, limited information is available on the functional role of sulfasalazine (SSZ), which could modulate the CD44v9‐xCT system in order to enhance cisplatin (CDDP)‐induced cytotoxicity and inhibit the metastatic potential of BC. CD44v9 protein expression was examined immunohistochemically in 63 muscle invasive BC (MIBC) patients who underwent radical cystectomy. CD44v9 expression was independently associated with disease recurrence and cancer‐specific death in MIBC. Cytotoxic effects, glutathione levels, and reactive oxygen species production by SSZ and CD44v9 and phospho‐p38MAPK protein expression by SSZ with or without CDDP were assessed in MBT‐2V cells with highly metastatic potential. Sulfasalazine exerted cytotoxic effects against MBT‐2V cells by inhibiting glutathione levels and inducing the production of reactive oxygen species. Sulfasalazine in combination with CDDP appeared to exert strong cytotoxic effects against MBT‐2V cells by inhibiting CD44v9 expression and upregulating phospho‐p38MAPK expression. The inhibitory effects of SSZ with or without CDDP were also investigated using an MBT‐2V lung metastatic model. In the murine lung metastatic BC model, SSZ significantly prolonged animal survival. Furthermore, the combination of SSZ with CDDP exerted stronger inhibitory effects on the establishment of lung tumor nodules than SSZ or CDDP alone. CD44v9 expression could be a clinical biomarker for predicting poor outcomes in MIBC patients. Sulfasalazine in combination with CDDP has potential as a novel therapy against metastatic BC.
Keywords: bladder cancer, CD44v9, cisplatin, metastasis, sulfasalazine
Abbreviations
- BC
bladder cancer
- BSO
L‐buthionine‐sulfoximine
- CD44v9
the variant isoform of CD44 containing v8‐v10
- CDDP
cisplatin
- CI, combination index; CSC
cancer stem cell
- CSS
cancer‐specific survival
DCF, dichlorodihydrofluorescein
- GSH
synthesis of glutathione
- HR
hazard ratio
- MIBC
muscle invasive bladder cancer
- NAC
N‐acetylcysteine
- RC
radical cystectomy
- RFS
recurrence‐free survival
- ROS
reactive oxygen species
- SE, standard error
SSZ
sulfasalazine
1. INTRODUCTION
Metastatic BC is one of the most aggressive malignancies and remains extremely challenging to treat. Cisplatin‐based combination chemotherapy has been the gold standard first‐line treatment for metastatic BC.1, 2 However, the initial response rate and median overall survival were previously reported to be low at approximately 60% and 13‐14 months, respectively.1 Furthermore, CDDP has been reported to cause severe side‐effects. Therefore, a new therapeutic strategy for the treatment of metastatic BC is strongly warranted.
Cancer stem cells, which possess stem cell‐like characteristics, including multilineage and self‐renewal potentials, have been identified in cancer tissues.3 Previous studies reported that tumor progression, metastasis, and resistance to anticancer therapy were driven by CSCs, which were identified by cell surface markers such as CD44.4, 5, 6, 7 CD44 exists in numerous variant isoforms generated through the alternative mRNA splicing of different combinations of 10 exons (v1‐10), and the variant isoforms of CD44 containing v8‐v10 (CD44v9) have been identified as new cell surface markers of CSCs.8 In the field of urothelial carcinoma, CD44v9 expression has been associated with poor prognosis and chemoresistance.9, 10, 11, 12 CD44v9 on the cell surface stabilizes xCT, which is a subunit of the cystine transporter that modulates its function. xCT acts as an Na+‐independent transporter mediated by the exchange of extracellular cystine for intracellular glutamate, which promotes the synthesis of GSH and subsequently reduces ROS production. Therefore, the interaction between CD44v9 and xCT contributes to the features of CSCs, such as metastasis and resistance to anticancer therapy.
We focused on SSZ, an anti‐inflammatory drug approved for the treatment of inflammatory bowel disease and rheumatoid arthritis.13 Sulfasalazine is an inhibitor of xCT‐mediated cystine transporters14 and exerts inhibitory effects against tumor growth, invasion, and metastasis in many types of cancers.4, 15, 16, 17, 18, 19 Furthermore, previous studies reported that SSZ enhanced the effects of CDDP therapy.20, 21 However, limited information is currently available on the functional role of SSZ, which could modulate the CD44v9‐xCT system in order to enhance CDDP‐induced cytotoxicity and inhibit the metastatic potential of BC.
In the present study, we investigated: (a) the prognostic role of CD44v9 for disease recurrence and cancer‐specific death in MIBC patients who underwent RC; (b) the functional role of SSZ in ROS production and its cytotoxic effects on MBT‐2V cells, which were established from a parent MBT‐2 tumor with multiple lung metastases;22 and (c) the therapeutic effects of SSZ with or without CDDP using an MBT‐2V lung metastatic BC model.
2. MATERIALS AND METHODS
2.1. Cell culture, agents, and animals
MBT‐2V cells were established and characterized from the parent MBT‐2 cells by Lerner Research Institute, Cleveland Clinic Foundation (Cleveland, OH, USA).22 MBT‐2V cells were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) with 10% FBS at 37°C in a humidified 5% CO2 atmosphere. Sulfasalazine, NAC, BSO, and CDDP were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Eight‐week‐old female C3H/HeN mice were purchased from CLEA Japan (Tokyo, Japan). Mice were maintained under standardized laboratory conditions with free access to food and water.
2.2. Tissue samples
A total of 91 patients underwent RC for MIBC at our institution between 1997 and 2007. We excluded four patients with data deficiencies and 24 diagnosed with pT0. The remaining 63 patients were assessed in the present study. The median follow‐up was 58 months (range, 7‐190 months), and the mean patient age was 69 years (range, 46‐83 years). Regional lymphadenectomy around the bilateral external and internal iliac vessels and obturator nerve was generally undertaken for all patients. Extended lymphadenectomy was not routinely carried out.
Specimens were fixed with 10% formalin and embedded in paraffin. All slides were rereviewed by a genitourinary pathologist, and tumor grades were assessed according to the 2004 WHO/International Society of Urologic Pathology consensus classification. Tumors were staged according to the American Joint Committee on Cancer‐UICC TNM classification. Lymphovascular invasion was defined as the presence of tumor cells within an endothelium‐lined space without underlying muscular walls. This study was undertaken subject to the guidelines of the Declaration of Helsinki and had no influence on the treatment of patients. Our institutional ethical committee granted ethical approval for this study.
