Abstract
Growth arrest associated lncRNA 1 (GASL1) is a newly discovered tumor suppressor long non-coding RNA (lncRNA) in osteosarcoma; however its role in other malignancies remains unknown. The aim of the present study was to investigate the involvement of GASL1 in gastric cancer. In the current study, gastric cancer tissue and adjacent healthy tissue samples were collected from patients with gastric carcinoma, and blood samples were collected from patients with gastric carcinoma and healthy controls to detect the expression of serum GASL1. All patients were followed up for 5 years and the diagnostic and prognostic value of GASL1 for gastric carcinoma was evaluated by ROC and survival curve analyses, respectively. The chi-square test was used to analyze the correlation between serum levels of GASL1 and the clinicopathological features of patients with gastric carcinoma. A GASL1 expression vector and GASL1 small interfering RNA were transfected into gastric cancer cell lines and the effects on β-catenin expression and cell proliferation were examined by western blot and cell proliferation assays, respectively. The expression level of lncRNA GASL1 was significantly downregulated in gastric cancer tissues compared with adjacent normal tissues from patients with gastric carcinoma. In addition, serum levels of GASL1 were significantly decreased in patients with gastric carcinoma when compared with healthy controls. Serum GASL1 levels distinguished patients with gastric carcinoma from healthy controls, and low expression levels of GASL1 were associated with decreased postoperative survival time. GASL1 overexpression downregulated, while GASL1 knockdown upregulated β-catenin expression. GASL1 overexpression inhibited, and GASL1 knockdown promoted gastric cancer cell proliferation. In addition, treatment with a Wnt agonist demonstrated no significant effect on GASL1 expression, however the inhibitory effect of GASL1 overexpression on cell proliferation was reduced following treatment with the Wnt agonist. In conclusion, the GASL1 lncRNA may inhibit tumor growth in patients with gastric carcinoma by inactivating the Wnt/β-catenin signaling pathway.
Keywords: gastric carcinoma, growth arrest associated lncRNA 1, Wnt/β-catenin signaling pathway, proliferation
Introduction
As one of the most common types of cancer, gastric carcinoma is one of leading causes of cancer-associated mortality worldwide (1). Gastric carcinoma primarily affects people residing in Central and Eastern Europe, East Asia and South Africa (2). In China, there are approximately 380,000 new cases of gastric cancer each year, which accounts for >40% of the total worldwide number of cancer diagnoses each year (3). Despite efforts to improve treatment outcomes for patients with gastric carcinoma, postoperative survival is extremely poor, and the overall 5-year survival rate of patients with gastric carcinoma is <10% (4). Early, accurate diagnosis and reliable prognosis prediction are key factors for improving the survival of patients with gastric carcinoma. Therefore, identifying effective diagnostic and prognostic biomarkers of gastric cancer is required.
It is widely accepted that the occurrence of gastric carcinoma is closely associated with several factors including mutations in tumor suppressor genes or oncogenes, bacterial and viral infections and diet (5–7). The Wnt/β-catenin signaling pathway serves a role in the pathogenesis of several types of cancer by promoting tumor growth or metastasis (8,9). In many cases, Wnt/β-catenin achieves its biological function by interacting with long non-coding RNAs (lncRNAs) (10); a subgroup of non-coding RNAs with roles in multiple human diseases (11). Growth arrest associated lncRNA 1 (GASL1) is a newly discovered tumor suppressor lncRNA with a functional role in osteosarcoma (12); however, its role in other malignancies remains unknown. Preliminary microarray data has demonstrated that lncRNA GASL1 expression is downregulated in patients with gastric carcinoma (data not shown). The current study demonstrated that lncRNA GASL1 may inhibit tumor growth in patients with gastric carcinoma by inactivating the Wnt/β-catenin signalingpathway.
