Abstract
Diabetic foot ulcers (DFU), which may lead to lower extremity amputation, is one of the severe and chronic complications of diabetic mellitus. This study aims to develop, and use dressings based on Silk fibroin (SF) as the scaffold material, gelatin microspheres (GMs) as the carrier for the neurotensin (NT), a neuropeptide that acts as an inflammatory modulator in wound healing and NT as accelerate wound healing drug to treat DFU. We evaluated the wound healing processes and neo-tissue formation in rat diabetic model by macroscopic observation, histological observation (H&E staining and Masson's trichrome staining) and immunofluorescence analysis at 3, 7, 14, 21 and 28 post-operation days. Our results show that the NT/GMs/SF group performance the best not only in macroscopic healing and less scars in 28 post-operation days, but also in fibroblast accumulation in tissue granulation, collagen expression and deposition at the wound site. From release profiles, we can know the GMs are a good carrier for control release drugs. The SEM results shows that the NT/GMs/SF dressings have an average pore size are 40–80 μm and a porosity of ∼85%, this pore size is suit for wound healing regeneration. These results suggest that the NT/GMs/SF dressings may work as an effective support for control release NT to promote DFU wound healing.
Keywords: Diabetic foot ulcers (DFU), Silk fibroin (SF), Gelatin microspheres (GMs), Neurotensin (NT), Rat diabetic model
Graphical abstract
Highlights
-
•
This study aims to develop, and use dressings based on Silk fibroin (SF) as the scaffold material, gelatin microspheres (GMs) as the carrier for the Neurotensin (NT), a neuropeptide that acts as an inflammatory modulator in wound healing and NT as accelerate wound healing drug to treat DFU.
-
•
The NT/GMs/SF dressings stimulated fibroblast accumulation in tissue granulation, collagen expression and deposition at the wound site, which lead to the production of a more organized collagen matrix.
-
•
This treatment effectively accelerating wound regeneration and re-epithelialization.
1. Introduction
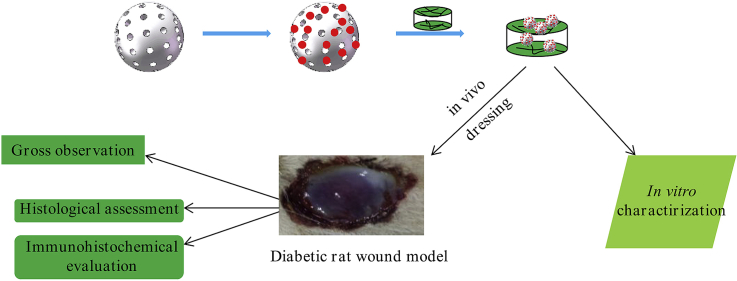
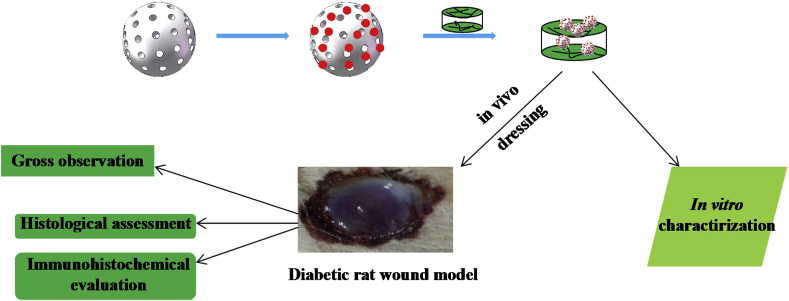
Diabetes mellitus is one of the most complicated chronic diseases that affect millions of people over the word [1]. One severe and chronic complication of diabetic is non-healing diabetic foot ulcers (DFU) that cause pain, suffering and decrease in quality of life. In most case, DFUs need culminate with lower extremity amputations [2,3]. Neurologic, peripheral vascular disease, and inflammatory alterations are recognized major factors leading to the development of DFUs, which have all the characteristic of a chronic wound [4]. Impaired diabetic wound healing is increased in skin areas that are affected by peripheral neuropathy and there is growing evidence that cutaneous peripheral nerves regulate immune and cytokine response via mediator such as neuropeptides that are important for proper wound healing [2,5]. (see Scheme 1)
Scheme 1.
Illustration of the experimental protocol.
Generally substance P and neuropeptide Y is the most common used as neuropeptides to improve diabetic wound healing [6,7]. Neurotensin (NT), a 13 amino acids peptide is mainly localized in endocrine N-cells of the gastrointestinal tract, is a bioactive neuropeptide widely existed in brain and peripheral systems that acts on immune cells such as leukocyte, mast cells and macrophages. NT can leads to cytokine release and chemotaxis necessary for a correct immunomodulation response [8,9]. In addition, NT affects new vessel formation micro-environment which can helps to improve angiogenesis during wound healing processes [10]. However, the major of those neuropeptides problem is that short half-life and little bioactivity concentration in the peptides-rich wound environment [11]. In order to solve this problem, we used silk fibroin dressing, a biocompatible material, for sustained controlled-release neuropeptide. This method not only can protect neuropeptides form rapid biodegradation but also the silk fibroin dressing can replicate skin micro-environment in order to promote the proliferation and migration of fibroblasts, keratinocytes and also enhance collagen synthesis, leading to the wound healing [12].
Silk fibroin (SF), which is a natural protein generated from silk cocoons [13], has been highlighted for diverse applications in the biomedical field such as wound healing [14], tissue regeneration [15] and drug delivery [16], because it has excellent mechanical property, controllable biodegradability, hemostatic properties, non-cytotoxicity, low antigenicity and non-inflammatory characteristic [17]. In addition, controlled-release drug or growth factors is important to the design of biomaterials. We, our previous studies have shown, use gelatin microspheres (GMs) implanted SF technique for controlled-release [18,19]. Usually, growth can be loaded into fibrous biomaterials by adsorption. Because SF have porous zone, many growths have good affinity to it. For example, the porous SF hydrogels made by Freeze-drying could provide sustained delivery systems of antibodies for over 38 days [16].
The aim of this study is to develop and apply silk fibroin dressings with GMs to control release neurotensin in diabetic rat model to evaluate wound healing, compared with normal healing, silk fibroin dressing, and silk fibroin with neurotensin. The wound healing process was investigated by wound size and healing rate; H&E; masson staining; NT and NT receptor expression.