2.3. Immunohistochemistry
Four‐micrometer‐thick paraffin sections from cystectomy specimens were deparaffinized in xylene and dehydrated in a graded ethanol series. After antigen retrieval with citric acid (pH = 6.0) at 120°C for 10 minutes, endogenous peroxidase activity was blocked with 1% hydrogen peroxide for 20 minutes followed by washing with distilled water. In order to bind nonspecific antigens, sections were incubated at room temperature for 15 minutes with 5% skim milk in PBS. Sections were then incubated at 4°C overnight with an anti‐CD44v9 rat mAb (1:5000 dilution; Cosmo Bio, Tokyo, Japan). After washing with PBS, tissue sections were incubated with an anti‐rat goat secondary Ab (Histofine Simple Stain MAX PO (rat); Nichirei Biosciences, Tokyo, Japan) for 30 minutes. The Ab was detected using the avidin‐biotin complex peroxidase method. Color was developed with 3,30‐diaminobenzidine tetrahydrochloride in 50 mmol/L Tris‐HCl (pH 7.5) containing 0.005% hydrogen peroxide. Sections were then counterstained with hematoxylin.
The results of CD44v9 immunostaining in RC specimens were evaluated as described previously.9 In order to assess CD44v9 staining, cancer cells with positive staining in the cell membrane were counted under a light microscope at ×400 magnification in at least 10 randomly selected representative fields, and the percentage of positive cancer cells was calculated. The density of CD44v9 (CD44v9 density) in tumor cells was scored as the average proportion of detectable immunoreactions in 10 representative fields (range, 0%‐100%) for each tumor. Two authors blinded to patient data independently evaluated immunoreactivity for CD44v9.
2.4. Survival analysis for 63 patients who underwent RC
The relationship between CD44v9 expression and clinicopathological features was assessed using the χ2 test. Recurrence‐free survival was defined as the time from RC until the presence of local recurrence and/or distant metastases. Cancer‐specific survival was defined as the time from RC until death due to BC. Both RFS and CSS rates were estimated using the Kaplan‐Meier method and compared with the log‐rank test. Univariate and multivariate Cox regression analyses were carried out in order to assess prognostic indicators for disease recurrence and survival. Only significant parameters detected in the univariate analysis were included in the final multivariate analysis using Cox's proportional hazards regression models with stepwise forward selection. The level of significance was set at P < .05. These analyses were undertaken with the SPSS version 23.0 statistical software package (IBM, Somers, NY, USA).
2.5. Cell proliferation assay
MBT‐2V cells were seeded on 96‐well black plates in a volume of 4 × 103 cells/100 μL/well culture medium and incubated at 37°C for 24 hours under 5% CO2 in a humidified incubator. Cells were treated with various concentrations of SSZ with or without NAC (3 μmol/L) or with or without CDDP (10 μmol/L) for 48 hours. The number of viable cells was evaluated with a Cell Titer‐Glo luminescence cell viability kit (Promega, Madison, WI, USA). The luminescence signal was detected with an Enspire 2300 (PerkinElmer, Waltham, MA, USA). Data were expressed as the percentage of viable cells relative to that of the control. The experiment was carried out in triplicate, and data were expressed as the mean ± standard error (SE) of relative cell viability. A combination index (CI) analysis was also used to measure of the extent of the drug interaction quantitatively. A combination index (CI) of less than, equal to, and more than 1 indicates synergy, additivity, and antagonism, respectively.23
2.6. Measurement of GSH and ROS levels
Intracellular GSH levels were examined using a GSH‐Glo Glutathione Assay kit (Promega). MBT‐2V cells treated with SSZ (300 or 400 μmol/L), BSO (100 μmol/L), or the vehicle control for 24 hours were harvested and diluted in PBS to 5 × 103 cells/well in 96‐well white plates. GSH‐Glo Reagent (Promega) was added to the plates and incubated for 30 minutes. Reconstituted Luciferin Detection Reagent (Promega) was added to the wells and incubated for 15 minutes. The luminescence signal was detected using an Enspire 2300. The experiment was carried out in triplicate, and data were expressed as the mean ± SE of intracellular GSH concentrations.
Intracellular ROS levels were assessed using dichlorodihydrofluorescein (DCF) fluorescence staining (C6827; Invitrogen, Tokyo, Japan). MBT‐2V cells treated with SSZ (300 or 400 μmol/L), BSO (100 μmol/L), or the vehicle control were harvested and diluted in PBS to 1 × 105 cells/well in 6‐well plates for 24 hours. These cells were incubated with 10 μmol/L dichloro‐dihydro‐fluorescein diacetate at 37°C for 15 minutes and washed twice with PBS. The mean intensity of DCF fluorescence in 1 × 104 cells was assessed using flow cytometry. The results obtained were analyzed using Image StreamX/Flow Sight (Merck Millipore, Burlington, MA, USA). The experiment was carried out in triplicate, and data were expressed as the mean ± SE of intracellular ROS levels.
2.7. Cell extraction and western blot analysis
In order to obtain whole cell extracts, we used RIPA buffer consisting of 50 mmol/L Tris‐HCl (pH 7.5), protease inhibitor, 1% NP‐40, 0.5% deoxycholate, 0.1% SDS, and 150 mmol/L NaCl.
In western blotting, 20 μg total protein from each sample was loaded onto 12.5% SDS‐polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was blocked at 4°C overnight in TBS containing 5% PhosphoBlocker Blocking Reagent (Cell Biolabs, San Diego, CA, USA) and 0.2% Tween‐20, and then incubated at 4°C overnight with the primary Abs for CD44v9 (1:5000 dilution; Cosmo Bio), phospho‐p38MAPK (1:500 dilution; Cell Signaling Technology, Danvers, MA, USA), p38MAPK (1:1000 dilution; Cell Signaling Technology), and β‐actin (1:5000 dilution; Sigma‐Aldrich). Membranes were then washed for 5 minutes in TBS with 0.2% Tween‐20 and incubated with a peroxidase‐labeled secondary Ab (Dako, Glostrup, Denmark) for 1 hour. The membranes were washed again and analyzed after visualization using enhanced chemiluminescence reagents with the ECL Plus Western Blotting Detection System (GE Healthcare, Chicago, IL, USA). Signal intensities were quantified using the LAS 4000 system (GE Healthcare). The experiment was carried out in triplicate, and data were expressed as the mean ± SE of the relative signal intensities.