Materials and methods
Clinical samples
Gastric cancer and adjacent normal tissue samples were collected from 88 patients (male, n=52; female, n=36; age range, 26–70 years; mean age, 47.7±6.3 years) who were diagnosed and treated at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) from March 2011 to January 2013. In addition, serum samples were also obtained from each patient. Inclusion criteria for the study included: i) patients diagnosed with gastric carcinoma for the first time, as confirmed by pathological assessment, and that had not previously undergone treatment; ii) patients with complete clinical data; iii) patients that had completed a 5-year follow-up following discharge; iv) patients and/or their familes willing to participate. Exclusion criteria for the study included: i) patients that had received any treatment prior to admission; ii) patients that had failed to complete treatment or the 5-year follow-up procedure; iii) patients with other malignancies and/or gastric diseases; iv) patients that had succumbed to other diseases or accidents during follow-up. In addition, serum samples from 72 healthy control patients (male, n=44; female, n=28; age range, 25–68 years; mean age, 47.1±6.1 years) were also included as a control group. Healthy patients received routine physiological examinations at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from March 2011 to January 2013. No significant differences in age and gender were found between the two groups. The current study was approved by the Ethics Committee at the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), and all patients and/or their families provided written informed consent.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cancer tissues, adjacent healthy tissues, serum and in vitro cultivated cells using TRIrizol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total RNA concentation was measured using a NanoDrop™ 2000 Spectrophotometer (Thermo Fisher Scientific, Inc.), and RNA samples with a A260/A280 ratio of 1.8–2.0 were reverse transcribed into cDNA. qPCR was subsequently performed using the SYBR® Green PCR Master Mix (Thermo Fisher Scientific, Inc.). The following primer pairs were used in the qPCR reactions: GASL1 forward, 5′-CATGTTCCAATATGATTCCACC-3′, and reverse, 5′-GATGGGATTTCCATTGATGAC-3′; β-actin forward, 5′- GACCTCTATGCCAACACAGT-3′, and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′. All PCR reactions were performed using an ABI PRISM 7500 sequence detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following thermocycling conditions were used for the qPCR reactions: Initial denaturation at 95°C for 45 sec; 40 cycles of 95°C for 12 sec and 60°C for 45 sec. GASL1 mRNA levels were quantified using the 2−ΔΔCq method (13) and normalized to the internal reference gene β-actin.
Cell culture and transfection
Human gatric carcinoma cell lines SNU-16 (ATCC® CRL-5974™) and NCI-N87 (ATCC® CRL-5822™) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured in ATCC-formulated RPMI-1640 medium (ATCC® 30-2001™) supplemented with 10% fetal bovine serum (ATCC® 30-2020™). GASL1 siRNA (CCUGAGGCUAGAGGGUCUAAGAGAA) and the siRNA Universal Negative Control were provided by Shanghai GenePharma Co., Ltd. (Shanghai, China). The full-length GASL1 cDNA clone surrounded by EcoRI restriction sites was obtained by PCR amplification and cloned into the linearized pIRSE2-EGFP vector (Clontech Laboratories, Inc., Mountainview, CA, USA) to generate a GASL1 expression vector. Prior to transfection, cells were cultured to reach 80–90% confluence. Cells (5×105 cells/well of a 6-well plate) were transfected with siRNAs (50 nM) and vectors (10 nM) using Lipofectamine® 2000 reagent (cat. no. 11668-019, Invitrogen; Thermo Fisher Scientific, Inc.). GASL1 downregulation and overexpression was confirmed by RT-qPCR before subsequent experimentation. Cells were harvested at 24 h following transfection for subsequent experiments. Untransfected cells were control cells, and cells transfected with empty vectors were negative control cells.
Cell proliferation assay
Cell proliferation was analyzed using the Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Brifely, SNU-16 and NCI-N87 cells in the logarithmic growth phase were harvested and single-cell suspensions with a density of 4×104 cells/ml were prepared. Subsequently, cells were seeded in 96-well plates at a density of 4×103 cells/well and incubated at 37°C in a 5% CO2-humidified incubator for 24, 48, 72 and 96 h. Following incubation, 10 µl CCK-8 reagent was added to each well and cells were incubated for an additional 5 h. Cell proliferation was determined by measuring the optical density OD at a wavelength of 450 nm using a microplate reader.
Western blot analysis
Total protein was extracted using radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Inc.). Total protein was quantified using a bicinchoninic acid assay and 20 µg protein/lane was separated by 10% SDS-PAGE. The separated proteins were transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and blocked with 5% skimmed milk at room temperature for 2 h. Following washing with PBS solution, membranes were incubated with primary antibodies against β-catenin (rabbit anti-human; cat. no. ab16051; dilution, 1:1,200) and GAPDH (rabbit anti-human; cat. no. ab9485; dilution, 1:1,000; both Abcam, Cambridge, UK) overnight at 4°C. Following washing, membranes were incubated with horseradish peroxidase-labeled anti-rabbit IgG secondary antibody (cat. no. MBS435036; dilution, 1:1,000; MyBioSource, Inc., San Diego, CA, USA) at room temperature for 2 h. Protein bands were visualized using the Amersham™ ECL™ western blotting reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). β-catenin protein expression was quantified using Image J v.1.47 software (National Institutes of Health, Bethesda, MD, USA) and normalized to the GAPDH loading control.