2. Materials and methods
2.1. Materials
Bovine gelatin, which isoelectric point of 5.0 was purchased form Acros Organics (Geel, Begium). B. mori silkworm cocoons used in study were donated by Sijia Min form Zhejiang University. NT was purchased form Sigma– Aldrich (St. Louis, USA). All other chemicals were of analytical reagent quality and used without further processing.
2.2. Preparation of the SF solution
Preparation of the SF solution technique has been described in other research [20]. Briefly, the cocoons of the silkworm B. mori were cut into small pieces and boiled in Na2CO3 aqueous solution (0.02 M) for 1 h and then washed with double distilled water (DDW) several times to remove the sericin proteins form the silk fibers. The extracted SF was dried in oven at 60 °C for 12 h and then dissolved in an aqueous solution of 9.3 M LiBr at 60 °C for 5 h to yield a 5% (w/v) solution. The regenerated SF solution was then dialyzed in a cellulose tube (10 kDa molecular weight (MW) cutoff) against deionized water for 72 h, with several changes of the deionized water to remove LiBr. The solution was collected by centrifugation (5000 rpm for 10 min at 25 °C), and the concentration was determined gravimetrically after freeze-drying the SF solution. The regenerated 3.5% (w/v) SF was stored at 4 °C until further use.
2.3. Preparation of the G microspheres
The G microspheres were prepared as described previously [21]. Briefly, 10 ml of 10% (w/v) G solution, preheated at 50 °C for 1 h, was added dropwise into 100 ml of olive oil (containing 0.1 g span-80) under stirring at 400 rpm with a mechanical stirrer to prepare a water-in-oil emulsion. The emulsion was immersed in an ice bath to maintain its temperature below 10 °C and stirred continuously for 20min to complete gelation of G in the water phase. After the addition of 30 ml of chilled acetone, the emulsion was stirred continuously for 1 h. The resulting G microspheres were collected by centrifuging at 5000 rpm for 10 min at 25 °C, and washing three times with acetone and one time with isopropyl alcohol. The G microspheres were immersed in a 2.5 wt% glutaraldehyde aqueous solution for 12 h at 4 °C. Deionized water was used to wash the cross-linked microspheres. The cross-linked microspheres were suspended in 50 mM glycine solution at 25 °C for 2 h to block the unreacted residual glutaraldehyde. The microspheres were then washed with deionized water. After immersing the microspheres in deionized water at 25 °C for 2 h, the microspheres were freeze-dried for 24 h to remove any remaining solvent.
2.4. Preparation of the NT-impregnated GMs (NT/GMs)
The NT impregnated GMs method has been previously designed as Vm/G microspheres in our study [19]. Briefly, NT/G microspheres were obtained by adding 4 μl of the NT solution (25 mg/ml) per 1 mg of freeze-dried G microspheres and then stored at 4 °C overnight. The microspheres absorbed all of the NT solution because the volume of the solution was much less than the swelling saturation volume; therefore, the incorporation efficiency of NT into the G microspheres was considered as 100%. The composite microspheres were then freeze-dried for 24 h to remove any remaining solvent.
2.5. Preparation of the SF, NT/SF and NT/GMs/SF dressing
The concentration of the SF was almost 4% (w/v), which was determined gravimetrically after freeze-drying for 24 h to remove any remaining solvent. The SF dressing produced is that SF solution pouring into a 24-well plate and freeze-drying for 24 h. The NT/SF dressing was fabricated by mixing 1 ml of 4% (w/v) SF solution and 4 μL of NT solution (25 mg/ml) and then same as SF dressing protocol. The NT/GMs/SF dressing was produced by mixing 1 ml of 4% (w/v) SF solution and 10 mg of NT/GMs, and then like SF produced. All dressings were immersed in 90% (v/v) methanol aqueous solution for 30 min to make water-insoluble dressing [22]. This methanol solution has negligible effect on NT, due to NT being insoluble in most organic liquids, including acetone and methanol [23].
2.6. Swelling of the GMs
To calculating the water content of the GMs, we have to know the degree of swelling. Dried and wet which saturated with deionized water for 4 h at room temperature, GMs were observed under a microscope (Axio Scope A1 FL; Carl Zeiss, Wetzlar, Germany). One hundred dried and wet GMs were viewed, respectively. Their diameter was measured by microscope. The swelling ratios were then calculated using the following formula: swelling ratio = .
2.7. Scanning electron microscopy
The morphologies of SF, NT/SF and NT/GMs/SF dressings were characterized by scanning electron microscopy (SEM; LEO1530 VP, Philips, Amsterdam, the Netherlands). The pore sizes of each dressing were evaluated by measurement of 25 random pores in SEM images and analysis by ImageJ software (NIH, Bethesda, MD, USA). The porosity of the SF scaffolds was measured according to a method published previously [18].
2.8. Animal model
Male Sprague-Dawley rats (200–230 g) were used in this work. The animals were maintained at normal room temperature (22–24 °C) on a 12 h light/dark cycle, with free access to commercial pellet diet and water. After the wounding procedure, the animals were kept in individual cages. All animal procedures were approved by the experimental animal administration committee of Sun Yat-Sen University.
Diabetic was induced by a single intraperitoneal injection of streptozocin (STZ) (150 mg/kg) in citrate buffer of pH 4.5. Four days after diabetic induction blood glucose levels were checked using an Accu-Chek Aviva glucometer (Roche Diagnostics GmbH, Germany). Animals with blood glucose levels higher than 300 mg/dl were considered diabetic. Rats were anesthetized by intraperitoneal injection of xylazine (13 mg/kg) and ketamine (66.7 mg/kg). The dorsal hair of all diabetic rat was shaved, and four 15 mm diameter full thickness wounds were created with a biopsy punch.
2.9. Measurement of wound healing area
To examine the ratios of dressing on the diabetic wound-healing process, we design the wounds were covered with none, SF, NT/SF and NT/GMs/SF dressings, respectively. Each wound dressing was exchanged every 2 days for the first week and then once a week for next 4 weeks. During this period, the DFU wound site was photographed (every 3, 7, 14, 21 and 28 days) and measured using IPP software.
