Abstract
We conducted a large‐scale surveillance study as a post–marketing commitment to investigate the safety and effectiveness of alectinib in patients with ALK‐positive non–small‐cell lung cancer (NSCLC) in Japan. Patients receiving 300 mg twice‐daily alectinib (September 2014 to June 2015) were monitored until termination of alectinib or completion of 18 months of treatment at 519 Japanese study sites. The primary endpoint was the incidence of adverse drug reactions (ADR), which are important identified risks for alectinib in Japanese patients. Overall survival (OS), a key secondary endpoint, was assessed according to information on outcome. Overall, 1251 patients were enrolled. The median patient age was 62.0 years; 12.9% of patients were aged ≥75 years. At baseline, 63.0% of patients had received crizotinib and 40.6% had brain metastases. Altogether, 1512 ADR occurred in 654 patients (53.6%), with 164 grade ≥3 ADR in 123 patients (10.1%). Commonly occurring ADR were hepatic disorders (all grades, 19.8%; grade ≥3, 2.0%), decreased neutrophil and/or white blood cell count (all grades, 7.6%; grade ≥3, 1.1%), and interstitial lung disease (all grades, 3.8%; grade ≥3, .7%). Median OS was not estimable. The 18‐month cumulative OS rate was longer in patients with ECOG performance status ≤1 (vs 2 or ≥3; 83.7% vs 44.5% or 27.2%), without prior crizotinib (vs with; 81.1% vs 73.4%), receiving first‐line alectinib (vs second and third or later line; 83.0% vs 79.2% or 71.9%), without brain metastases (vs with; 79.5% vs 71.5%). These data confirm the favorable safety and effectiveness of alectinib in patients with ALK‐positive NSCLC in Japan.
Keywords: alectinib, ALK‐positive, Japan, NSCLC, real‐world study
1. INTRODUCTION
Non–small‐cell lung cancer (NSCLC) with anaplastic lymphoma kinase fusion gene‐rearrangement (ALK‐positive) is a distinct subset of lung cancer occurring in approximately 5% of advanced adenocarcinoma cases.1, 2, 3 The central nervous system (CNS) active and highly selective ALK inhibitor alectinib is approved by the US Food and Drug Administration and the European Medicines Agency, and is recommended as a preferred first‐line treatment option for ALK‐positive NSCLC in the NCCN guidelines.4
Approval of alectinib in Japan was based on data from a phase I/II study of ALK inhibitor‐naïve patients with ALK‐positive NSCLC who received alectinib 300 mg twice daily (AF‐001JP).5, 6 Using data from the AF‐001JP study, the Japanese Risk Management Plan (J‐RMP) identified interstitial lung disease (ILD), liver function disorder, and decreased neutrophil and/or white blood cell (WBC) counts as important risks for Japanese patients receiving alectinib.
To investigate the safety and effectiveness of alectinib in patients with ALK‐positive NSCLC in Japan, including adverse drug reactions (ADR; treatment‐related adverse events [AE]) of particular concern, a large‐scale surveillance study was implemented in Japan as part of a post–marketing commitment. Here, we report the study outcomes.
2. PATIENTS AND METHODS
2.1. Study design
This multicenter surveillance study examined the safety and effectiveness of alectinib in all patients with ALK‐positive NSCLC in Japan (UMIN‐CTR Clinical Trial number: UMIN000014989, Protocol number ALC1401). Patients receiving alectinib 300 mg orally twice daily between 5 September 2014 and 30 June 2015, at 519 hospitals and clinics in Japan were monitored until study drug termination or completion of 18 months of treatment.
The primary study endpoint was the incidence of ADR, including ILD, hepatic disorders, and decreased neutrophil and/or WBC counts, which are important identified risks for alectinib treatment in Japanese patients. Overall survival (OS) was a key secondary endpoint and was assessed according to information on outcome.
The study was conducted in accordance with Good Post–marketing Study Practice regulations from the Japanese Ministry of Health, Labour, and Welfare, and the study protocol was approved by the relevant ethics committees at the participating sites.