Statistical analysis
Data are presented as the mean ± standard deviation. All statistical analyses were performed using GraphPad Prism software (version 6.0; GraphPad Software, La Jolla, CA, USA).GASL1 and β-catenin expression data were analyzed using an unpaired t-test, whilst the statistical significance between GASL1 expression levels in tumor and adjacent healthy tissue samples were analyzed using a paired Student's t-test. One-way analysis of variance followed by the least significant difference test was used to analyze differences among multiple groups. The overall survival of patients with gastric cancer was evaluated using the Kaplan-Meier method and the log-rank test was used to compare the survival distribution between the two groups. The correlation between serum levels of GASL1 and clinicopathological data from patients with gastric carcinoma were analyzed using a Chi-square test. P<0.05 was considered to indicate a statistically significant difference.
Results
GASL1 expression in gastric cancer tissue and adjacent normal tissue samples from 88 patients with gastric carcinoma
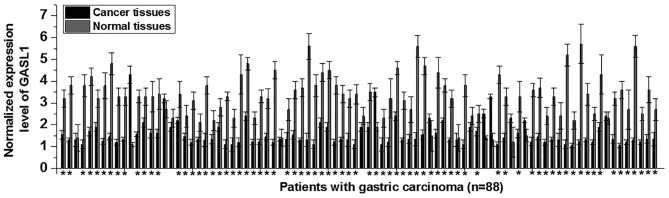
The differential expression of a gene in cancer tissues and adjacent normal tissues typically indicates the involvement of this gene in a malignancy. Therefore, the expression level of GASL1 in cancer and normal adjacent tissue samples from all 88 patients with gastric carcinoma was examined. The expression level of GASL1 was significantly decreased in gastric cancer tissues when compared with normal adjacent tissues in 76/88 patients with gastric carcinoma (Fig. 1). These results suggest that downregulation of GASL1 may be involved in the pathogenesis of gastric carcinoma.
Figure 1.
GASL1 expression in gastric cancer and adjacent normal tissue samples from 88 patients with gastric carcinoma. Relative GASL1 expression levels were determined by reverse transcription-quantitative polymerase chain reaction analysis in gastric cancer and adjacent normal tissue samples from patients with gastric cancer. *P<0.05 vs. Normal tissues. GASL1, growth arrest associated lncRNA 1.
Comparison between GASL1 serum levels in patients with gastric cancer and healthy controls and the potential diagnostic value
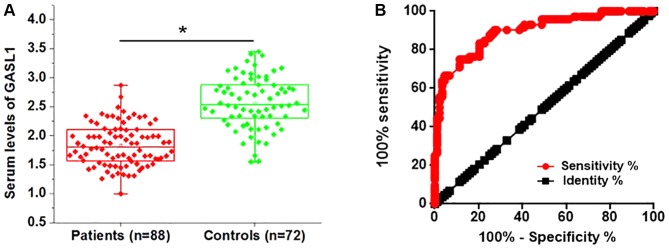
LncRNAs are present in the circulatory system and serve as molecular signals participating in several biological processes (11). In the current study, serum levels of GASL1 were detected in all patients with gastric carcinoma. The serum level of GASL1 was significantly decreased in patients with gastric carcinoma compared with healthy controls (Fig. 2A). Receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic value of serum lncRNA GASL1 in discriminating patients with gastric carcinoma from normal controls. The area under the curve was 0.8945 (95% confidence interval, 0.8452–0.943; standard error, 0.02313) (Fig. 2B). These results suggest that low serum GASL1 may serve as a diagnostic biomarker for gastric carcinoma.
Figure 2.
Comparison between GASL1 serum levels in patients with gastric cancer and healthy controls. (A) Relative GASL1 expression levels in serum samples from patients with gastric cancer and healthy controls were determined by reverse transcription-quantitative polymerase chain reaction. (B) Receiver operating characteristic curve analysis of GASL1 expression in serum samples. *P<0.05 vs. Control. GASL1, growth arrest associated lncRNA 1.