2.10. Histological examination
The entire of wound area was then wrapped with sterile gauze, sample were harvested at days 3, 7,14, 21, and 28 postoperatively. For histological analyses, the harvest samples were fixed in 4% formaldehyde in PBS at 4 °C, dehydrated in a graded series of ethanol, and then embedded in paraffin for routine haematoxylin-eosin (H&E) staining and Masson's trichrome staining for collagen fibers. H&E− and Masson's-trichrome-stained sections were observed with a light microscope (Axio Scope A1 FL; Carl Zeiss, Wetzlar, Germany).
2.11. Immunofluorescence assay
Immunofluorescence was performed following the procedures previously described. Briefly, paraffin sections (5 mm) were deparaffinised, washed three times in PBS for 5 min, blocked with 5% serum for 30 min and incubated overnight at 4 °C with anti-NT primary antibody (1:100; United States Biological, Massachusetts, USA), anti-NTR2 primary antibody (1:50; GeneTex, California, USA) and anti-NTR3 primary antibody (1:50; Bioss, Massachusetts, USA). After rinsing three times with PBS, the slides were incubated with FITC-conjugated secondary antibody (1:50; Dako, Carpinteria, CA, USA) for 30 min. After washing three times in PBS, the cell nuclei were stained by DAPI (Sigma, Buchs, Switzerland) for 10 min at room temperature. Images were acquired with a fluorescence microscope (IX 81; Olympus, Tokyo, Japan).
2.12. Statistical analysis
Values are expressed as the mean of at least three replicates ± standard deviation (SD). Statistical comparisons were performed using ANOVA (t-test). All statistical computations were performed using the SPSS for Windows software (ver. 16.0; SPSS Inc., Chicago, IL, USA). Differences with a value of P < 0.05 were considered statistically significant. All experiments were performed in triplicate and the results are presented as the mean ± SD of six measurements in the latest experiment; statistical significance was calculated by Student's t-test.
3. Results
3.1. Characterization of GMs
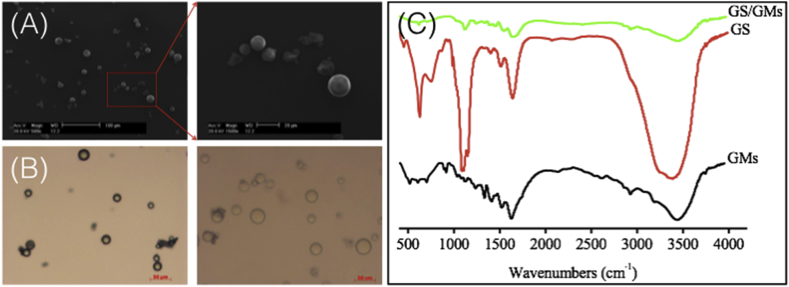
Though SEM examined the surface morphology characterization of GMs (Fig. 1 A). The results were shown in Fig. 1 A and Table 1, all the GMs were spherical, uniformly sized and smooth. Form the optical images of the GMs before and after swelling in DI water for 4 h at room temperature (Fig. 1 B). After immersion in DI water, the GMs swelled and became transparent. The average diameter of the wet GMs was 30.6 ± 5.4 μm, with a range of 21–48 μm, the swelling ratio of the GMs based on volume was 4.3 ± 0.6.
Fig. 1.
Characteristic of GMs, (A) SEM of GMs, (B) before and after immersion in DI water 4 h, (C) FTIR spectra of GMs, GS, GS/GMs composites.
Table 1.
Particle sizes and swelling ratio of GMs.
| Dry diameter ± SD (μm) | Wet diameter ± SD (μm) | Swelling ratio ± SD (volume) | |
|---|---|---|---|
| GMs | 20.8 ± 6.5 | 30.6 ± 5.4 | 4.3 ± 0.6 |
The FTIR analyses of GMs, NT and NT/GMs composites, as shown in Fig. 1 C, indicated the presence of NT inside the GMs; characteristic GS peaks at 619, 1120 and 1242 cm−1 were observed in all spectra.
3.2. Characterization of SF and GMs/SF dressings
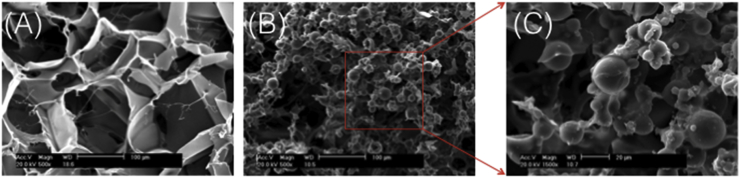
The SF and GMs/SF dressings SEM images have shown that Fig. 2A and B, typical SF dressings exhibited uniform pore distribution with an average pore size of 90–120 μm. The porosity of the SF scaffold was 92 ± 0.5% with marked interconnectivity. In NT/GMs/SF dressings (Fig. 2B), the embedded NT/GMs were well distributed and integrated throughout the SF structure and were fixed firmly to the walls or intersections of the scaffold. Throughout, all embedded NT/GMs appeared to be wrapped in a thin layer of SF. The porosity (about 85%) and interconnectivity of the pores were well maintained and not affected by the embedded NT/GMs. However, the average pore size of the NT/GMs/SF dressings decreased to 40–80 μm.
Fig. 2.
Scanning electron micrographs of (A) SF dressing, (B) NT/GMs/SF dressing, (C) magnification 1500 NT/GMs/SF dressing.
