Abstract
The aim of the present prospective comparative cohort study was to analyze changes in Streptococcus (S.) mutans and Lactobacillus in the saliva of patients with fixed orthodontics. Salivary parameters, including secretory immunoglobulin A (sIgA), myeloperoxidase (MPO) and lactate dehydrogenase (LDH), were investigated during orthodontic treatment, as well as immune response and inflammatory processes. A total of 15 patients were included and treated with fixed appliances. Whole saliva was obtained at four time-points: Prior to bonding (T1), 3 months after bonding (T2), 6 months after bonding (T3) and 18 months after bonding (T4). Quantitative polymerase chain reaction was performed to evaluate changes in total bacteria, S. mutans and Lactobacillus DNA in saliva. ELISA was applied to measure sIgA, MPO and LDH levels. The level of total bacteria remained stable during the 18-month treatment period, and the quantity of Lactobacillus exhibited a slight but non-significant increase. S. mutans remained stable over the first 6 months and significantly increased at T4 (P<0.05), and there was a significant difference in S. mutans levels between two types of braces. A higher level of S. mutans was found in patients with conventional braces, as compared to those with self-ligating braces (P<0.05) who exhibited an unchanged level of S. mutans during this period. The amount of sIgA, MPO and LDH remained constant during the orthodontic treatment period. No correlation was detected between sIgA and bacterial quantity. In conclusion, S. mutans in patients with conventional braces increased significantly in the late period of treatment, which indicated that white spot lesions may occur after long-term orthodontic treatment. As the type of braces may be considered a latent influencing factor, self-ligating braces should be preferred. However, the effect of fixed orthodontic treatment on Lactobacillus and sIgA, MPO and LDH in the oral microenvironment was insignificant (Chinese Clinical Trial Registry no. ChiCTR-RCH-13003295).
Keywords: fixed orthodontic treatment, Streptococcus mutans, Lactobacillus, saliva, white spot lesion
Introduction
White spot lesions (WSLs) are among the most common side effects of orthodontic treatment, and the labial surfaces around braces and gingival margins are the most common sites for WSLs (1). The incidence of new WSLs during orthodontic treatment has been reported to be 32.0–72.9%, and the activity of cavitated lesions has been observed to increase over the orthodontic treatment period (2,3). The insertion of fixed appliances may considerably alter the oral microenvironment, since it provides bacterial colonization and accumulation areas for dental biofilm and fungi (4). It has been demonstrated that Streptococcus (S.) mutans and Lactobacillus are the major constituents of the pathogenic bacterial flora in WSLs and dental caries (1).
Saliva is regarded as a microbial repository and transport medium, which is affected by oral health status, as well as the quantity and types of bacteria. Saliva is also considered to contain innate immune factors and various salivary defense proteins. Since secretory immunoglobulin A (sIgA) is a key antibody in the salivary defense system (5), it may respond to changes in the oral microenvironment during orthodontic treatment. Furthermore, myeloperoxidase (MPO) and lactate dehydrogenase (LDH) act as salivary markers of periodontitis that are involved in periodontal metabolism, and the activity of MPO and LDH is promoted in the initial period of orthodontic treatment (6–8). However, the immune response during long-term treatment has yet to be fully elucidated.
Various clinical studies have indicated that changes in S. mutans or Lactobacillus are complex and unpredictable during the first 2–6 months of orthodontic treatment (4,9,10). However, follow-up times have not previously been extended beyond 6 months, despite a typical orthodontic treatment lasting 18–36 months. The association between bacterial levels and innate immune factors during long-term orthodontic therapy remains poorly understood. The aim of the present prospective cohort study was to observe the major pathogens and immune-associated proteins relevant to long-term orthodontic treatment, and the association between them. The present study illustrates that characteristics of the oral microbial environment are affected by long-term orthodontic treatment, and may provide information for the development of potential management strategies for the side effects of orthodontic treatment.