Association between GASL1 serum levels and clinicopathological characteristics of patients with gastric cancer
Patients were divided into the high expression group (n=44) and low expression group (n=44) according to the median serum level of GASL1. The correlation between the serum levels of GASL1 and clinicopathological data from patients with gastric carcinoma were analyzed using a Chi-square test. As presented in Table I, no significant correlation between the serum level of GASL1 and age, gender, distant tumor metastasis and the smoking and alcohol consumption of patients was observed. However, serum levels of GASL1 were significantly correlated with tumor size. Therefore, GASL1 may participate in the regulation of tumor growth in gastric carcinoma.
Table I.
Association between serum GASL1 and clinicopathological characteristics of patients with gastric cancer.
| GASL1 expression level | |||||
|---|---|---|---|---|---|
| Characteristics | No. of cases | High (n=44) | Low (n=44) | χ2 | P-value |
| Age (years) | |||||
| >45 | 48 | 22 | 26 | 0.73 | 0.39 |
| <45 | 40 | 22 | 18 | ||
| Gender | |||||
| Male | 52 | 23 | 29 | 1.69 | 0.19 |
| Female | 36 | 21 | 15 | ||
| Drinking | |||||
| Yes | 47 | 22 | 25 | 0.41 | 0.52 |
| No | 41 | 22 | 19 | ||
| Smoking | |||||
| Yes | 42 | 19 | 23 | 0.73 | 0.39 |
| No | 46 | 25 | 21 | ||
| Tumor size (cm) | |||||
| >5 | 45 | 17 | 28 | 5.50 | 0.02 |
| <5 | 43 | 27 | 16 | ||
| Tumor distant metastasis | |||||
| Yes | 41 | 18 | 23 | 1.14 | 0.29 |
| No | 47 | 26 | 21 | ||
GASL1, growth arrest associated lncRNA 1.
Association between GASL1 serum levels and the overall survival of patients with gastric cancer
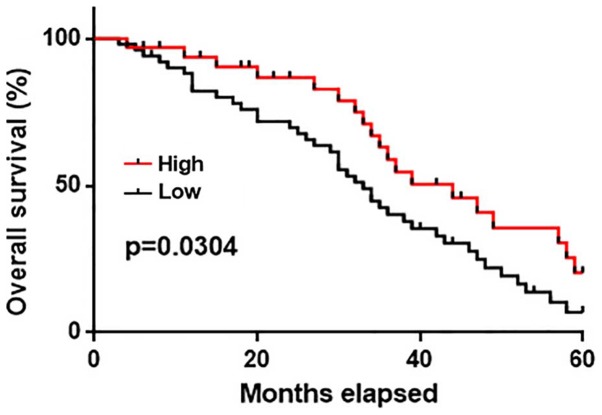
No significant correlation between the serum levels of GASL1 and distant tumor distant metastasis was observed. Distant metastasis is a major cause of death in gastric carcinoma patients; therefore, the 5-year follow-up data from patients with and without metastasis were analyzed. The survival curves of patients in the high and low expression groups were plotted using the Kaplan-Meier method and compared by log-rank test. The overall survival of patients in the high expression group was significantly longer when compared with patients in the low expression group (P<0.05; Fig. 3). These results suggest that serum GASL1 levels may also serve as a potential prognostic biomarker for patients with gastric cancer.
Figure 3.
Comparison of the survival distribution between high and low GASL1 expression groups. Overall survival of patients with gastric cancer was analyzed using the Kaplan-Meier method. GASL1, growth arrest associated lncRNA 1.