3.3. The animal model wound healing
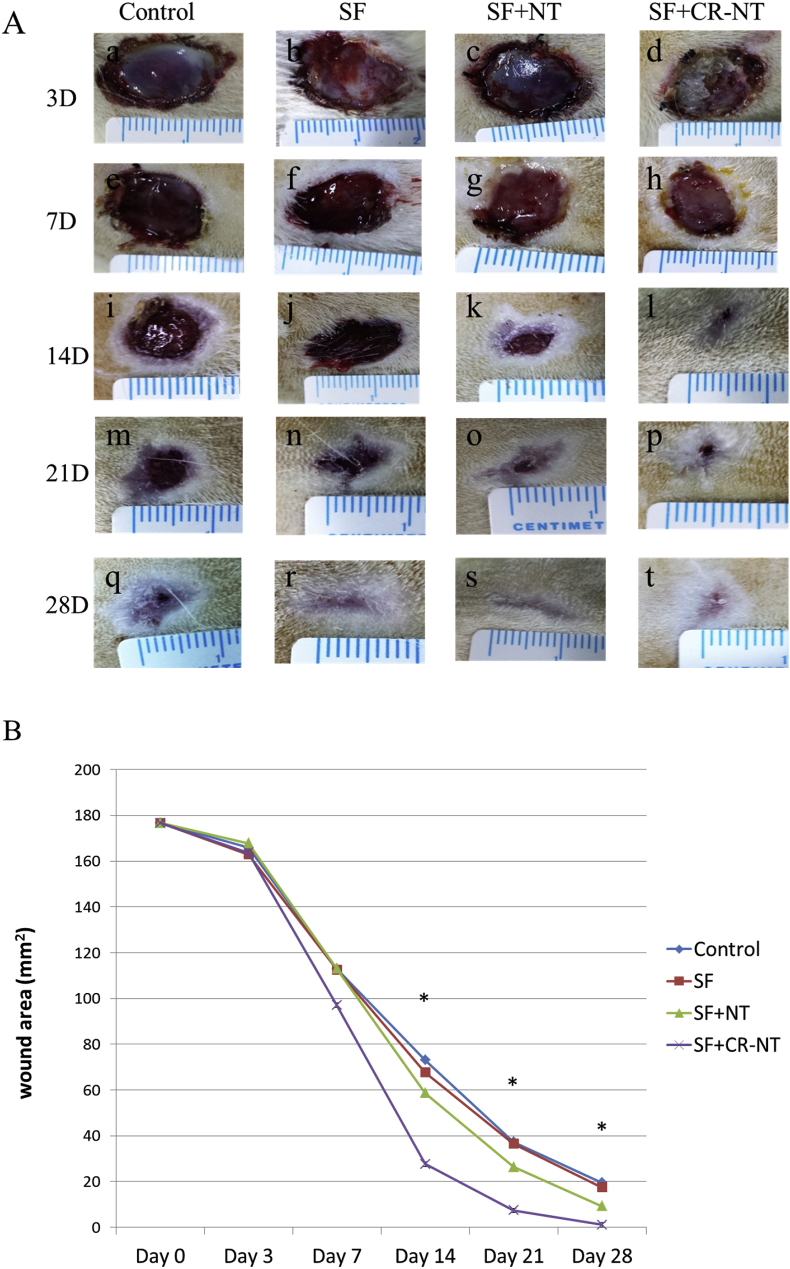
To determine the effect of SF, NT/SF and NT/GMs/SF dressings on the DFU wound healing process, respectively. The dressings were applied on top of the DFU wound (D = 15 mm) immediately after the wound was made. The wound areas were measured at 3, 7, 14, 21, 28 days after surgical procedure. In this regard, Fig. 3 A shows residual wound area change with healing time. In control group (A, F, I, M, Q), wound area reduced gradually but still existed on day 28. Purulent secretion could be found on wound surface in the healing process. In experimental group, wound area reduced apparently, especially in day 14. Compared with SF group (B, F, J, N, R), the wound was significantly reduced in NT/SF group (C, G, K, O, S) and NT/GMs/SF group (D, H, L, P, T). The wound almost healed completely in NT/GMs/SF group only little scar left. The wound areas in the photographs were analyzed by IPP software. The data showed that the velocity of wound area reduction in NT/GMs/SF group (Fig. 3 B).
Fig. 3.
The different effect of wound healing by four procedures in diabetic rat. (A) The wound areas were measured by metric ruler and photograph were taken at postoperative days 3, 7, 14, 21, 28 days. (B) Residual wound area changed with healing time (28 days). p value is for the difference between Control and SF + CR-NT groups.
3.4. Histological observation of wound healing process
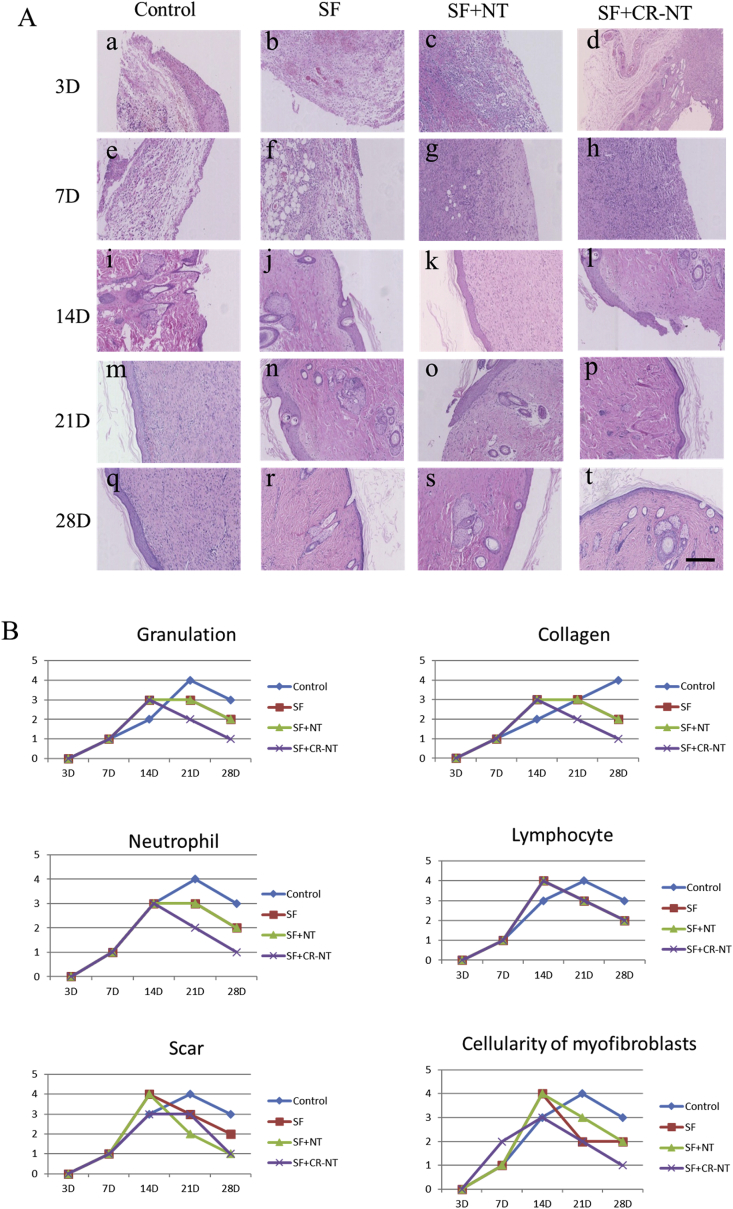
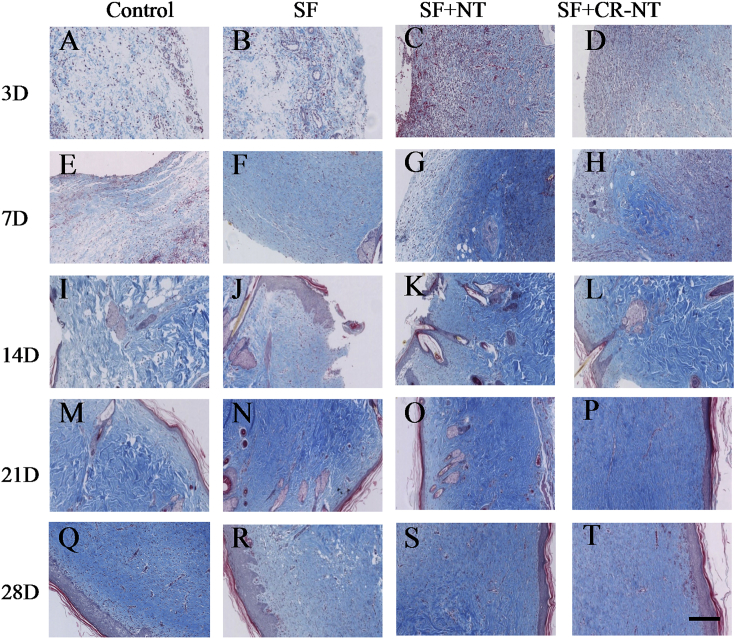