2.8. In vivo experiment
All animal experiments were reviewed and approved by our Institutional Animal Care Committees. Lung tumor nodules were generated by injecting 2 × 105 MBT‐2V cells in 200 μL PBS into the tail veins of female C3H/HeN mice on day 0.
The first set of in vivo experiments was undertaken in order to examine the survival benefit of the SSZ treatment. The i.p. administration (2 days on/1 day off) of SSZ (500 mg/kg) or the vehicle control of PBS in a total volume of 200 μL was started on day 3 (n = 18 in each group). Survival rates were calculated by the Kaplan‐Meier method, and differences between the SSZ treatment group and vehicle control group were analyzed with the log‐rank test.
The second set of in vivo experiments was undertaken in order to examine the effects of SSZ alone, CDDP alone, and their combinations in the murine animal model. Mice were classified into 4 groups: vehicle control, SSZ alone, CDDP alone, and the combination of SSZ and CDDP treatment (n = 10 in each group). The SSZ‐treated group received i.p. administration (2 days on/1 day off) of SSZ (500 mg/kg) in a total volume of 200 μL starting on day 3. The CDDP‐treated group received i.p. administration (every fifth day) of CDDP (2 mg/kg) in a total volume of 200 μL starting on day 3. The combination treatment group also received these schedules of SSZ and CDDP treatments. Mice were then killed on day 15, and the number of tumor nodules on the lung surface was counted macroscopically. Lung tissues were fixed in 10% formalin, embedded in paraffin, and assessed pathologically with H&E staining. Furthermore, CD44v9 expression in lung tissues was evaluated immunohistochemically. The primary and secondary Abs used for the immunohistochemical analysis were an anti‐mouse CD44v9 rat mAb (1:5000 dilution; Cosmo Bio) and anti‐rat rabbit secondary Ab (Vector Laboratories, Burlingame, CA, USA), respectively. For the automated image analysis, stained slides were scanned into high‐resolution digital images with NanoZoomer‐XR (Hamamatsu Photonics, Shizuoka, Japan). The percentage of positive cancer cells with positive staining in the cell membrane was automatically calculated in 10 randomly selected representative fields by HistoQuest version 3.5 (NOVEL SCIENCE, Kanagawa, Japan). The density of CD44v9 in lung tumor nodules was scored as the average proportion of detectable immunoreactions in 10 representative fields for each tumor.
In order to assess the potential toxicity of SSZ, we undertook laboratory tests for creatinine, aspartate transaminase, and alanine transaminase and investigated the histological appearance of the major organs, including the heart, liver, kidneys, spleen, pancreas, and ovaries.
3. RESULTS
3.1. CD44v9 expression in 63 patients with MIBC
The mean ± SE of the density of CD44v9 expression was 16.5 ± 26.9%. We used a cut‐off level of 5%, the median of the density of CD44v9 expression. Based on the cut‐off level of 5%, patients were classified into high (n = 36, 57.1%) and low CD44v9 expression groups (n = 27, 42.9%). Representative CD44v9 immunohistochemical staining is shown in Figure 1A‐C. Low and high CD44v9 expression in tumor specimens are shown in Figure 1A,B, respectively. In Figure 1C, showing the high‐power field of Figure 1B, CD44v9 was expressed in the epithelium of tumor glands with a heterogeneous expression pattern, and its expression was also detected along the cell membrane.
Figure 1.
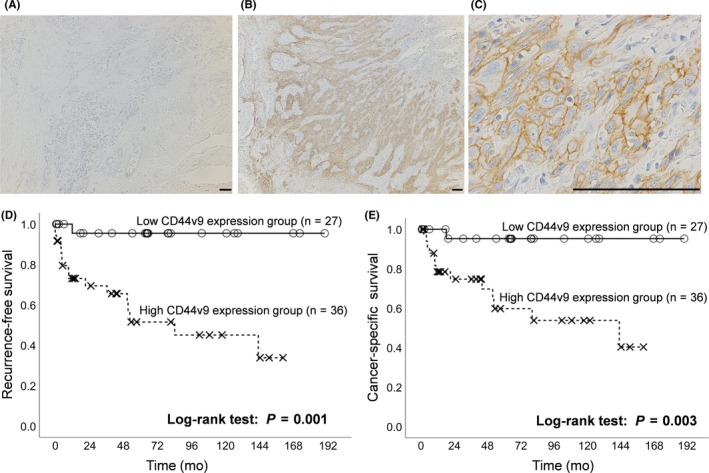
Representative immunostaining for CD44v9 in radical cystectomy specimens of muscle invasive bladder cancer (MIBC) patients and prognostic significance of CD44v9 expression on disease recurrence and cancer‐specific death. A, Low expression of CD44v9 in radical cystectomy specimens of MIBC. B,C, High expression of CD44v9 in MIBC. Scale bar = 100 μm. D,E, Kaplan‐Meier curves of recurrence‐free survival (D) and cancer‐specific survival (E) according to CD44v9 expression (low expression of CD44v9 vs high expression of CD44v9)
The relationship between CD44v9 expression and clinicopathological features is shown in Table 1. Patients with high CD44v9 expression had a higher incidence of high grade tumors (P = .031) and equal or more pT3 stage tumors (P = .003) than those with low CD44v9 expression.
Table 1.
Relationship between CD44v9 expression and clinicopathological features in 63 patients with muscle invasive bladder cancer
| CD44v9 expression | |||
|---|---|---|---|
| Low (n = 27) | High (n = 36) | P value | |
| Sex | |||
| Male | 19 (70.4) | 29 (80.6) | .260 |
| Female | 8 (29.6) | 7 (19.4) | |
| Age at surgery (years) | |||
| <70 | 13 (48.1) | 18 (50.0) | .415 |
| 70≤ | 14 (51.9) | 18 (50.0) | |
| Tumor grade | |||
| Low | 8 (29.6) | 3 (8.3) | .031 |
| High | 19 (70.4) | 33 (91.7) | |
| Pathological T stage | |||
| <pT3 | 15 (55.6) | 7 (19.4) | .003 |
| pT3≤ | 12 (44.4) | 29 (80.6) | |
| Pathological N stage | |||
| pN0 | 24 (88.9) | 28 (77.8) | .210 |
| pN1≤ | 3 (11.1) | 8 (22.2) | |
| Adjuvant chemotherapy | |||
| Administered | 6 (22.2) | 12 (33.3) | .248 |
| Not administered | 21 (77.8) | 24 (66.7) | |
| LVI status | |||
| Negative | 13 (48.1) | 10 (27.8) | .081 |
| Positive | 14 (51.9) | 26 (72.2) | |
Data are shown as n (%).