2.2. Assessments
Demographic and baseline data were collected, including age, gender, histologic tumor type, Eastern Cooperative Oncology Group performance status (ECOG PS), smoking history, line of treatment, brain metastases, previous treatment, medical history and concomitant diseases. Safety data were collected at 6 and 18 months after the start of alectinib. AE were graded using the National Cancer Institute Common Terminology Criteria for AE version 4.0 and coded using the Medical Dictionary for Regulatory Activities version 20.0 preferred terms. OS was defined as the time from starting alectinib until the time of death, regardless of cause.
2.3. Statistical analyses
The planned sample size was 1000 patients, which was the number required to identify ADR with an incidence of .3% in at least 1 patient with a probability of ≥95%. The safety population comprised all patients who received at least 1 dose of alectinib and had available case report form data. Patients without data for the duration of the observation period, or from the time of treatment initiation, were excluded from the analyses of treatment duration. The effectiveness population comprised all patients in the safety population, except those prescribed off‐label alectinib, or with a history of alectinib pretreatment. Median OS and cumulative survival rates were estimated using Kaplan‐Meier methodology. Statistical analyses were performed using Statistical Analysis Software version 9.2 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient disposition
A total of 1251 patients with ALK‐positive NSCLC (determined by immunohistochemistry [IHC], fluorescence in situ hybridization [FISH] or reverse transcriptase‐polymerase chain reaction [RT‐PCR]) were enrolled, of whom 1221 patients formed the safety population and 1194 patients formed the effectiveness population (Figure S1). Baseline patient characteristics are summarized in Table 1. There were slightly more women than men (53.8% women [n = 657], 46.1% men [n = 563]). The median patient age was 62.0 years and 12.9% of patients (n = 158) were aged ≥75 years. ECOG PS score was ≥2 in 16.1% of patients (n = 197) and ≥3 in 7.3% of patients (n = 89). Baseline brain metastases were present in 40.6% of patients (n = 496).
Table 1.
Baseline patient characteristics (safety population)
| Characteristic | Category | Patients (N = 1221) |
|---|---|---|
| Sex, n (%) | Male | 563 (46.1) |
| Female | 657 (53.8) | |
| Unknown | 1 (.1) | |
| Age, ya | Mean ± SD | 59.8 ± 13.2 |
| Median | 62.0 | |
| Range | 22‐91 | |
| Age, n (%)a | <75 y | 1062 (87.0) |
| ≥75 y | 158 (12.9) | |
| Unknown | 1 (.1) | |
| ECOG PS, n (%) | 0 | 512 (41.9) |
| 1 | 510 (41.8) | |
| 2 | 108 (8.8) | |
| 3 | 63 (5.2) | |
| 4 | 26 (2.1) | |
| Unknown | 2 (.2) | |
| Line of treatment, n (%) | First line | 223 (18.3) |
| Second line | 386 (31.6) | |
| Third line | 278 (22.8) | |
| Fourth line or later | 327 (26.8) | |
| Unknown | 7 (.6) | |
| Prior crizotinib, n (%) | No | 451 (36.9) |
| Yes | 769 (63.0) | |
| Unknown | 1 (.1) | |
| Metastatic foci, n (%) | Lung | 392 (32.1) |
| Brain | 496 (40.6) | |
| Pleura | 353 (28.9) | |
| Liver | 219 (17.9) | |
| Bone | 449 (36.8) | |
| Lymph node | 535 (43.8) | |
| Other | 171 (14.0) | |
| ALK fusion gene testing, n (%) | FISH+, IHC+ | 770 (63.1) |
| FISH−, IHC+ | 37 (3.0) | |
| FISH not conducted, IHC+ | 95 (7.8) | |
| FISH+, IHC− | 45 (3.7) | |
| FISH+, IHC not conducted | 209 (17.1) | |
| RT‐PCR+ | 32 (2.6) | |
| Unknown | 33 (2.7) |
ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; RT‐PCR, reverse transcriptase‐polymerase chain reaction; SD, standard deviation.
n = 1220.
The results of ALK fusion gene testing were positive by both FISH and IHC in 63.1% of patients (n = 770; Table 1). Overall, 63.0% of patients (n = 769) had a history of crizotinib pretreatment. Alectinib was administered as first‐line treatment in 18.3% of patients (n = 223), second line in 31.6% of patients (n = 386), third line in 22.8% of patients (n = 278), and as fourth line or later in 26.8% of patients (n = 327) (Table 1), reflecting the real‐world setting of this study.