Materials and methods
Subjects and clinical procedures
The present study included 15 subjects who were scheduled for fixed orthodontic therapy at the Department of Orthodontics of West China Hospital of Stomatology (Sichuan University, Chengdu, China). They had first presented at the department between July 2013 and March 2014. In a preliminary study, the mean bacterial DNA levels and standard deviations were defined. The hypothesized mean value and standard deviations were calculated with the assumption that the statistical significance level was 0.05, and an adequate statistical power was typically regarded as 0.9. Subsequently, the sample size required was estimated to be 6 per group, and 15 cases were therefore included in the present study to achieve an adequate sample size. All experimental procedures were in accordance with the Declaration of Helsinki. The study was entered in the Chinese Clinical Trial Registry (no. ChiCTR-RCH-13003295). Prior to enrolment, informed consent forms were signed by the patients, or by their guardians if they were <18 years old.
The inclusion criteria were as follows: i) Patients with >24 permanent teeth; ii) patient age between 14 and 20 years; and iii) a treatment period of >18 months. The exclusion criteria were as follows: i) Any systemic or infectious diseases; ii) any active carious lesions; iii) a history of smoking; and iv) any antibiotic or hormone therapy within 1 month prior to or during orthodontic treatment.
Patients were treated with fixed orthodontic appliances (straight wire technique) and divided into two groups. Treatment with either of two types of fixed orthodontic appliances was performed by the orthodontist according to each patient's situation and individual preference. The groups received two different types of braces: Conventional braces (CB; HX, Shinye Inc., Hangzhou, China; n=6) and self-ligating braces (SLB; Tomy, Inc., Japan or Damon Q, Ormco, Orange, CA, USA; n=9). The orthodontic procedure started with the implementation of NiTi wire for the alignment and initial leveling stages, followed by stainless steel wires for the subsequent stages. All subjects were required to use fluoride-containing toothpaste according to oral hygiene instructions.
Sample collection
For each patient, a self-contrast approach was designed to assess changes in oral microbiota and salivary proteins during the treatment period. A volume of 4 ml unstimulated whole saliva was collected at four time-points: Prior to bonding (T1), 3 months after bonding (T2), 6 months after bonding (T3) and 18 months after bonding (T4), according to a previous study (4). The saliva was collected between 1:00 and 4:00 p.m. Participants were suggested to have lunch prior to clinical visiting, and tooth brushing was required following eating. For sample collection, participants were asked to refrain from eating and drinking for ≥1 h prior to sample collection, and they were required to rinse their mouths and sit quietly prior to collection. Saliva was collected in sterile centrifugal tubes within 15 min, and divided into 2 tubes: 1 ml for quantitative polymerase chain reaction (qPCR) analysis and 3 ml for ELISA. The saliva was then centrifuged at 22,673 × g for 10 min at 4°C, and then stored at −80°C for subsequent detection.
DNA isolation and qPCR
The sediment of centrifuged saliva samples (1 ml) was collected. Bacterial chromosomal DNA was extracted using a DNA kit (cat. no. 56304; Qiagen GmbH, Hilden, Germany), according to the manufacturer's protocol. A Nanodrop 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to assess the quality and purity of the extracted DNA. DNA extracted from S. mutans UA159 and Lactobacillus (L.) acidophilus 4356 provided by the State Key Laboratory of Oral Disease, Sichuan University (Chengdu, China) were used as standards with 16S ribosomal RNA as a reference. The standard curves were generated using a series of 10-fold dilutions from 10–106 copies (Fig. 1).
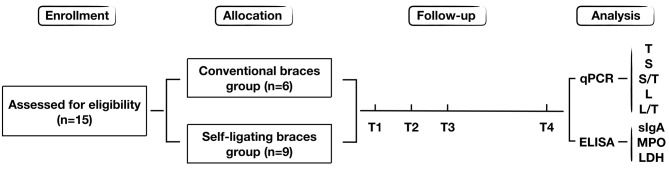
Figure 1.
Study flow diagram for bacterial quantification. Flow diagram for the enrollment and progression of the patients in the present study. Time-points: T1, Prior to bonding; T2, 3 months after bonding; T3, 6 months after bonding; and T4, 18 months after bonding. T, total bacteria DNA; S, Streptococcus mutans DNA; L, Lactobacillus DNA; sIgA, secretory immunoglobulin A; MPO, myeloperoxidase; LDH, lactate dehydrogenase.