LVI, lymphovascular invasion.
3.2. Prognostic significance of CD44v9 expression on disease recurrence and cancer‐specific death
Disease recurrence was noted in 17 patients (26.9%) patients during the follow‐up: 16 with high CD44v9 expression and 1 with low CD44v9 expression. A Kaplan‐Meier curve revealed that the 5‐year RFS rate of the high CD44v9 expression group was 51.5%, which was significantly lower than that of the low CD44v9 expression group (95.5%, P = .001, Figure 1D). A univariate Cox analysis identified the pathological T stage (P = .016), pathological N stage (P = .008), and CD44v9 expression in tumor specimens (P = .001) as significant prognostic factors for disease recurrence (Table 2). A multivariate Cox regression analysis showed that high CD44v9 expression in tumor specimens (HR 6.18, P = .016) was independently associated with disease recurrence in addition to pN1 or greater tumors (HR 3.19, P = .036).
Table 2.
Univariate and multivariate Cox regression analyses for disease recurrence and cancer‐specific death in 63 muscle invasive bladder cancer patients
| Disease recurrence | Cancer‐specific death | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| P value | HR (95% CI) | P value | P value | HR (95% CI) | P value | |
| Sex | .136 | .037 | .379 | |||
| Male | 1.00 | |||||
| Female | 2.37 (0.34‐13.20) | |||||
| Age at surgery | .276 | .178 | ||||
| <70 | ||||||
| 70≤ | ||||||
| Tumor grade | .054 | .09 | ||||
| Low | ||||||
| High | ||||||
| Pathological T stage | .016 | .39 | .011 | .679 | ||
| <pT3 | 1.00 | 1.00 | ||||
| pT3≤ | 2.10 (0.22‐5.37) | 1.61 (0.17‐15.50) | ||||
| Pathological N stage | .008 | .036 | .004 | .148 | ||
| pN0 | 1.00 | 1.00 | ||||
| pN1≤ | 3.19 (1.08‐9.43) | 3.50 (0.67‐14.10) | ||||
| LVI | .329 | .053 | ||||
| Negative | ||||||
| Positive | ||||||
| Adjuvant chemotherapy | .181 | .508 | ||||
| Administered | ||||||
| Not administered | ||||||
| CD44v9 expression | .001 | .016 | .003 | .031 | ||
| Low | 1.00 | 1.00 | ||||
| High | 6.18 (1.59‐19.20) | 5.67 (1.26‐20.40) | ||||
CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion.
Cancer‐specific death was noted in 14 patients (22.2%) during the follow‐up: 13 in the high CD44v9 expression group and 1 in the low CD44v9 expression group. A Kaplan‐Meier curve revealed that the 5‐year CSS rate of the high CD44v9 expression group was 59.8%, which was significantly lower than that of the low CD44v9 expression group (95.2%, P = .003, Figure 1E). A univariate Cox analysis identified sex (P = .037), pathological T stage (P = .011), pathological N stage (P = .004), and CD44v9 expression in tumor specimens (P = .003) as significant prognostic factors for cancer‐specific death (Table 2). A multivariate Cox regression analysis revealed that only high CD44v9 expression in tumor specimens (HR 5.67, P = .031) was independently associated with cancer‐specific death.
3.3. Effects of SSZ on cytotoxicity and function of the CD44v9‐xCT system in MBT‐2V cells
We initially assessed the cytotoxic effects of various concentrations of SSZ in MBT‐2V cells (Figure 2A). The means ± SE of relative cell viability in MBT‐2V cells treated with 300, 400, 500, or 600 μmol/L SSZ were 86.8 ± 7.8%, 20.7 ± 7.2%, 6.1 ± 11.4%, and 3.5 ± 7.9%, respectively. Figure 2B shows relative cell viabilities in MBT‐2V cells treated with SSZ with or without 3 μmol/L NAC. The means ± SE of relative cell viability in MBT‐2V cells treated with 400, 600, or 800 μmol/L SSZ alone were 16.7 ± 16.2%, 2.3 ± 4.4%, and 1.6 ± 5.4%, which were significantly lower than those in MBT‐2V cells treated with 400, 600, or 800 μmol/L SSZ in addition to 3 μM NAC (108.5 ± 7.3%, 109.6 ± 7.8%, and 78.9 ± 5.4%, respectively, P < .001 for each).
Figure 2.