Effect of GASL1 overexpression and knockdown on β-catenin expression
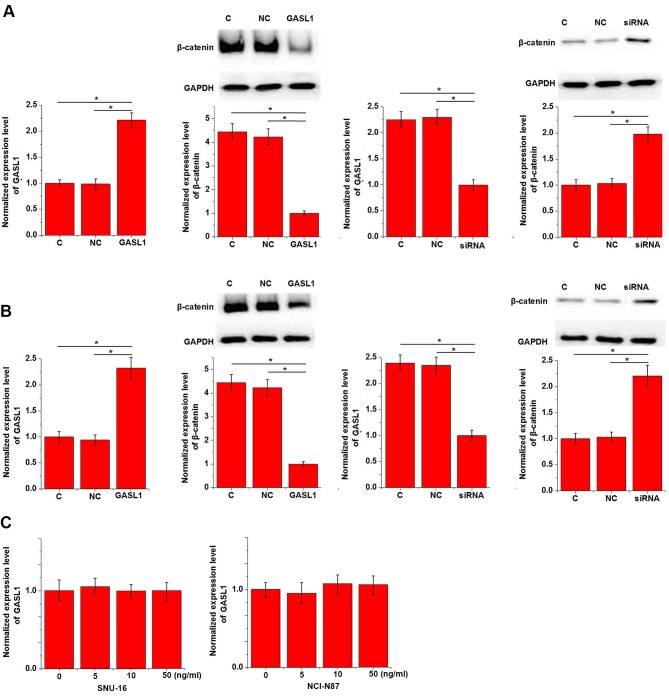
The results presented in Table I indicate that GASL1 may be involved in the regulation of gastric tumor growth. The Wnt/β-catenin signaling pathway promotes cancer cell proliferation in several types of cancer, including gastric cancer (8). To investigate the potential mechanism underlying the function of GASL1, the effect of GASL1 expression on the Wnt/β-catenin signaling pathway was examined in vitro using gastric carcinoma cell lines, SNU-16 and NCI-N87. GASL1 overexpression inhibited and GASL1 knockdown promoted the expression of β-catenin in both SNU-16 (Fig. 4A) and NCI-N87 (Fig. 4B) gastric cell lines. In addition, treatment with a Wnt agonist, a potent and selective activator of Wnt signaling, demonstrated no significant effect on GASL1 expression at doses of 5, 10 and 50 ng/ml (Fig. 4C).
Figure 4.
Effects of GASL1 overexpression and knockdown on β-catenin expression. GASL1 overexpression inhibited and GASL1 knockdown promoted the expression of β-catenin in both (A) SNU-16 and (B) NCI-N87 gastric cell lines. (C) In addition, treatment with a Wnt agonist, a potent and selective activator of Wnt signaling, demonstrated no significant effect on GASL1 expression at doses of 5, 10 and 50 ng/ml. *P<0.05, as indicated. GASL1, growth arrest associated lncRNA 1; siRNA, small interfering RNA.
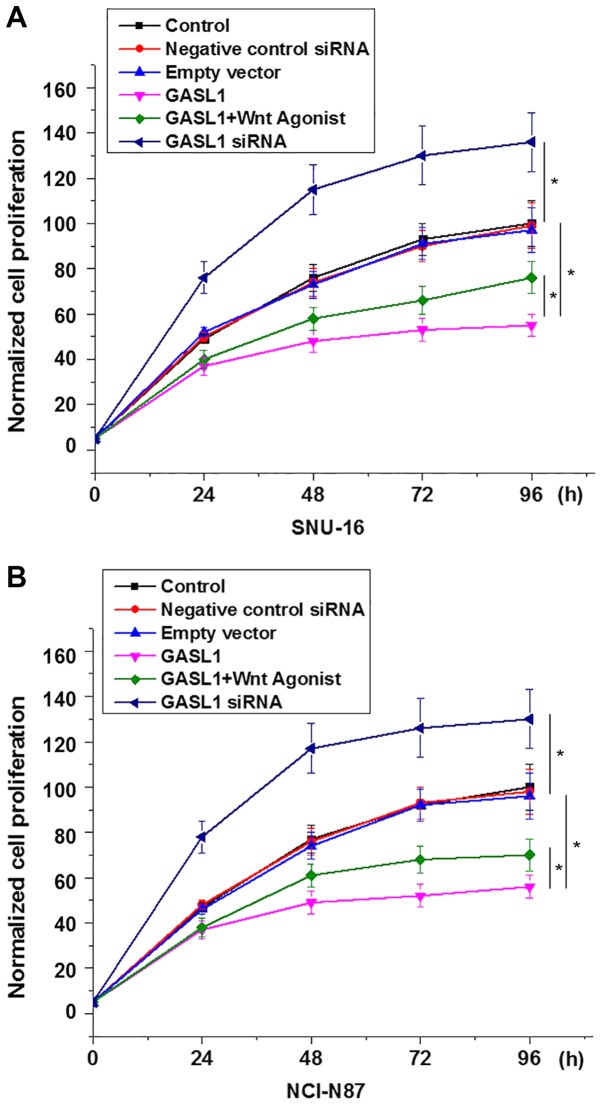
Effects of GASL1 overexpression and knockdown on cell proliferation
A CCK-8 assay was performed to investigate the effects of GASL1 overexpression and knockdown on the proliferation of gastric carcinoma cell lines, SNU-16 and NCI-N87. GASL1 overexpression inhibited and GASL1 knockdown promoted the proliferation of SNU-16 (Fig. 5A) and NCI-N87 (Fig. 5B) cells. In addition, treatment with a Wnt agonist (10 ng/ml) significantly reduced the inhibitory effect of GASL1 overexpression on the proliferation of both cell lines (Fig. 5). Furthermore, GASL1 overexpression inhibited and GASL1 knockdown demonstrated no significant effect on gastric carcinoma cell migration and invasion (data not shown), which suggests the specific involvement of GASL1 in gastric carcinoma growth but not metastasis.