A total of 20 ng DNA was used for each qPCR analysis. qPCR was performed using the ABI 7300 system (Applied Biosystems; Thermo Fisher Scientific, Inc.), and 16S ribosomal RNA was used to quantify total bacteria (11). The specific primer for S. mutans was designed according to the glucosyltransferase-I gene (12). The Lacto-forward and Lacto-reverse primers were used as the primary Lactobacillus primers to represent all lactobacilli, as previously described (13) (Table I). Reaction mixtures were subjected to a standard qPCR program (n=3), according to the following thermocycling conditions: 95°C for 5 min, then 40 cycles of 95°C for 10 sec and 60°C for 30 sec, followed by 72°C for 10 min.
Table I.
Primers for polymerase chain reaction.
| Type | Name/direction | Sequence (5′-3′) |
|---|---|---|
| Total bacteria | 16S-F | GGTTAAGTCCCGCAACGAGC |
| 16S-R | AGGGGCATGATGATTTGACG | |
| Streptococcus mutans | gtfB-F | CTACACTTTCGGGTGGCTTG |
| gtfB-R | GAAGCTTTTCACCATTAGAAGCTG | |
| Lactobacilli | Lacto-F | TGGAAACAGRTGCTAATACCG |
| Lacto-R | GTCCATTGTGGAAGATTCCC |
16S, 16S ribosomal RNA; F, forward; R, reverse; gtfB, glucosyltransferase-I.
ELISA
For salivary sIgA, MPO and LDH determination, the supernatant was collected from the centrifuged saliva sample (3 ml). Standard curves were generated and sample preparations were performed according to the protocols of the Human Secretory Immunoglobulin A/Myeloperoxidase/Lactate Dehydrogenase ELISA kits (cat. nos. KB10115, KB11580 and KB12774, respectively; Shanghai Jiang Lai Biotechnology, Inc. Shanghai, China). The absolute sample densities were determined using linear regression equations with multiplication of the results by the dilution factor.
Statistical analysis
Prior to bonding, a Student's t-test was used to compare the amount of total bacteria (T), the ratio of S. mutans (S) to total bacteria (S/T) and that of Lactobacillus (L) to total bacteria (L/T) between males and females, and between the two types of braces, in order to evaluate the baseline characteristics. A repeated-measures analysis of variance (ANOVA) was used to determine the time-dependent differences in T, S, S/T, L and L/T with regard to sex or type of braces. Independent comparisons of the T, S, S/T, L and L/T, as well as salivary sIgA, MPO and LDH between different time-points were performed using one-way ANOVA and least significant difference analysis. Statistical analysis was performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Pearson's correlation coefficient was determined to evaluate correlations between sIgA and T, S/T or L/T. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 15 patients who met the inclusion criteria and were enrolled in the present study. The mean age of the patients was 16.64±2.03 years (17.00±1.91 years for the CB group and 16.44±1.83 years for the SLB group). The male-to-female ratio in the CB group and the SLB group was 1:2. At baseline, there were no significant differences in T, S/T and L/T within the groups between males and females.
qPCR analysis of salivary bacteria
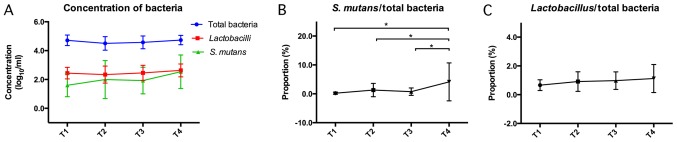
S. mutans and Lactobacillus levels in saliva samples were evaluated using qPCR. The absolute levels of T, S/T and L/T were evaluated from T1 to T4 (Fig. 2 and Table II). The results demonstrated that there were no significant differences in the amount of total bacteria over the 18-month orthodontic treatment (Fig. 2A). However, a slight but insignificant increase was observed in the proportion of Lactobacillus from T1 to T4 (Fig. 2C). A significant increase in the proportion of S. mutans was observed from month 6 to month 18 (P<0.05; Fig. 2B).
Figure 2.