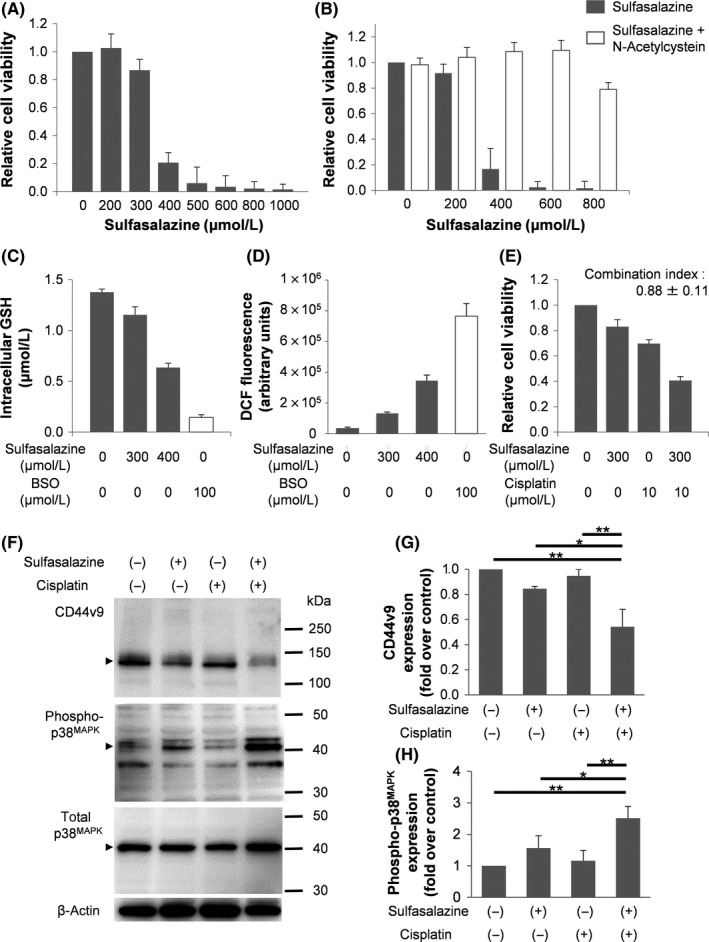
Sulfasalazine selectively inhibits cell proliferation, decreases glutathione (GSH) synthesis, increases reactive oxygen species (ROS) levels, and enhances cisplatin‐induced cytotoxic effects in MBT‐2V cells. A, Cytotoxic effects of sulfasalazine (SSZ) in MBT‐2V cells. Cells were exposed to various concentrations of SSZ for 48 h. B, Cytotoxic effects of SSZ in the presence or absence of N‐acetylcysteine (NAC, an antioxidant). Cells were exposed to various concentrations of SSZ with or without NAC (3 μmol/L) for 48 h. C, Intracellular GSH levels of MBT‐2V cells treated with the vehicle control, 300 or 400 μmol/L SSZ, and 100 μmol/L L‐buthionine‐sulfoximine (BSO) for 24 h. D, Quantitative analysis of ROS production by MBT‐2V cells treated with the vehicle control, 300 or 400 μmol/L SSZ, and 100 μmol/L BSO for 24 h. E, Cytotoxic effects of SSZ (300 μmol/L), cisplatin (CDDP) (10 μmol/L), and their combinations in MBT‐2V cells for 48 h. F, Expression of CD44v9, phospho‐p38MAPK, and total p38MAPK protein in MBT‐2V cells treated with the vehicle control, SSZ alone (300 μmol/L), CDDP alone (10 μmol/L), and their combinations detected by western blotting. G,H, Signal intensities of CD44v9 and phospho‐p38MAPK protein expression in each group was quantified. All data are shown as mean ± SE. *P < .01, **P < .001
We then confirmed whether the SSZ treatment affected the functional role of the CD44v9‐xCT system and ROS production. Figure 2C shows intracellular GSH levels in MBT‐2V cells treated with SSZ or BSO, an inhibitor of GSH synthesis. Intracellular GSH levels in MBT‐2V cells treated with 300 or 400 μmol/L SSZ were 1.16 ± 0.08 and 0.64 ± 0.04 μmol/L, respectively, which were significantly lower than those in MBT‐2V cells treated with the vehicle control (1.38 ± 0.03 μmol/L, P < .01 and P < .001, respectively). Furthermore, the greater production of ROS was observed in MBT‐2V cells treated with 300 or 400 μmol/L SSZ (Figure 2D).
3.4. Effects of the combination of SSZ and CDDP on cell proliferation and expression of CD44v9 and phospho‐p38MAPK in MBT‐2V cells
We examined whether SSZ enhances the cytotoxic effects of CDDP in MBT‐2V cells. As shown in Figure 2E, relative cell viability in MBT‐2V cells treated with the combination of 300 μmol/L SSZ and 10 μmol/L CDDP was 40.7 ± 3.1%, which was significantly lower than that in MBT‐2V cells treated with SSZ alone (83.1 ± 5.4%, P < .001) or CDDP alone (69.8 ± 3.0%, P < .001). The CI value of the SSZ and CDDP combination treatment was 0.88 ± 0.11, which exerted a synergistic effect against MBT‐2V cells.
We then analyzed CD44v9, phospho‐p38MAPK, and total p38MAPK expression using western blotting in MBT‐2V cells treated with 300 μmol/L SSZ and 10 μmol/L CDDP (Figure 2F). No significant differences were observed in CD44v9 protein expression between MBT‐2V cells treated with the vehicle control and those treated with CDDP alone; however, CD44v9 protein expression in MBT‐2V cells treated with the combination of SSZ and CDDP was significantly lower than that in MBT‐2V cells treated with CDDP or SSZ alone (Figure 2G). Regarding phospho‐p38MAPK protein expression, no significant differences were noted in phospho‐p38MAPK protein expression between MBT‐2V cells treated with the vehicle control and those treated with CDDP alone; however, phospho‐p38MAPK protein expression in MBT‐2V cells treated with the combination of SSZ and CDDP was significantly higher than that in MBT‐2V cells treated with CDDP or SSZ alone (Figure 2H). However, no significant differences were noted in total p38MAPK protein expression among the 4 groups.
3.5. Antitumor effects of the combination of SSZ with or without CDDP in the murine lung metastasis model
In the first set of in vivo experiments, the effects of SSZ on survival in the murine lung metastasis model were investigated. The SSZ treatment resulted in an animal survival rate of 58.8% over a 25‐day follow‐up period, whereas this rate was only 18.8% in the vehicle control group (P = .011; Figure 3A).
Figure 3.