3.2. Treatment duration
The median time to treatment failure was 482.0 days (range 1‐775). This was lower in patients with ECOG PS ≥3 (n = 89, median 133.0 days [range 1‐573]), in those who received prior crizotinib (n = 753, median 453.0 days [range 1‐775]) and in patients with baseline brain metastases (n = 485, median 446.0 days [range 1‐775]) (Table S1).
3.3. Adverse drug reactions
The incidence of ADR in patient subgroups defined by baseline characteristics was comparable with the overall safety population (Table S2). A total of 1512 ADR occurred in 654/1221 patients (53.6%), with 164 grade ≥3 ADR reported in 123/1221 patients (10.1%; Table 2). The most common all‐grade ADR were increased blood creatinine (8.2%), increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (both 7.1%), increased blood bilirubin (6.3%) and constipation (5.9%). The most frequent grade ≥3 ADR (≥5 patients or .4%) were anemia (1.1%), decreased neutrophil count (.9%), increased blood creatine phosphokinase (.7%), ILD (.6%), increased AST and ALT (.5% each), hepatic function abnormal, rash, maculo‐papular rash and increased blood bilirubin (.4% each). Anemia, decreased appetite, ILD, and constipation were common grade ≥3 events in patients with ECOG PS ≥2 (n = 197). The overall incidence of grade ≥3 ADR in patients with ECOG PS ≥2 was 15.7% (vs 9.0% for ECOG PS ≤1), of which 72.1% recovered or improved.
Table 2.
Incidence of adverse drug reactionsa in patients receiving alectinib (N = 1221)
| System organ class | Any grade, patients, n (%) | Grade ≥3, patients, n (%) |
|---|---|---|
| Overall | 654 (53.6) | 123 (10.1) |
| Investigations | 338 (27.7) | 42 (3.4) |
| Gastrointestinal disorders | 136 (11.1) | 15 (1.2) |
| Skin and subcutaneous tissue disorders | 102 (8.4) | 12 (1.0) |
| General disorders and administration site conditions | 81 (6.6) | 4 (.3) |
| Hepatobiliary disorders | 76 (6.2) | 9 (.7) |
| Nervous system disorders | 74 (6.1) | 1 (.1) |
| Respiratory, thoracic and mediastinal disorders | 73 (6.0) | 12 (1.0) |
| Blood and lymphatic system disorders | 49 (4.0) | 18 (1.5) |
| Metabolism and nutrition disorders | 44 (3.6) | 13 (1.1) |
| Musculoskeletal and connective tissue disorders | 41 (3.4) | 1 (.1) |
| Renal and urinary disorders | 29 (2.4) | 2 (.2) |
| Infections and infestations | 22 (1.8) | 3 (.2) |
| Eye disorders | 19 (1.6) | 0 |
| Vascular disorders | 13 (1.1) | 3 (.2) |
| Cardiac disorders | 12 (1.0) | 3 (.2) |
| Psychiatric disorders | 6 (.5) | 1 (.1) |
| Reproductive system and breast disorders | 2 (.2) | 0 |
| Immune system disorders | 1 (.1) | 0 |
| Endocrine disorders | 1 (.1) | 0 |
| Ear and labyrinth disorders | 1 (.1) | 0 |
Adverse drug reactions based on current precautions (Medical Dictionary for Regulatory Activities Japanese version 20.0).
Frequently occurring ADR of important identified risk were hepatic disorders (all grades, 19.8%; grade ≥3, 2.0%), decreased neutrophil and/or WBC count (all grades, 7.6%; grade ≥3, 1.1%) and ILD (all grades, 3.8%; grade ≥3, .7%) (Table 3).
Table 3.
Adverse drug reactions of identified or potential risk with alectinib based on the J‐RMP
| Adverse drug reaction | Any grade, patients, n (%) | Grade ≥3, patients, n (%) |
|---|---|---|
| Identified risk | ||
| Interstitial lung diseasea | 47 (3.8) | 9 (.7) |
| Hepatic disorders | 242 (19.8) | 24 (2.0) |
| Decreased neutrophil and/or WBC count | 93 (7.6) | 14 (1.1) |
| Potential risk | ||
| Prolongation of QT interval | 3 (.2) | 1 (.1) |
| Bradycardia | 8 (.7) | 3 (.2) |
| Visual disorders | 9 (.7) | 0 |
| Gastrointestinal perforation | 3 (.2) | 3 (.2) |
| Arterial thromboembolism | 1 (.1) | 0 |
| Venous thromboembolism | 7 (.6) | 1 (.1) |
ILD, interstitial lung disease; J‐RMP, Japanese Risk Management Plan; WBC, white blood cell.