Figure 5.
Effects of GASL1 overexpression and knockdown on cell proliferation. Cell proliferation was examined in (A) SNU-16 and (B) NCI-N87 cell lines following transfection with GASL1 expression construct with and without treatment with Wnt agonist and GASL1 siRNA. *P<0.05, as indicated. GASL1, growth arrest associated lncRNA 1; siRNA, small interfering RNA.
Discussion
In the current study, the expression of GASL1 in patients with gastric carcinoma and healthy controls was detected and the funtional role of GASL1 in gastric carcinoma was investigated. The present study demonstrated that GASL1, a known tumor suppressor lncRNA in osteosarcoma (12), inhibits gastric carcinoma cell proliferation. In addition, the GASL1 lncRNA may exert its tumor suppressor function in gastric carcinoma by inactivating the Wnt/β-catenin signaling pathway.
The occurrence and development of gastic carcinoma is associated with alterations in the expression pattern of a large number of lncRNAs (14), indicating the involvement of lncRNAs in gastric carcinoma. LncRNA expression profiles in tumor tissue and adajcent normal tissues serve important roles by inhibiting or promoting cancer progression (11). It has been reported that the expression of lncRNA-H19 is significantly upregulated in gastric carcinoma tissues compared with adjacent normal tissues, and overexpression of lncRNA-H19 promotes distant tumor metastasis (15). By contrast, the expression of the maternally expressed gene 3 lncRNA is downregulated in patients with gastric carcinoma and was reported to be an indicator of poor prognosis (16). GASL1 lncRNA expression is downregulated in osteosarcoma (12). In the current study, the expression level of GASL1 was significantly decreased in gastric cancer tissues when compared with adjacent normal tissues in the majority of patients, indicating that downregulation of GASL1 may be involved in the pathogenesis of gastric carcinoma.
The survival of patients with gastric carcinoma remains low, and this is in part due to the low rate of early diagnosis (17). The gold standard for the diagnosis of gastric carcinoma is pathological examination, however application is often limited due to the invasive nature of the procedure (18). The development of human disease is often associated with alterations in certain substances (such as circulating proteins, hormones and RNAs) in the circulatory system, and monitoring changes in these substances may facilitate the diagnosis of disease (19). In the current study, serum levels of GASL1 were significantly decreased in patients with gastric carcinoma when compared with healthy controls. ROC curve analysis demonstrated that low levels of serum GASL1 effectively distinguished patients with gastric carcinoma from healthy controls. In addtion, survival analysis revealed that low serum GASL1 levels were closely correlated with poor survival. GASL1 expression in other diseases remains unkown. Therefore, multiple biomarkers should be combined to improve the diagnostic and prognostic accuracy of serum GASL1 levels as a potential diagnostic marker for gastric carcinoma. In addition, serum levels of GASL1 do not always reflect the expression levels of GASL1 in tumor tissues. In the current study, 4 patients in the high GASL1 serum level group also demonstrated relatively high expression levels of GASL1 in tumor tissues.
The survival rate of patients with gastric carcinoma in the early stages of disease is significnatly higher than those with distant tumor metastasis (20). In the current study, the serum levels of GASL1 demonstrated no significant correlation with distant tumor metastases, indicating that GASL1 may not be involved in the invasion and migration of gastric cancer cells. Therefore, the 5-year follow-up records included data from patients with and without distant metastases. Taken together, these results suggest that serum GASL1 may serve as a potential prognostic biomarker for gastric carcinoma.