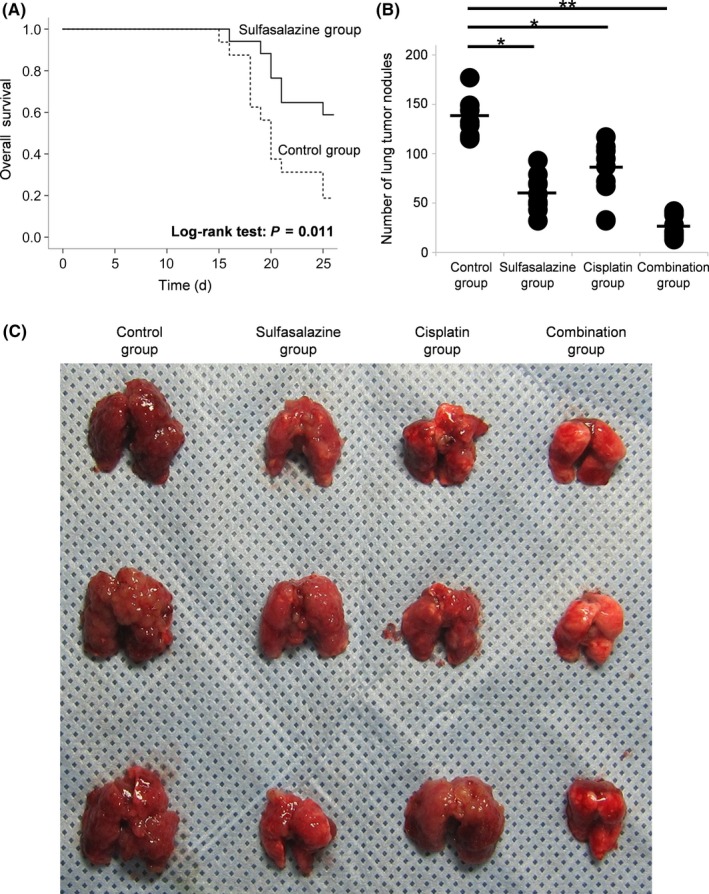
Survival analysis of sulfasalazine (SSZ) treatment and antitumor effects for lung tumor nodules of SSZ alone, cisplatin alone, and their combinations in a murine lung metastasis model. A, Lung tumor nodules were generated by injecting 2 × 105 MBT‐2V cells into the tail veins of female C3H/HeN mice on day 0. The i.p. administration (2 days on/1 day off) of SSZ (500 mg/kg) or the vehicle control (PBS) was started on day 3 (n = 18 each group). Survival analysis was evaluated by Kaplan‐Meier curve between the SSZ treatment group and the control group. B, In the lung metastasis model, mice were classified into 4 groups: vehicle control, SSZ alone (500 mg/kg), cisplatin alone (2 mg/kg, every fifth day), and their combinations (n = 10 each group). Mice were killed on day 15, and the number of lung tumor nodules was counted macroscopically. *P < .01, **P < .001. C, Representative lungs extracted from mice treated with the vehicle control, SSZ alone, cisplatin alone, and their combination
In the second set of in vivo experiments evaluating antitumor responses to SSZ alone, CDDP alone, or their combinations in the murine lung metastasis model, the number of tumor nodules (mean ± SE) on the lung surface in mice treated with the vehicle control was 114.3 ± 37.9, which was significantly higher than that in mice treated with SSZ alone (50.6 ± 19.1, P = .003), those treated with CDDP alone (69.2 ± 30.9, P = .042), and those treated with the combination of SSZ and CDDP (15.5 ± 10.8, P < .001; Figure 3B). Furthermore, the number of lung tumor nodules in mice treated with the combination of SSZ and CDDP was significantly lower than that in mice treated with SSZ or CDDP alone (P < .001 for each, respectively). Representative samples are shown in Figure 3C.
In Figure 4A, a histopathological examination of H&E‐stained sections showed that lung tumor nodules in mice treated with the vehicle control were more prominent than those in mice treated with SSZ alone, CDDP alone, and their combinations. Lung tumor nodules in mice treated with the combination of SSZ and CDDP were the smallest among the 4 treatment groups. CD44v9 immunohistochemical staining on tumor tissue is also shown in Figure 4B. The density of CD44v9 (mean ± SE) in lung tumor nodules in mice treated with the vehicle control was 89.6 ± 9.77%, which was significantly higher than that in mice treated with SSZ alone (56.9 ± 17.2%, P = .002), those treated with CDDP alone (75.9 ± 16.8%, P = .034), and those treated with the combination of SSZ and CDDP (36.7 ± 6.33%, P < .001; Figure 4C).
Figure 4.
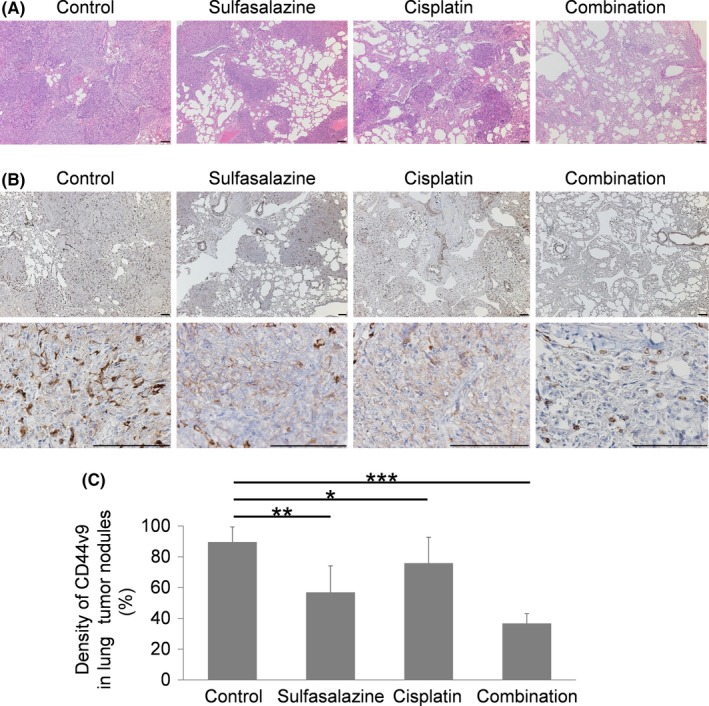
Representative microscopic findings of lungs extracted from mice treated with the vehicle control, sulfasalazine alone, cisplatin alone, and their combinations in a murine lung metastasis model. A,B, H&E staining (A) and immunostaining (B) for CD44v9 in lung tissue of mice treated with the vehicle control, sulfasalazine alone, cisplatin alone, and their combinations. Scale bar = 100 μm. C, Density of CD44v9 in lung tumor nodules of the 4 treatment groups. All data are shown as mean ± SE. *P < .05, **P < .01, ***P < .001
Regarding the side‐effects of SSZ, no abnormal laboratory data were obtained from mice treated with SSZ alone, CDDP alone, or their combinations. We also histologically analyzed the major organs, including the heart, liver, kidneys, spleen, pancreas, and ovaries, using H&E‐stained sections. No significant differences were observed in the histological appearance of or damage to these organs among the 4 treatment groups.