Preferred terms in the MedDRA Japanese version, based on the definition of ILD, were interstitial lung disease, eosinophilic pneumonia acute, eosinophilic pneumonia, pulmonary fibrosis and pneumonitis.
3.4. Interstitial lung disease
According to physician‐assessed data, 48 ILD events were reported in 47/1221 patients (3.8%). All of these were serious, except for 1 event of pulmonary fibrosis, which was classed as non–serious. Overall, 44/48 (91.7%) of these ILD events recovered or improved, 23/48 events (47.9%) were fully recovered and 21/48 events (43.8%) improved.
Grade 1 ILD was reported in 19/1221 patients (1.6%), grade 2 in 20/1221 patients (1.6%), grade 3 in 9/1221 patients (.7%) and grade 4 in 1 patient (.1%). Of the grade ≥3 events, 5 recovered, 3 improved and 1 did not recover. Based on the assessment of an independent ILD medical expert, the possibility that 1 patient died as a result of an ILD could not be refuted.
Figure 1 illustrates the incidence of first onset of ILD per 100 patient‐weeks. The median time from start of treatment to onset of ILD was 119.5 days (range 3‐532), and the median time from onset to outcome was 57.5 days (range 8‐379) (Table 4).
Figure 1.
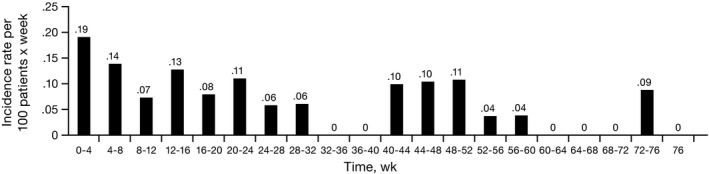
Incidence of interstitial lung disease (ILD) from start of alectinib administration to onset of ILD
Table 4.
Time to onset and outcome of adverse drug reactions of important identified risk with alectinib
| Adverse drug reaction | Events, n | Median time to onset, days (range) | Events that recovered or improved, % (n) | Median time to recovery or improvement, days (range) |
|---|---|---|---|---|
| Interstitial lung disease | 48 | 119.5 (3‐532) | 91.7 (44/48) | 57.5 (8‐379) |
| Hepatic disorders | 390 | 28.5 (1‐624) | 82.6 (322/390) | 36.0 (2‐627) |
| Decreased neutrophil and/or WBC count | 163 | 12.0 (1‐550) | 93.3 (152/163) | 28.5 (1‐617) |
WBC, white blood cell.
3.5. Hepatic disorders
In total, 390 hepatic disorder events were reported in 242/1221 patients (19.8%); 5 of these events in 4 patients were serious. Grade 1 events were reported in 174/1221 patients (14.3%), grade 2 events in 64/1221 patients (5.2%) and grade ≥3 events in 24/1221 patients (2.0%). Of the grade ≥3 events, 19 recovered, 9 improved and 5 did not recover. None of these events resulted in death.
The median time from start of treatment to onset of hepatic disorder events was 28.5 days (range 1‐624). Overall, 82.6% of events recovered or improved, including all serious events. The median time from onset to outcome was 36.0 days (range 2‐627) (Table 4).
3.6. Decreased neutrophil and/or white blood cell count
Overall, 163 decreased neutrophil and/or WBC count events were reported in 93/1221 patients (7.6%), of which 10 events in 5 patients were serious. Grade 1 events were reported in 50/1221 patients (4.1%), grade 2 events in 41/1221 patients (3.4%) and grade ≥3 events in 14/1221 patients (1.1%). Of the grade ≥3 ADR, 17 recovered, 3 improved and 1 did not recover. None of these events resulted in death.
The median time from start of treatment to onset of these events was 12.0 days (range 1‐550). Overall, 93.3% of events recovered or improved, with a median time from onset to outcome of 28.5 days (range 1‐617) (Table 4).