Changes of salivary bacteria levels from T1 to T4. Bacterial DNA was extracted from 1 ml saliva samples at each time-point. The concentration of (A) total bacterial, S. mutans and Lactobacillus DNA, as well as the ratio of (B) S. mutans and (C) Lactobacillus were evaluated. *P<0.05. Time-points: T1, Prior to bonding; T2, 3 months after bonding; T3, 6 months after bonding; and T4, 18 months after bonding. S. mutans, Streptococcus mutans.
Table II.
Bacterial levels at T1-T4.
| Parameter | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| T (Log10/ml) | 4.72±0.37 | 4.50±0.47 | 4.57±0.44 | 4.73±0.33 |
| S (Log10/ml) | 1.55±0.74 | 1.95±1.80 | 1.89±0.89 | 2.52±1.17 |
| L (Log10/ml) | 2.44±0.40 | 2.34±0.59 | 2.45±0.52 | 2.62±0.45 |
| S/T (%) | 0.21±0.38a | 1.29±2.30a | 0.75±1.28a | 4.14±6.56 |
| S/T-CB (%) | 0.45±0.52a | 2.84±2.95a | 1.50±1.75a | 8.64±8.14 |
| S/T-SLB (%) | 0.04±0.04 | 0.15±0.26 | 0.18±0.22 | 0.77±1.55 |
| L/T (%) | 0.66±0.37 | 0.91±0.68 | 0.97±0.61 | 1.12±0.97 |
Time-points: T1, Prior to bonding; T2, 3 months after bonding; T3, 6 months after bonding; and T4, 18 months after bonding. CB, conventional braces group; SLB, self-ligating braces group. T, total bacteria; S, S. mutans DNA; L, Lactobacillus DNA.
P<0.05 vs. T4.
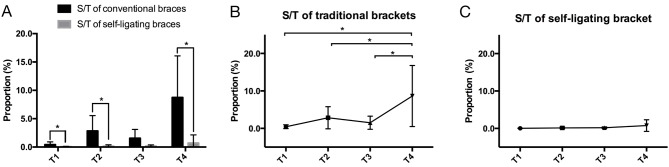
At T1, T2 and T4, there was a significant difference in the proportion of S. mutans between the CB and SLB groups (P<0.05; Fig. 3). The proportion of S. mutans in patients with self-ligating braces was lower compared with that in patients with conventional braces (Table II).
Figure 3.
Changes in S. mutans/total bacteria depending on types of braces. (A) Levels of S. mutans/total bacteria in different braces at each time-point. Changes of S. mutans/total bacteria in patients stratified into (B) the conventional braces group and (C) self-ligating braces group from T1 to T4. A significant difference existed regarding bracket types (P<0.05). *P<0.05. Time-points: T1, Prior to bonding; T2, 3 months after bonding; T3, 6 months after bonding; and T4, 18 months after bonding. S. mutans, Streptococcus mutans; S/T, ratio of S. mutans/total bacteria.
Analysis of salivary proteins
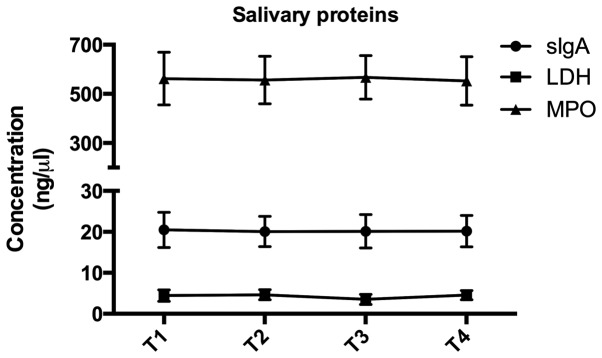
Standard curves and linear regression coefficients of salivary sIgA, MPO and LDH were generated based on standard densities and optical density values obtained by ELISA. All of these parameters remained constant over the orthodontic treatment period (P>0.05). The average concentration of salivary sIgA, MPO and LDH was 20.22±3.97, 561.86±97.49 and 4.20±1.28 µg/ml, respectively (Fig. 4). Furthermore, Pearson's correlation analysis indicated no correlation between sIgA and T, S/T or L/T (data not shown).
Figure 4.