4. DISCUSSION
In the present study, we examined 63 MIBC patients who underwent RC and analyzed the prognostic role of CD44v9 expression, a marker of CSCs, by immunohistochemistry. The results obtained revealed that high CD44v9 expression in tumor specimens was a significant indicator for higher disease recurrence and cancer‐specific death. The prognostic role of the expression of CD44v9 in human tissue samples has also been reported in various cancers, such as gastric,24 hepatocellular,25 and head and neck cancers.26 In the field of urothelial cancer, Miyake et al12 showed that an elevated ratio of CD44v8‐10 to standard CD44 indicated shorter disease‐free survival, and Hagiwara et al9 reported that the expression of CD44v9 was an independent predictor of tumor recurrence as well as cancer death in muscle invasive upper tract urothelial carcinoma. Furthermore, Kobayashi et al11 reported that higher CD44v9 expression was associated with a worse prognosis in patients with non‐MIBC and those with MIBC. However, due to the small number of patients examined, a multivariate analysis to confirm the independent prognostic role of CD44v9 has not yet been undertaken. The present results suggest that CD44v9 expression in tumor specimens has an independent prognostic role for identifying poor survival outcomes in MIBC patients who underwent RC, and we speculate that tumors strongly expressing CD44v9 have a highly malignant potential.
The initial finding of SSZ as a potent inhibitor of xCT was investigated in lymphoma cells14 and increasing numbers of studies reported the anticancer effects of SSZ in various cancers, such as glioma,27 prostate cancer,28 and pancreatic cancer.16 Furthermore, SSZ was shown to enhance the therapeutic efficacy of chemotherapies.21, 29 In the present study, we examined the effectiveness of SSZ in BC cells using the MBT‐2V cell line, which has a highly metastatic potential with high CD44v9 expression. CD44v9 was recently found to interact with xCT by combining together on the tumor cell surface. This interaction stabilizes xCT at the cell membrane, thereby promoting the cellular uptake of cysteine and subsequent synthesis of GSH.8 Glutathione is a major antioxidant that is essential for protecting cells against ROS. Reductions in ROS production inactivate the ROS‐p38MAPK signal, and p38MAPK is a class of MAPK that negatively controls carcinogenesis and contributes to the suppression of carcinoma development by inhibiting cell growth and the induction of cell death. Therefore, the inhibition of xCT contributes to oxidative stress in cancer cells and inhibits their proliferation.
Metastatic cancer cells are often required to adapt to the selective pressure of the tumor microenvironment.30 A recent study reported that neutrophils inhibited metastatic seeding in lungs by generating H2O2. Granot et al31 indicated that neutrophils accumulated in lung tissues before the arrival of metastatic cells and inhibited lung metastasis by inducing oxidative stress, and the neutralization of oxidative stress at potential sites of metastasis was required for cancer cells to establish metastatic lesions. Therefore, xCT‐dependent ROS defenses allow cancer cells to evade metastatic stress.7 Chen et al4 found that the disruption of xCT activated p38MAPK and inhibited cancer cell metastasis through the caveolin‐1/β‐catenin pathway. The present study clearly showed that by reducing intracellular GSH levels and enhancing ROS production, the SSZ treatment upregulated the expression of the phospho‐p38MAPK protein in MBT‐2V cells, which could result in inhibitory metastatic activity in the lung metastatic murine model.
Previous studies reported that SSZ sensitized cancer cells to CDDP‐based chemotherapy. Ma et al21 showed that the combination of SSZ and CDDP resulted in greater ROS production and stronger cytotoxic effects in colorectal cancer cells than SSZ alone. In the field of BC, Drayton et al20 indicated the effectiveness of SSZ in combination with CDDP in BC cells in an in vitro study. We herein clearly showed that the combination of SSZ and CDDP enhanced the production of ROS and upregulated the expression of phospho‐p38MAPK in MBT‐2V cells. We also demonstrated marked inhibitory effects on the establishment of lung tumor nodules in the murine animal model. This is the first study to show the therapeutic effects of not only SSZ, but also the combination of SSZ and CDDP in a BC metastatic model, suggesting the potential of SSZ and CDDP combination therapy as a new therapeutic approach for metastatic BC. A case report by Takayama et al32 recently showed that a patient with metastatic BC positive for CD44v9 expression achieved a complete response by multidisciplinary therapy including CDDP‐based chemotherapy in combination with SSZ. Future clinical studies are warranted in order to evaluate the efficacy and safety of SSZ in combination with CDDP‐based chemotherapy for metastatic BC.
The use of female mice in our in vivo study is one of the limitations of the present study. There were several reasons for using female mice, but not male mice. We previously developed and used a metastatic bladder cancer animal model using female mice with high reproducibility.33 Furthermore, we are undertaking another in vivo study using an MBT‐2 orthotopic BC model. Female C3H/HeN mice are suitable for developing the orthotopic BC model due to the ease of catheterization with a short urethra. In addition, previous studies showed that female bladder cancer patients had greater metastatic potential and a poorer prognosis.34, 35
In summary, the expression of CD44v9 could be a useful clinical biomarker for predicting poor outcomes in MIBC patients. Sulfasalazine could modulate the CD44v9‐xCT system by inhibiting GSH levels, inducing ROS production, and upregulating phospho‐p38MAPK expression, and might also enhance CDDP‐induced cytotoxic effects against BC cells and the subsequent inhibitory effects in the MBT‐2V murine lung metastatic model. Sulfasalazine in combination with CDDP might have potential as a novel therapeutic approach for metastatic BC patients.
CONFLICT OF INTEREST
Eiji Kikuchi received honoraria for lecture fees from MSD, Chugai Pharmaceutical, Ono Pharmaceutical, Taiho Pharmaceutical, Astra Zeneca, Astellas Pharma, Japan BCG Laboratory, and Nippon Kayaku. The other authors have no conflict of interest.
ACKNOWLEDGMENTS
This study was supported in part by the Japan Society for the Promotion of Science KAKENHI (grant nos. JP17K16812 and JP17K11157) and a Grant‐in‐Aid for the Encouragement of Young Medical Scientists from Keio University (#02‐002‐0013).
Ogihara K, Kikuchi E, Okazaki S, et al. Sulfasalazine could modulate the CD44v9‐xCT system and enhance cisplatin‐induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 2019;110:1431–1441. 10.1111/cas.13960
Funding information
Japan Society for the Promotion of Science, Grant Award Numbers JP17K11157 and JP17K16812; Keio University School of Medicine, Grant Award Number #02‐002‐0013.