To observe the DFU wound healing effect of SF, NT/SF, and NT/GMs/SF dressings treatment, we examined histology changes in STZ rat skin (Fig. 4 A). HE staining showed the wound got healed and repaired gradually in each group and the most comprehensive repair in NT/GMs/SF group which manifested scar formation, inflammatory cells extinction, skin integrity and intact absorption of accessories. The NT/GMs/SF group had shorter repair process and faster repair speed compared with other groups. On day 14, repair scar appeared, and inflammatory reaction significantly reduced in NT/GMs/SF group. We also analyzed Granulation tissue degree, collagen contents, number of neutrophils and lymphocytes, scar extent, cellularity of myofibroblasts were divided into 0–4 grades. Form Fig. 4 B showed the indicators increased in all group in two weeks. But the NT/GMs/SF group presented decreased content of granulation and collagen, reduction number of inflammatory cells, weakened degree of scar and lower cellularity of myofibroblasts which prompted the wound healed rate was accelerated and the degree of wound healing was improved. Fig. 5 shows the collagen deposition at the wound site at day 28. Collagen array in the NT/GMs/SF dressing treated groups were relatively denser and more continuous compared with other groups.
Fig. 4.
(A) Haematoxylin and eosin staining images of DFU wound tissues, the bar corresponds to 200 μm; (B) Granulation tissue degree, collagen content, number of neutrophils and lymphocytes, scar extent, cellularity of myofibroblasts were divided into 0–4 grades and analyzed.
Fig. 5.
Collagen deposition in DFU wound. Collagen was stained with MT (blue). The bar corresponds to 200 μm.
3.5. Immunofluorescence analysis
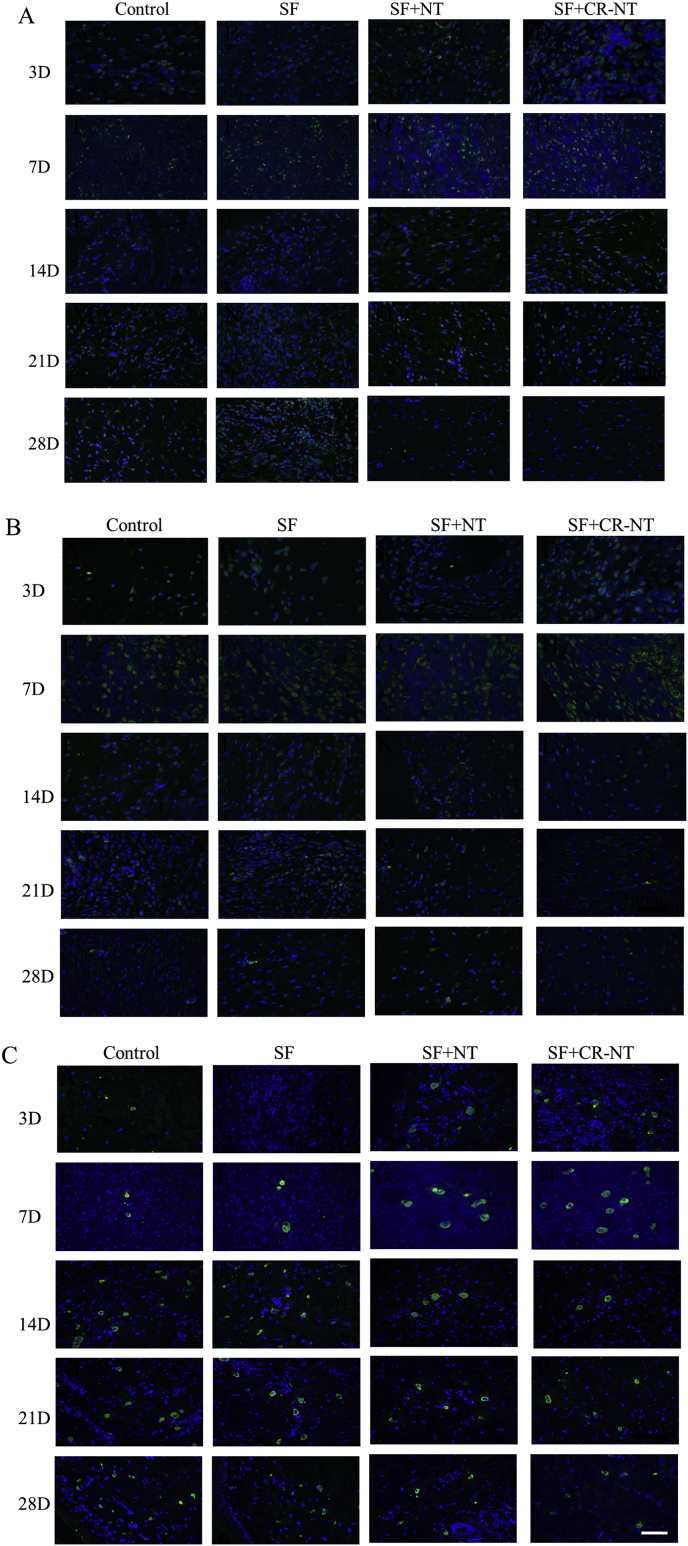
NT, NTR2 and NTR3 expression were observed after staining the tissue surrounding the DFU wound areas. Fig. 6 A B C shows immunoreactive cells at 28 days after treatment. Along with the wound healing, only little NT, NTR2, NTR3 expressed under the new epithelia in each group.