Changes of salivary proteins from T1 to T4. Salivary sIgA, LDH and MPO were assessed in salivary samples using ELISA at each time-point. Time-points: T1, Prior to bonding; T2, 3 months after bonding; T3, 6 months after bonding; and T4, 18 months after bonding. sIgA, secretory immunoglobulin A; MPO, myeloperoxidase; LDH, lactate dehydrogenase.
Discussion
In the present study, the levels of salivary total bacteria, S. mutans and Lactobacillus of patients who received fixed orthodontic treatment for ≥18 months were evaluated. Salivary samples were harvested at four observation time-points, at which salivary parameters, including sIgA, LDH and MPO, were assessed. To the best of our knowledge, the present study was the first to investigate the association between bacteria and immune-associated proteins in patients undergoing long-term fixed orthodontic treatment.
Orthodontic appliances include braces, wires and attachments, which retain more food particles and provide retentive sites for dental plaque. This makes it more difficult to maintain oral hygiene, increasing the likelihood for WSLs and dental caries to develop. Since S. mutans and Lactobacillus are critical pathogenic bacteria in WSLs and dental caries, demineralization was observed at 1 month after bonding (14). S. mutans and Lactobacillus have been previously demonstrated to markedly increase from month 3 to month 6 of orthodontic treatment (4,15). Other studies have demonstrated no significant difference in the levels of S. mutans and total bacteria within the first 3 months (9,10). The observation times of these previous investigations were restricted to the early stages of orthodontic treatment. However, the majority of observations of WSL or dental caries have been recorded following ~2 years of orthodontic treatment, so that bacterial alterations may have been missed in studies with a shorter duration. The present study was designed as a long-term observation. The detection of S. mutans and Lactobacillus in samples was performed by qPCR, which is more rapid and sensitive for detecting specific bacteria than the bacilli culture methods used in previous studies, and they allow for the calculation of absolute numbers of bacteria (11).
The number of total bacteria was predominately stable over the 18-month period, suggesting that total bacterial homeostasis was maintained during the orthodontic treatment. The increase in S. mutans at 3 months was in line with a number of previous studies (4,9,10,15). However, the present study demonstrated that S. mutans increased significantly over an 18-month treatment period in the CB group, even when total bacteria did not increase. The accumulation of S. mutans may cause WSLs and caries, and the present results indicate that it is difficult to maintain oral hygiene over a long treatment duration; WSLs occurred in some patients at this time-point. S. mutans binds to the tooth surface by producing water-insoluble glucans; its glucosyltransferases may serve critical roles in the development of virulent dental plaque, and enable S. mutans to thrive in the acidic environment and escape the buffer function of saliva (16). In addition, surface roughness of dental materials and S. mutans biofilm adhesion increase with extended periods of treatment (17). As a result, the incidence of caries increases with a longer treatment time, possibly due to the sustained action and constant adhesion of S. mutans. This indicates that long-term treatment is a critical risk factor for the development of caries. Although it is difficult to define the precise time range for the occurrence of dental caries, this result suggests that particular attention should be paid to oral hygiene, since the factors described above, including the acidogenic ability, adhesion frequency and time of S. mutans, contribute to the occurrence of caries when dental plaque accumulates above a certain threshold.
Repeated measurement analyses demonstrated significant differences between conventional and self-ligating braces. Arch wires were placed in conventional braces using ligature wires or elastomeric rings. Self-ligating braces restrict wires into slots using self-ligating components that markedly reduce friction and provide less space for plaque accumulation around braces. It is easier to clear bacteria from self-ligating braces, and differences in wire or accessory roughness may affect biofilm adhesion, leading to a lower incidence of dental caries in patients with self-ligating braces. In the present study, the proportion of S. mutans in the CB and SLB groups exhibited a slight but insignificant increase over the first 1–3 months, which was consistent with previous studies (18,19). Of note, the results of the present study demonstrated a significant increase in the proportion of S. mutans only in patients with conventional braces over the 18-month treatment period, while the proportion of S. mutans in the SLB group remained at a much lower level without any significant increasing. Similarly, previous studies have demonstrated that the prevalence of patients who develop WSLs after orthodontic treatment with self-ligating braces was lower compared with that of patients with conventional braces, and lower amounts of total bacteria, particularly oral streptococci, were identified in the plaque of teeth bonded with self-ligating braces (3,20). The present study provided evidence from a long-term investigation that the self-ligating orthodontic system is likely to have advantages over conventional braces in terms of reduced biofilm adhesion and plaque accumulation, and allows for better maintenance of oral hygiene through improving the convenience of cleaning.