3.7. Survival
Of the 1194 patients in the effectiveness population, 1191 were included in the OS analysis, and 247 events occurred. Three patients were excluded from the OS analysis because of missing data (ie, information was missing on the initial dosing date of alectinib, outcome or survival). Median OS could not be estimated during the observation period.
The cumulative OS rate at 6, 12 and 18 months, was 89.0% (95% confidence interval [CI], 87.0‐90.7), 82.4% (95% CI, 80.0‐84.6) and 76.2% (95% CI, 73.5‐78.7), respectively. OS differed significantly according to ECOG PS (log‐rank test P < .0001): the 18‐month cumulative OS rate was 83.7% (95% CI, 81.0‐86.1) in patients with ECOG PS ≤1, 44.5% (95% CI, 33.7‐54.7) for PS 2, and 27.2% (95% CI, 17.4‐37.8) for PS ≥3 (Figure 2). Similar results were observed in patients receiving first‐line alectinib: the 18‐month cumulative OS rate was 87.9% (95% CI, 81.6‐92.1) for patients with ECOG PS ≤1, 67.5% (95% CI, 16.2‐91.9) for PS 2 and 49.9% (95% CI, 28.8‐67.9) for PS ≥3 (Figure S2).
Figure 2.
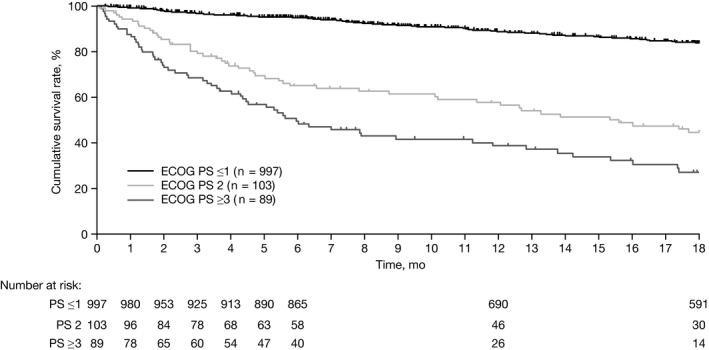
Cumulative survival rate according to baseline ECOG PS. ECOG PS, Eastern Cooperative Oncology Group performance status
The 18‐month cumulative OS rate was also significantly longer in patients without previous crizotinib treatment (81.1% [95% CI, 76.8‐84.7] vs 73.4% [95% CI, 69.8‐76.7] with prior crizotinib; P = .0122), receiving first‐line alectinib (83.0% [95% CI, 76.8‐87.6] vs 79.2% [95% CI, 74.4‐83.3] second line and 71.9% [95% CI, 67.7‐75.6] third or later line; P = .0013) and without baseline brain metastases (79.5% [95% CI, 76.0‐82.5] vs 71.5% [95% CI, 66.8‐75.6] with; P = .0014) (Figure 3).
Figure 3.
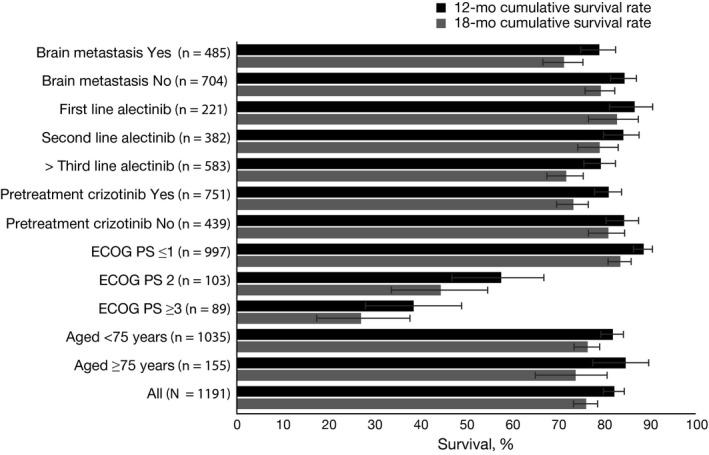
Cumulative survival rates at 12 and 18 mo by baseline patient characteristics. ECOG PS, Eastern Cooperative Oncology Group performance status
4. DISCUSSION
Data from this large‐scale, real‐world surveillance study confirm the acceptable safety and effectiveness profile of alectinib in patients with ALK‐positive NSCLC in Japan. We observed no increased incidence of ADR compared with the pivotal AF‐001JP phase I/II alectinib study5 or the Japanese bioequivalence study.7 The ADR were generally mild in intensity and managed effectively when alectinib was used according to the utility guidance and treatment handbook for patients. However, these results must be interpreted with caution due to the different study populations enrolled; that is, the pre–approval studies did not include patients with ECOG PS ≥2, or with crizotinib pretreatment, and included fewer patients aged ≥75 years. Furthermore, we did not see any increased incidence of ADR in patients with ECOG PS ≥2 vs ≤1, suggesting that alectinib may be tolerable in patients with poor PS, as previously reported,8 or in those who had been pretreated with crizotinib vs crizotinib‐naïve patients. Visual disturbances and gastrointestinal effects, which are frequently reported with crizotinib,9 occurred at a low rate with alectinib, as observed in AF‐001JP.5
There was no marked difference in the incidence of ILD in the present study (3.8%) and that reported in the AF‐001JP (1.7%)5 and phase III J‐ALEX (8%)10 Japanese alectinib studies, or in the global PROFILE crizotinib studies (3.7%),11 or the ASCEND‐1/2 ceritinib studies (1%‐4%).12 However, as an independent ILD medical expert could not refute the possibility that 1 patient died as a result of an ILD, it is important to continually monitor for signs of ILD during alectinib treatment. Again, caution should be exercised in comparing these results with previous study findings due to differences in patient characteristics and study observation periods.