Fig. 6.
NT (A), NTR2 (B) and NTR3 (C) expression in the DFU wound tissue surrounding the affected area at 28 days. The bar corresponds to 200 μm.
4. Discussion
With diabetes affecting millions of people every year, most people developing chronic diseases such as lower extremity ulcer which ultimately result in amputation. Given the morbidity and mortality of amputations which survival rate only have 50%–60% in 2 years [24], it is imperative to develop better therapies to treat diabetic foot ulcers (DFU). The economic costs associated with diabetic ulcers are enormous and include hospital costs, disability, decreased productivity, and loss of independence [25,26]. So, before amputation to development of novel, more efficacious modalities of treatment would have tremendous benefit to both individual patients and society. Clinically, the current treatment in topical DFU management before amputations includes topical antibiotics and artificial topical dressing [11]. In the present study, one of the main objectives of this work was to evaluate the capacity of silk fibroin-base wound dressings as biocompatible and biodegradable loaded gelatin microspheres (GMs) supports for the sustained delivery of neurotensin (NT) to improve wound healing.
NT, a tridecapeptide, is localized predominantly in the CNS (predominantly hypothalamus and pituitary) and in endocrine cells (N cells) of the jejunum and ileum. In the CNS, NT functions are to inhibit dopaminergic pathways. In the periphery, NT stimulates growth of various GI tissues as well as adrenal gland, hepatocytes and fibroblasts [27]. NT also has three receptors including NTR1 is the predominant NT receptor, NTR2, and NTR3 is an intracellular receptor with a single transmembrane domain [28]. All three receptors are found all throughout the CNS. NT has been shown to play a role in the pathogenesis of diabetes. Though raised concentrations and total contents of NT were observed in the pancreas of obese mice and the intestine of both and diabetic mice [29]. A lot of researches have been reported that NT can modulate cell functions of both innate and adaptive immunity [[30], [31], [32]]. It is apparent that NT is an important immunomodulator. At previous studies have shown that NT would enhance wound healing by increasing IL-8 expression and/or initiating mast cell degranulation [23].
Wound closure results from our study showed that the topical application of NT reduced significantly the wound area in NT/SF and NT/GMs/SF compared with control and SF groups. These results are in agreement with previous data reports in the literature showing that different neuropeptides, namely substance P, induce diabetic wound healing [33,34]. Moreover, form our wound closure results shows that SF; NT/SF and NT/GMs/SF are healing more quickly and less scars, compared with control group. That tells us artificial dressings, especially SF loaded with GMs (control release working), can helpful in wound healing.
Currently, using hydrogels, scaffolds and microspheres systems to delivery drugs and neuropeptides are very commonly [16,35,36]. In our study, we choose SF loaded with GMs to control release NT. Because the SF is a natural protein and have excellent mechanical property, controllable biodegradability, hemostatic properties, non-cytotoxicity, low antigenicity and non-inflammatory characteristic as scaffold [17]. And the use of SF as a scaffold material not only improved cell proliferation and differentiation but also adherence and migration [37,38]. GMs as a drug control release microspheres system due to it good biocompatibility and degradation to nontoxic and readily excreted products [39]. Fig. 1 A shows GMs SEM analyses spherical morphology with an average diameter of 18.8 μm and a smooth surface. For the water absorption profile of GMs can be used to evaluate the merits of specific GMs as drug carriers. Fig. 1B and Table 1 shows that the swelling ratio of the GMs used in this study was 4.3 ± 0.6, which was same as other study values [40]. Moreover, the GMs have high water absorption capability and smooth surface suggest that they can be easily loaded with NT achieve control release function. Fig. 1 C shows that the FTIR analyses NT signal was weaker than NT/GMs, which may be attributed to the small quantity of NT, compared to the GMs quantity [41]. Hence, compared with others microspheres from the FTIR spectra analyses, the NT can impregnated into GMs via hydration which means soaking in NT solution, not need chemical linkage procedure [42]. Furthermore, the wound healing area (Fig. 3) and the Immunofluorescence (Fig. 6) indicated that GMs can be used to control release NT and other drugs.
The NT/GMs/SF dressings were composed of the following three types of compound: NT as accelerate wound healing drug, GMs as the carrier for the NT, and SF as the scaffold material. As the previous study have demonstrated that dressings have the optimum pore for adult skin regeneration is ranging from 20 to 125 μm. Fig. 2 shows that the NT/GMs/SF dressings have an average pore size are 40–80 μm and a porosity of ∼85%, which suit for wound healing regeneration.
In chronic diabetes, DFU wound healing is a complex process involving interaction among various cytokines, cells and extracellular matrices. In our study, we evaluated the wound healing processes and neo-tissue formation in rat diabetic model by macroscopic observation, histological observation (H&E staining and Masson's trichrome staining) and immunofluorescence analysis at the indicated time points (Fig. 3, Fig. 4, Fig. 5, Fig. 6). At all time points, the NT/GMs/SF group exhibited a quicker healing rate performance in macroscopic observation, histological observation and immunofluorescence analysis, which would be favorable to wound repair. Moreover, NT/GM/SF dressings have the appropriate pore size and a highly porous structure, which should promote matrix swelling and absorption of wound exudates. These features may activate the generation of vascular endothelial cells and fibroblasts, enhancing neovascularization and neo-tissue formation.