In dental caries, >7 dominant species of Lactobacillus were identified as pathogenic bacteria (21). In the present study, Lactobacillus levels also displayed a slight increase from T1 to T4, which was consistent with the results of previous short-term studies (22,23). In previous research, the association between Lactobacillus and dental caries was established, and caries appears to be important for the sustained colonization of Lactobacillus (24). In advanced dental caries, 18 different phylotypes of Lactobacillus were detected, and L. gasseri and L. ultunensis were of highest loads (13). Despite pathogenic Lactobacillus, certain probiotic Lactobacillus may also be extracted from the digestive system, and food containing probiotic Lactobacillus may increase the resistance to caries. Although the amount of total Lactobacillus remained stable throughout the treatment period in the present study, specific Lactobacillus types should be evaluated when active caries or possible dentinal caries occur, and the roles and levels of probiotic and pathogenic Lactobacillus during the orthodontic treatment should be confirmed in future studies.
Salivary sIgA is considered to be a critical antibody in the mucosal immune response. However, the association between sIgA levels and dental caries remain controversial: Conflicting studies have identified sIgA to be decreased in pediatric patients with active caries (25), increased in pediatric patients with active caries (26), or to have no association with dental caries or S. mutans (27). In adult patients receiving orthodontic therapy, the association between sIgA and the level of oral pain was evaluated, and the results indicated no significant change in the initial arch phase (28). In the present study, no statistically significant correlation was identified between sIgA and S. mutans throughout the orthodontic treatment, and sIgA levels remained stable.
In addition, two key enzymes, LDH and MPO, were evaluated in the present study. LDH is released during cell death and is associated with gingival inflammation (6). Salivary LDH activity is also an indicator of oral mucosa pathologies and the periodontal status (29). Previous studies have indicated that LDH levels were higher in the initial 7 days to 10 weeks after tooth movement. Similarly, the activity of MPO enzyme serves as a biochemical marker of oral inflammation. MPO was demonstrated to be elevated from 2 h to 7 days after bonding in the saliva and gingival crevicular fluid, and returned to the baseline at day 14 (7,8). In addition to the initial changes described above, the present study aimed to identify changes of LDH and MPO during long-term orthodontic treatment. The results indicated a stable level of salivary LDH and MPO from T1 to T4. The maintained level in the later stages may be attributed to the oral secretion balance and environmental adaptation over a longer follow-up period compared with that in the aforementioned studies. Collectively, the expression of the salivary proteins indicated a relatively stable microenvironment with respect to the oral salivary immune response and inflammatory processes during the 18-month orthodontic treatment period.
A limitation of the present study was that the evaluation period was from 3 to 18 months. A longer period with more observation time-points may be required to better detect any fluctuations in S. mutans levels. Furthermore, the sample size was relatively small, and the data may only marginally reflect the variation trend of these microenvironmental indexes and not be representative of the average concentrations in larger cohorts. Another limitation was that WSLs were not systematically recorded. A further study assessing the correlation between WSLs and S. mutans in a larger sample of orthodontic patients is under consideration.
In conclusion, the present study demonstrated an increase of the pathogen S. mutans as an alteration in the oral bacterial flora in patients with long-term fixed orthodontic treatment, especially when using conventional braces. S. mutans may become a potential therapeutic target to maintain a healthy oral environment in further research. Oral hygiene should be emphasized during the entire treatment period, and self-ligating braces should be preferred over conventional braces.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from National Natural Science Foundation of China (grant nos. 81771048 and 81800964).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author, on reasonable request.