The cumulative OS rate achieved in our study did not differ considerably from that reported in the AF‐001JP study,5 in which the 2‐year and 3‐year survival rates were 79% (95% CI, 63‐89) and 78% (95% CI, 63‐88), respectively. The 18‐month cumulative survival rate seen in patients with baseline metastases was also comparable with that reported in study AF‐001JP.5 However, results cannot be compared directly due to differences in patient baseline characteristics and the duration of the observation period.
A strength of the current study was its very large size, but it was limited by the lack of a comparator group or control arm, and the absence of a strict observation period or patient selection criteria. However, this could also be considered a strength as it is more reflective of real‐world treatment. The inclusion of a small number of patients with ECOG PS ≥3 allowed us to assess the benefit of alectinib treatment in this population who do not usually receive active treatment. Alectinib was administered as third line treatment or later in a relatively large proportion of patients, reflecting the real‐world setting of this study. The study was conducted at multiple sites but there was no central data review. Furthermore, median OS could not be estimated despite the existence of accumulated survival rate data.
Data from this large‐scale surveillance study confirm that alectinib has a favorable safety and effectiveness profile in patients with ALK‐positive NSCLC in Japan. As with all tyrosine kinase inhibitors, health‐care providers should carefully observe patients for signs of ILD during treatment with alectinib, as onset of ILD occurred throughout the observation period. After suspicion of onset of ILD and diagnosis by computed tomography scan, patients should be closely monitored and their risk level assessed.
CONFLICT OF INTEREST
Noriyuki Masuda has acted in a consulting/advisory role to Chugai Pharmaceutical Co. Ltd; Yuichiro Ohe has acted in a consulting/advisory role to AstraZeneca, Chugai Pharmaceutical Co. Ltd, Lilly Japan, Ono Pharmaceutical Co. Ltd, and Novartis, received honoraria from AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb Japan, Chugai Pharmaceutical Co. Ltd, Daiichi Sankyo, Kyorin, Kyowa Hakko Kirin, Lilly Japan, Merck Sharp & Dohme, Nippon Kayaku, Novartis, Ono Pharmaceutical Co. Ltd, Pfizer, and Taiho Pharmaceuticals Co. Ltd, received research funding from AstraZeneca, Bristol‐Myers Squibb Japan, Chugai Pharmaceutical Co. Ltd, Dainippon Sumitomo Pharmaceuticals, Kyorin, Lilly Japan, Novartis, Ono Pharmaceutical Co. Ltd, Pfizer, and Taiho Pharmaceuticals Co. Ltd, and owns stocks in Ono Pharmaceutical Co. Ltd; Akihiko Gemma has acted in a consulting/advisory role to Chugai Pharmaceutical Co. Ltd; Masahiko Kusumoto has acted in a consulting/advisory role to AstraZeneca Japan, Chugai Pharmaceutical Co. Ltd, Merck Sharp & Dohme, and Ono Pharmaceutical Co. Ltd; Ikuyo Yamada is an employee of Chugai Pharmaceutical Co. Ltd; Tadashi Ishii is an employee of Chugai Pharmaceutical Co. Ltd; Nobuyuki Yamamoto has acted in a consulting/advisory role to AstraZeneca, Boehringer‐Ingelheim, Chugai Pharmaceutical Co. Ltd, Merck Sharp & Dohme, Ono Pharmaceutical Co. Ltd, Pfizer, Taiho Pharmaceuticals Co. Ltd, and Takeda, participated in Speakers’ Bureaus for AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb Japan, Chugai Pharmaceutical Co. Ltd, Kyowa Hakko Kirin, Lilly Japan, Ono Pharmaceutical Co. Ltd, Pfizer, and Taiho Pharmaceuticals Co. Ltd, received honoraria from AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb Japan, Chugai Pharmaceutical Co. Ltd, Daiichi Sankyo, Kyorin, Kyowa Hakko Kirin, Lilly Japan, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical Co. Ltd, Pfizer, Taiho Pharmaceuticals Co. Ltd, and Takeda, and received research funding from AstraZeneca, Chugai Pharmaceutical Co. Ltd, Lilly Japan, and Nippon Boehringer‐Ingelheim.