For DFU wounds, conventional wound dressings are changed to limit positive components/drugs for promote regeneration and can't achieve components/drugs stable concentration in wound site. The NT/GMs/SF dressings can be used to achieve longer-term release of effective concentrations of NT, thereby reducing the risk of the lower extremity amputation and can improve the diabetic patient life quantify. Of course, our study has also limitation is that we don't detect inflammatory cytokine about NT in DFU wound, only according to some previous research [18,23,43].
5. Conclusions
We prepared NT/GMs/SF dressings for use to treat DFU wound regeneration and investigated NT loaded GMs control release activities in a full-thickness wound in a diabetic rat model. The NT/GMs/SF dressings stimulated fibroblast accumulation in tissue granulation, collagen expression and deposition at the wound site, which lead to the production of a more organized collagen matrix. This treatment effectively accelerating wound regeneration and re-epithelialization. Hence, the NT/GMs/SF dressings are applicable as a novel, control release NT regeneration template for DFU patients.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study was supported financially by the Natural Science Foundation of China (51303064 and 31271019), the Science and Technology Program of Guangzhou (201601010270, 2017010160489, 201704030083), the Pearl River S&T Nova Program of Guangzhou (201710010155, 201806010072), and the Science and Technology Project of Guangdong province (2015A010101313, 2017A050506011, 2017B090911012, 2018A050506040, 2018A050506019, 2018A050506021).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Canbin Zheng, Email: zhengcanbin@qq.com.
Rui Guo, Email: guorui@jnu.edu.cn.
References
- 1.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.da Silva L., Carvalho E., Cruz M.T. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Expert Opin. Biol. Ther. 2010;10:1427–1439. doi: 10.1517/14712598.2010.515207. [DOI] [PubMed] [Google Scholar]
- 3.Boulton A.J., Vileikyte L., Ragnarson-Tennvall G., Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 4.Dinh T.L., Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int. J. Low. Extrem. Wounds. 2005;4:154–159. doi: 10.1177/1534734605280130. [DOI] [PubMed] [Google Scholar]
- 5.Pradhan L., Nabzdyk C., Andersen N.D., LoGerfo F.W., Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev. Mol. Med. 2009;11:e2. doi: 10.1017/S1462399409000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pradhan L., Cai X., Wu S., Andersen N.D., Martin M., Malek J. Gene expression of pro-inflammatory cytokines and neuropeptides in diabetic wound healing. J. Surg. Res. 2011;167:336–342. doi: 10.1016/j.jss.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlovic S., Daniltchenko M., Tobin D.J., Hagen E., Hunt S.P., Klapp B.F. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J. Investig. Dermatol. 2008;128:434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus L.H., Brown M.R., Perrin M.H. Distribution, localization and characteristics of neurotensin binding sites in the rat brain. Neuropharmacology. 1977;16:625–629. doi: 10.1016/0028-3908(77)90033-8. [DOI] [PubMed] [Google Scholar]
- 9.Sundler F., Hakanson R., Hammer R.A., Alumets J., Carraway R., Leeman S.E. Immunohistochemical localization of neurotensin in endocrine cells of the gut. Cell Tissue Res. 1977;178:313–321. doi: 10.1007/BF00218696. [DOI] [PubMed] [Google Scholar]
- 10.Kalafatakis K., Triantafyllou K. Contribution of neurotensin in the immune and neuroendocrine modulation of normal and abnormal enteric function. Regul. Pept. 2011;170:7–17. doi: 10.1016/j.regpep.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Sweitzer S.M., Fann S.A., Borg T.K., Baynes J.W., Yost M.J. What is the future of diabetic wound care? Diabetes Educat. 2006;32:197–210. doi: 10.1177/0145721706286897. [DOI] [PubMed] [Google Scholar]
- 12.Ju H.W., Lee O.J., Lee J.M., Moon B.M., Park H.J., Park Y.R. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016;85:29–39. doi: 10.1016/j.ijbiomac.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood D.N., Preda R.C., Yucel T., Wang X., Lovett M.L., Kaplan D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil E.S., Panilaitis B., Bellas E., Kaplan D.L. Functionalized silk biomaterials for wound healing. Adv. Healthc. Mater. 2013;2:206–217. doi: 10.1002/adhm.201200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Yang Q., Mao C., Zhang S. Osteogenic differentiation of bone marrow mesenchymal stem cells on the collagen/silk fibroin bi-template-induced biomimetic bone substitutes. J. Biomed. Mater. Res. A. 2012;100:2929–2938. doi: 10.1002/jbm.a.34236. [DOI] [PubMed] [Google Scholar]
- 16.Guziewicz N., Best A., Perez-Ramirez B., Kaplan D.L. Lyophilized silk fibroin hydrogels for the sustained local delivery of therapeutic monoclonal antibodies. Biomaterials. 2011;32:2642–2650. doi: 10.1016/j.biomaterials.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauney J.R., Nguyen T., Gillen K., Kirker-Head C., Gimble J.M., Kaplan D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan Y., Li W., Jiao Y., Guo R., Zhang Y., Xue W. Therapeutic efficacy of antibiotic-loaded gelatin microsphere/silk fibroin scaffolds in infected full-thickness burns. Acta Biomater. 2014;10:3167–3176. doi: 10.1016/j.actbio.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Lan Y., Li W., Guo R., Zhang Y., Xue W., Zhang Y. Preparation and characterisation of vancomycin-impregnated gelatin microspheres/silk fibroin scaffold. J. Biomater. Sci. Polym. Ed. 2014;25:75–87. doi: 10.1080/09205063.2013.836951. [DOI] [PubMed] [Google Scholar]