Authors' contributions
All authors have read and approved the manuscript. Study design: DJ and ZZ. Study conduction: JD, JH, YS, GT and YY. Data collection: JD, SY and TG. Data analysis: DJ, YS, GT and JH. Data interpretation: DJ, SY, TG and JH. Drafting of manuscript: DJ, LL and ZZ. Revision of manuscript content: LL and ZZ. Approval of final version of manuscript: DJ, JH, YS, GT, LL and ZZ.
Ethical approval and consent to participate
All experimental procedures were in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of West China Hospital of Stomatology (Chengdu, China; approval no. WCHSIRB-D-2013-070). Prior written informed consent was obtained from all patients, or by the guardians of the patients if they were <18 years old. The study was added to the Chinese Clinical Trial Registry (registration no. ChiCTR-RCH-13003295).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tanner AC, Sonis AL, Lif Holgerson P, Starr JR, Nunez Y, Kressirer CA, Paster BJ, Johansson I. White-spot lesions and gingivitis microbiotas in orthodontic patients. J Dent Res. 2012;91:853–858. doi: 10.1177/0022034512455031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter AE, Arruda AO, Peters MC, Sohn W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am J Orthod Dentofacial Orthop. 2011;139:657–664. doi: 10.1016/j.ajodo.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Akin M, Tezcan M, Ileri Z, Ayhan F. Incidence of white spot lesions among patients treated with self- and conventional ligation systems. Clin Oral Investig. 2015;19:1501–1506. doi: 10.1007/s00784-014-1382-3. [DOI] [PubMed] [Google Scholar]
- 4.Topaloglu-Ak A, Ertugrul F, Eden E, Ates M, Bulut H. Effect of orthodontic appliances on oral microbiota-6 month follow-up. J Clin Pediatr Dent. 2011;35:433–436. doi: 10.17796/jcpd.35.4.61114412637mt661. [DOI] [PubMed] [Google Scholar]
- 5.Smith DJ, Taubman MA. Cariogenic microflora and the immune response. Inter Oral Health Sci. 2010:394–399. [Google Scholar]
- 6.Alfaqeeh SA, Anil S. Lactate dehydrogenase activity in gingival crevicular fluid as a marker in orthodontic tooth movement. Open Dent J. 2011;5:105–109. doi: 10.2174/1874210601105010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcaccini AM, Amato PA, Leão FV, Gerlach RF, Ferreira JT. Myeloperoxidase activity is increased in gingival crevicular fluid and whole saliva after fixed orthodontic appliance activation. Am J Orthod Dentofacial Orthop. 2010;138:613–616. doi: 10.1016/j.ajodo.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Navarro-Palacios A, García-López E, Meza-Rios A, Armendariz-Borunda J, Sandoval-Rodríguez A. Myeloperoxidase enzymatic activity is increased in patients with different levels of dental crowding after initial orthodontic activation. Am J Orthod Dentofacial Orthop. 2014;146:92–97. doi: 10.1016/j.ajodo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Kupietzky A, Majumdar AK, Shey Z, Binder R, Matheson PB. Colony forming unit levels of salivary LactobacilliStreptococcus mutans in orthodontic patients. J Clin Pediatr Dent. 2005;30:51–53. doi: 10.17796/jcpd.30.1.wxp4870177030826. [DOI] [PubMed] [Google Scholar]
- 10.Jurela A, Repic D, Pejda S, Juric H, Vidakovic R, Matic I, Bosnjak A. The effect of two different bracket types on the salivary levels of S mutansS sobrinus in the early phase of orthodontic treatment. Angle Orthod. 2013;83:140–145. doi: 10.2319/030612-187.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein MI, Scott-Anne KM, Gregoire S, Rosalen PL, Koo H. Molecular approaches for viable bacterial population and transcriptional analyses in a rodent model of dental caries. Mol Oral Microbiol. 2012;27:350–361. doi: 10.1111/j.2041-1014.2012.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung WS, Kim H, Park SY, Cho EJ, Ahn SJ. Quantitative analysis of changes in salivary mutans streptococci after orthodontic treatment. Am J Orthod Dentofacial Orthop. 2014;145:603–609. doi: 10.1016/j.ajodo.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. 2004;42:3128–3136. doi: 10.1128/JCM.42.7.3128-3136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly MM, Featherstone JD. Demineralization and remineralization around orthodontic appliances: An in vivo study. Am J Orthod Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 15.Chang HS, Walsh LJ, Freer TJ. The effect of orthodontic treatment on salivary flow, pH, buffer capacity, and levels of mutans streptococci and lactobacilli. Aust Orthod. 1999;15:229–234. [PubMed] [Google Scholar]