REFERENCES
- 1. Sternberg CN, Yagoda A, Scher HI, et al. M‐VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol. 1988;139:461‐469. [DOI] [PubMed] [Google Scholar]
- 2. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068‐3077. [DOI] [PubMed] [Google Scholar]
- 3. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105‐111. [DOI] [PubMed] [Google Scholar]
- 4. Chen RS, Song YM, Zhou ZY, et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin‐1/beta‐catenin pathway. Oncogene. 2009;28:599‐609. [DOI] [PubMed] [Google Scholar]
- 5. Nagano O, Okazaki S, Saya H. Redox regulation in stem‐like cancer cells by CD44 variant isoforms. Oncogene. 2013;32:5191‐5198. [DOI] [PubMed] [Google Scholar]
- 6. Pongcharoen P, Jinawath A, Tohtong R. Silencing of CD44 by siRNA suppressed invasion, migration and adhesion to matrix, but not secretion of MMPs, of cholangiocarcinoma cells. Clin Exp Metastasis. 2011;28:827‐839. [DOI] [PubMed] [Google Scholar]
- 7. Yae T, Tsuchihashi K, Ishimoto T, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. [DOI] [PubMed] [Google Scholar]
- 8. Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(‐) and thereby promotes tumor growth. Cancer Cell. 2011;19:387‐400. [DOI] [PubMed] [Google Scholar]
- 9. Hagiwara M, Kikuchi E, Kosaka T, Mikami S, Saya H, Oya M. Variant isoforms of CD44 expression in upper tract urothelial cancer as a predictive marker for recurrence and mortality. Urol Oncol. 2016;34(337):e19‐e26. [DOI] [PubMed] [Google Scholar]
- 10. Hagiwara M, Kikuchi E, Tanaka N, et al. Variant isoforms of CD44 involves acquisition of chemoresistance to cisplatin and has potential as a novel indicator for identifying a cisplatin‐resistant population in urothelial cancer. BMC Cancer. 2018;18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi K, Matsumoto H, Matsuyama H, et al. Clinical significance of CD44 variant 9 expression as a prognostic indicator in bladder cancer. Oncol Rep. 2016;36:2852‐2860. [DOI] [PubMed] [Google Scholar]
- 12. Miyake H, Eto H, Arakawa S, Kamidono S, Hara I. Over expression of CD44V8‐10 in urinary exfoliated cells as an independent prognostic predictor in patients with urothelial cancer. J Urol. 2002;167:1282‐1287. [PubMed] [Google Scholar]
- 13. Zenlea T, Peppercorn MA. Immunosuppressive therapies for inflammatory bowel disease. World J Gastroenterol. 2014;20:3146‐3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)‐ cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633‐1640. [DOI] [PubMed] [Google Scholar]
- 15. Guan J, Lo M, Dockery P, et al. The xc‐ cystine/glutamate antiporter as a potential therapeutic target for small‐cell lung cancer: use of sulfasalazine. Cancer Chemother Pharmacol. 2009;64:463‐472. [DOI] [PubMed] [Google Scholar]
- 16. Lo M, Ling V, Wang YZ, Gout PW. The xc‐ cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer. 2008;99:464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thanee M, Loilome W, Techasen A, et al. CD44 variant‐dependent redox status regulation in liver fluke‐associated cholangiocarcinoma: a target for cholangiocarcinoma treatment. Cancer Sci. 2016;107:991‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshikawa M, Tsuchihashi K, Ishimoto T, et al. xCT inhibition depletes CD44v‐expressing tumor cells that are resistant to EGFR‐targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855‐1866. [DOI] [PubMed] [Google Scholar]
- 19. Zhang W, Trachootham D, Liu J, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol. 2012;14:276‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drayton RM, Dudziec E, Peter S, et al. Reduced expression of miRNA‐27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 2014;20:1990‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma MZ, Chen G, Wang P, et al. Xc‐ inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH‐dependent mechanism. Cancer Lett. 2015;368:88‐96. [DOI] [PubMed] [Google Scholar]
- 22. Horinaga M, Fukuyama R, Nishiyama T, et al. Novel enhanced lung‐colonizing variant of murine MBT‐2 bladder cancer cells. Urology. 2005;66:676‐681. [DOI] [PubMed] [Google Scholar]
- 23. Zhao L, Wientjes MG, Au JL. Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res. 2004;10:7994‐8004. [DOI] [PubMed] [Google Scholar]
- 24. Hirata K, Suzuki H, Imaeda H, et al. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kakehashi A, Ishii N, Sugihara E, Gi M, Saya H, Wanibuchi H. CD44 variant 9 is a potential biomarker of tumor initiating cells predicting survival outcome in hepatitis C virus‐positive patients with resected hepatocellular carcinoma. Cancer Sci. 2016;107:609‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aso T, Matsuo M, Kiyohara H, et al. Induction of CD44 variant 9‐expressing cancer stem cells might attenuate the efficacy of chemoradioselection and Worsens the prognosis of patients with advanced head and neck cancer. PLoS ONE. 2015;10:e0116596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haryu S, Saito R, Jia W, et al. Convection‐enhanced delivery of sulfasalazine prolongs survival in a glioma stem cell brain tumor model. J Neurooncol. 2018;136:23‐31. [DOI] [PubMed] [Google Scholar]
- 28. Doxsee DW, Gout PW, Kurita T, et al. Sulfasalazine‐induced cystine starvation: potential use for prostate cancer therapy. Prostate. 2007;67:162‐171. [DOI] [PubMed] [Google Scholar]
- 29. Song Y, Jang J, Shin TH, et al. Sulfasalazine attenuates evading anticancer response of CD133‐positive hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2017;36:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814‐2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takayama T, Kubo T, Morikawa A, Morita T, Nagano O, Saya H. Potential of sulfasalazine as a therapeutic sensitizer for CD44 splice variant 9‐positive urogenital cancer. Med Oncol. 2016;33:45. [DOI] [PubMed] [Google Scholar]
- 33. Takeda T, Kikuchi E, Mikami S, et al. Prognostic role of KiSS‐1 and possibility of therapeutic modality of metastin, the final peptide of the KiSS‐1 gene, in urothelial carcinoma. Mol Cancer Ther. 2012;11:853‐863. [DOI] [PubMed] [Google Scholar]
- 34. Kluth LA, Rieken M, Xylinas E, et al. Gender‐specific differences in clinicopathologic outcomes following radical cystectomy: an international multi‐institutional study of more than 8000 patients. Eur Urol. 2014;66:913‐919. [DOI] [PubMed] [Google Scholar]
- 35. Soave A, Dahlem R, Hansen J, et al. Gender‐specific outcomes of bladder cancer patients: a stage‐specific analysis in a contemporary, homogenous radical cystectomy cohort. Eur J Surg Oncol. 2015;41:368‐377. [DOI] [PubMed] [Google Scholar]