- 20.Kundu J., Dewan M., Ghoshal S., Kundu S.C. Mulberry non-engineered silk gland protein vis-a-vis silk cocoon protein engineered by silkworms as biomaterial matrices. J. Mater. Sci. Mater. Med. 2008;19:2679–2689. doi: 10.1007/s10856-008-3398-1. [DOI] [PubMed] [Google Scholar]
- 21.Kawai K., Suzuki S., Tabata Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499. doi: 10.1016/s0142-9612(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 22.Wongpanit P., Ueda H., Tabata Y., Rujiravanit R. In Vitro and in vivo release of basic fibroblast growth factor using a silk fibroin scaffold as delivery carrier. J Biomat Sci-Polym E. 2010;21:1403–1419. doi: 10.1163/092050609X12517858243706. [DOI] [PubMed] [Google Scholar]
- 23.Moura L.I., Dias A.M., Suesca E., Casadiegos S., Leal E.C., Fontanilla M.R. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim. Biophys. Acta. 2014;1842:32–43. doi: 10.1016/j.bbadis.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Cutson T.M., Bongiorni D.R. Rehabilitation of the older lower limb amputee: a brief review. J. Am. Geriatr. Soc. 1996;44:1388–1393. doi: 10.1111/j.1532-5415.1996.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 25.Ramsey S.D., Newton K., Blough D., McCulloch D.K., Sandhu N., Reiber G.E. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey S.D., Newton K., Blough D., McCulloch D.K., Sandhu N., Wagner E.H. Patient-level estimates of the cost of complications in diabetes in a managed-care population. Pharmacoeconomics. 1999;16:285–295. doi: 10.2165/00019053-199916030-00005. [DOI] [PubMed] [Google Scholar]
- 27.Evers B.M. Neurotensin and growth of normal and neoplastic tissues. Peptides. 2006;27:2424–2433. doi: 10.1016/j.peptides.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Gross K.J., Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm. Bowel Dis. 2007;13:918–932. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard M.C., Bailey C.J., Flatt P.R., Swanston-Flatt S.K., Shennan K.I. Immunoreactive neurotensin in spontaneous syndromes of obesity and diabetes in mice. Acta Endocrinol. 1985;108:532–536. doi: 10.1530/acta.0.1080532. [DOI] [PubMed] [Google Scholar]
- 30.Lemaire I. Neurotensin enhances IL-1 production by activated alveolar macrophages. J. Immunol. 1988;140:2983–2988. [PubMed] [Google Scholar]
- 31.Ramez M., Bagot M., Nikolova M., Boumsell L., Vita N., Chalon P. Functional characterization of neurotensin receptors in human cutaneous T cell lymphoma malignant lymphocytes. J. Investig. Dermatol. 2001;117:687–693. doi: 10.1046/j.0022-202x.2001.01439.x. [DOI] [PubMed] [Google Scholar]
- 32.Evers B.M., Bold R.J., Ehrenfried J.A., Li J., Townsend C.M., Jr., Klimpel G.R. Characterization of functional neurotensin receptors on human lymphocytes. Surgery. 1994;116:134–139. discussion 9-40. [PubMed] [Google Scholar]
- 33.Scott J.R., Tamura R.N., Muangman P., Isik F.F., Xie C., Gibran N.S. Topical substance P increases inflammatory cell density in genetically diabetic murine wounds. Wound Repair Regen.: off. publ.Wound Heal. Soc. & Eur. Tissue Repair Soc. 2008;vol. 16:529–533. doi: 10.1111/j.1524-475X.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibran N.S., Jang Y.C., Isik F.F., Greenhalgh D.G., Muffley L.A., Underwood R.A. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J. Surg. Res. 2002;108:122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 35.Guziewicz N.A., Massetti A.J., Perez-Ramirez B.J., Kaplan D.L. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials. 2013;34:7766–7775. doi: 10.1016/j.biomaterials.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J., Fang T., Wang Y., Dong J. The controlled release of vancomycin in gelatin/beta-TCP composite scaffolds. J. Biomed. Mater. Res. A. 2012;100:2295–2301. doi: 10.1002/jbm.a.34170. [DOI] [PubMed] [Google Scholar]
- 37.Guan G., Bai L., Zuo B., Li M., Wu Z., Li Y. Promoted dermis healing from full-thickness skin defect by porous silk fibroin scaffolds (PSFSs) Bio Med. Mater. Eng. 2010;20:295–308. doi: 10.3233/BME-2010-0643. [DOI] [PubMed] [Google Scholar]
- 38.Luan X.Y., Wang Y., Duan X., Duan Q.Y., Li M.Z., Lu S.Z. Attachment and growth of human bone marrow derived mesenchymal stem cells on regenerated antheraea pernyi silk fibroin films. Biomed. Mater. 2006;1:181–187. doi: 10.1088/1748-6041/1/4/001. [DOI] [PubMed] [Google Scholar]
- 39.Vandelli M.A., Rivasi F., Guerra P., Forni F., Arletti R. Gelatin microspheres crosslinked with D,L-glyceraldehyde as a potential drug delivery system: preparation, characterisation, in vitro and in vivo studies. Int. J. Pharm. 2001;215:175–184. doi: 10.1016/s0378-5173(00)00681-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X.H., Tabata Y., Wang C.H., Tong Y.W. Delivery of basic fibroblast growth factor from gelatin microsphere scaffold for the growth of human umbilical vein endothelial cells. Tissue Eng. 2008;14:1939–1947. doi: 10.1089/ten.tea.2007.0346. [DOI] [PubMed] [Google Scholar]
- 41.Cui X., Gu Y., Li L., Wang H., Xie Z., Luo S. In vitro bioactivity, cytocompatibility, and antibiotic release profile of gentamicin sulfate-loaded borate bioactive glass/chitosan composites. J. Mater. Sci. Mater. Med. 2013;24:2391–2403. doi: 10.1007/s10856-013-4996-0. [DOI] [PubMed] [Google Scholar]
- 42.Iemma F., Spizzirri U.G., Puoci F., Muzzalupo R., Trombino S., Picci N. Radical cross-linked albumin microspheres as potential drug delivery systems: preparation and in vitro studies. Drug Deliv. 2005;12:229–234. doi: 10.1080/10717540590952690. [DOI] [PubMed] [Google Scholar]
- 43.Moura L.I.F., Dias A.M.A., Leal E.C., Carvalho L., de Sousa H.C., Carvalho E. Chitosan-based dressings loaded with neurotensin-an efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014;10:843–857. doi: 10.1016/j.actbio.2013.09.040. [DOI] [PubMed] [Google Scholar]