- 16.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taha M, El-Fallal A, Degla H. In vitro and in vivo biofilm adhesion to esthetic coated arch wires and its correlation with surface roughness. Angle Orthod. 2016;86:285–291. doi: 10.2319/122814-947.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.do Nascimento LE, Pithon MM, dos Santos RL, Freitas AO, Alviano DS, Nojima LI, Nojima MC, Ruellas AC. Colonization of Streptococcus mutans on esthetic brackets: Self-ligating vs conventional. Am J Orthod Dentofacial Orthop. 2013;143(Suppl 4):S72–S77. doi: 10.1016/j.ajodo.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Uzuner FD, Kaygisiz E, Cankaya ZT. Effect of the bracket types on microbial colonization and periodontal status. Angle Orthod. 2014;84:1062–1067. doi: 10.2319/111813-844.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellegrini P, Sauerwein R, Finlayson T, McLeod J, Covell DA, Jr, Maier T, Machida CA. Plaque retention by self-ligating vs elastomeric orthodontic brackets: Quantitative comparison of oral bacteria and detection with adenosine triphosphate-driven bioluminescence. Am J Orthod Dentofacial Orthop. 2009;135:426–427.e1-e9. doi: 10.1016/j.ajodo.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Caufield PW, Schön CN, Saraithong P, Li Y, Argimón S. Oral Lactobacilli and Dental Caries. J Dent Res. 2015;94(Suppl 9):S110–S118. doi: 10.1177/0022034515576052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peros K, Mestrovic S, Anic-Milosevic S, Slaj M. Salivary microbial and nonmicrobial parameters in children with fixed orthodontic appliances. Angle Orthod. 2011;81:901–906. doi: 10.2319/012111-44.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lara-Carrillo E, Montiel-Bastida NM, Sánchez-Pérez L, Alanís-Tavira J. Effect of orthodontic treatment on saliva, plaque and the levels of Streptococcus mutansLactobacillus. Med Oral Patol Oral Cir Bucal. 2010;15:e924–e929. doi: 10.4317/medoral.15.e924. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, He J, Xue J, Wang Y, Li K, Zhang K, Guo Q, Liu X, Zhou Y, Cheng L, et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 2015;17:699–710. doi: 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
- 25.Chawda JG, Chaduvula N, Patel HR, Jain SS, Lala AK. Salivary SIgA and dental caries activity. Indian Pediatr. 2011;48:719–721. doi: 10.1007/s13312-011-0113-y. [DOI] [PubMed] [Google Scholar]
- 26.Shifa S, Muthu MS, Amarlal D, Rathna Prabhu V. Quantitative assessment of IgA levels in the unstimulated whole saliva of caries-free and caries-active children. J Indian Soc Pedod Prev Dent. 2008;26:158–161. doi: 10.4103/0970-4388.44031. [DOI] [PubMed] [Google Scholar]
- 27.Koga-Ito CY, Martins CA, Balducci I, Jorge AO. Correlation among mutans streptococci counts, dental caries, and IgA to Streptococcus mutans in saliva. Braz Oral Res. 2004;18:350–355. doi: 10.1590/S1806-83242004000400014. [DOI] [PubMed] [Google Scholar]
- 28.da Silva Campos MJ, Souza Alves CC, Barbosa Raposo NR, Ferreira AP, Farinazzo Vitral RW. Influence of salivary secretory immunoglobulin A level on the pain experienced by orthodontic patients. Med Sci Monit. 2010;16:CR405–CR409. [PubMed] [Google Scholar]
- 29.De La Peña VA, Diz Dios P, Tojo Sierra R. Relationship between lactate dehydrogenase activity in saliva and oral health status. Arch Oral Biol. 2007;52:911–915. doi: 10.1016/j.archoralbio.2007.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author, on reasonable request.