Supporting information
ACKNOWLEDGMENTS
The authors thank all patients who participated in the study and clinical personnel involved in data collection, investigators and study site staff. Third‐party medical writing assistance, under the direction of the authors, was provided by Fiona Fernando, PhD, of Gardiner‐Caldwell Communications, and was funded by Chugai Pharmaceutical Co. Ltd.
Masuda N, Ohe Y, Gemma A, et al. Safety and effectiveness of alectinib in a real‐world surveillance study in patients with ALK‐positive non–small‐cell lung cancer in Japan. Cancer Sci. 2019;110:1401–1407. 10.1111/cas.13977
Clinical Trial Registration: UMIN‐CTR Clinical Trial number: UMIN000014989.
REFERENCES
- 1. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small‐cell lung cancer: meta‐analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24:2371‐2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014;40:300‐306. [DOI] [PubMed] [Google Scholar]
- 3. Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685‐700. [DOI] [PubMed] [Google Scholar]
- 4. NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines), Non‐Small Cell Lung Cancer, version 6.2018, August 17, 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed August 01, 2018.
- 5. Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK‐rearranged advanced non‐small‐cell lung cancer (AF‐001JP study): a single‐arm, open‐label, phase 1‐2 study. Lancet Oncol. 2013;14:590‐598. [DOI] [PubMed] [Google Scholar]
- 6. Tamura T, Kiura K, Seto T, et al. Three‐year follow‐up of an alectinib phase I/II study in ALK‐positive non‐small‐cell lung cancer: AF‐001JP. J Clin Oncol. 2017;35:1515‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hida T, Nakagawa K, Seto T, et al. Pharmacologic study (JP28927) of alectinib in Japanese patients with ALK+ non‐small‐cell lung cancer with or without prior crizotinib therapy. Cancer Sci. 2016;107:1642‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iwama E, Goto Y, Murakami H, et al. Alectinib for patients with ALK rearrangement‐positive non‐small cell lung cancer and a poor performance status (Lung Oncology Group in Kyushu 1401). J Thoracic Oncol. 2017;12:1161‐1166. [DOI] [PubMed] [Google Scholar]
- 9. Crizotinib summary of product characteristics. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002489/human_med_001592.jsp&mid=WC0b01ac058001d124. Accessed May 01, 2018.
- 10. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK‐positive non‐small‐cell lung cancer (J‐ALEX): an open‐label, randomised phase 3 trial. Lancet. 2017;390:29‐39. [DOI] [PubMed] [Google Scholar]
- 11. Yoneda KY, Scranton JR, Cadogan MA, et al. Interstitial lung disease associated with crizotinib in patients with advanced non‐small cell lung cancer: independent review of four PROFILE trials. Clin Lung Cancer. 2017;18:472‐479. [DOI] [PubMed] [Google Scholar]
- 12. Califano R, Greystoke A, Lal R, et al. Management of ceritinib therapy and adverse events in patients with ALK‐rearranged non‐small cell lung cancer. Lung Cancer. 2017;111:51‐58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