Correlation analysis demonstrated a significant correlation between the serum levels of GASL1 and tumor size. In addition, in vitro cell proliferation assays revealed that GASL1 inhibits cancer cell proliferation in gastric carcinoma. The Wnt/β-catenin signaling pathway promotes cancer cell proliferation in several types of cancer, including gastric cancer (8). The current study confirmed that GASL1 inhibits the Wnt/β-catenin signaling pathway. By contrast, activation of the Wnt/β-catenin signaling pathway did not affect GASL1 expression, suggesting that GASL1 may be an upstream inhibitor of the Wnt/β-catenin signaling pathway in gastric carcinoma. Activation of the Wnt/β-catenin signaling pathway promotes tumor growth in gastric cancer (21), and rescue experiments in the current study revealed that activation of Wnt/β-catenin can reduce the inhibitory effects of GASL1 overexpression on gastric cancer cell proliferation. Therefore, GASL1 may inhibit gastric cancer cell proliferation by inactivating the Wnt/β-catenin signaling pathway.
Further studies are required to investigate the potential in vivo association between GASL1 and the Wnt/β-catenin signaling pathway, which include analyzing the correlation between GASL1 and Wnt/β-catenin expression levels in tumor tissues. In addition, the number of clinicopathologic factors evaluated in the current study were relatively low and therefore more factors should be included in future studies.
In conclusion, the GASL1 lncRNA is significantly downregulated in gastric carcinoma. Downregulation of serum GASL1 distinguishes patients with gastric carcinoma from healthy controls, and low expression also indicated poor survival. GASL1 may function as an inhibitor of tumor growth in gastric carcinoma through inactivation of the Wnt/β-catenin signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
CP, XL, YY and JC were responsible for the conception and design of the study. CP, XL and YY performed the experiments. CP and XL analyzed the data. CP and YY prepared the manuscript. CP and JC revised the final draft of the manuscript.
Ethics approval and consent to participate
The protocol used in the present study was approved by the Ethics Review Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), and all patients and/or their families provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hohenberger P, Gretschel S. Gastic cancer. Lancet. 2003;362:305–315. doi: 10.1016/S0140-6736(03)13975-X. [DOI] [PubMed] [Google Scholar]
- 2.Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230–236. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 3.Zhu X, Li J. Gastric carcinoma in China: Current status and future perspectives. Oncol Lett. 2010;1:407–412. doi: 10.3892/ol_00000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rugge M, Fassan M, Graham DY. Gastric Cancer. Springer; Cham: 2015. Epidemiology of gastric cancer; pp. 23–34. [DOI] [Google Scholar]
- 5.Canedo P, Durães C, Pereira F, Regalo G, Lunet N, Barros H, Carneiro F, Seruca R, Rocha J, Machado JC. Tumor necrosis factor alpha extended haplotypes and risk of gastric carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:2416–2420. doi: 10.1158/1055-9965.EPI-08-0413. [DOI] [PubMed] [Google Scholar]
- 6.Song HJ, Kim KM. Pathology of epstein-barr virus-associated gastric carcinoma and its relationship to prognosis. Gut Liver. 2011;5:143–148. doi: 10.5009/gnl.2011.5.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjödahl K, Lu Y, Nilsen TI, Ye W, Hveem K, Vatten L, Lagergren J. Smoking and alcohol drinking in relation to risk of gastric cancer: A population-based, prospective cohort study. Int J Cancer. 2007;120:128–132. doi: 10.1002/ijc.22566. [DOI] [PubMed] [Google Scholar]
- 8.Akaboshi S, Watanabe S, Hino Y, Sekita Y, Xi Y, Araki K, Yamamura K, Oshima M, Ito T, Baba H, Nakao M. HMGA1 is induced by Wnt/beta-catenin signaling pathway and maintains cell proliferation in gastric cancer. Am J Pathol. 2009;175:1675–1685. doi: 10.2353/ajpath.2009.090069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang GB, Kim JY, Cho SD, Park KS, Jung JY, Lee HY, Hong IS, Nam JS. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep. 2015;5:12465. doi: 10.1038/srep12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R, Cao Z, Singh B, Franklin JL, Wang J, Hu H, et al. lncRNA MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance via Wnt/β-catenin signaling. Nat Med. 2017;23:1331–1341. doi: 10.1038/nm.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalevée S, Feil R. Long noncoding RNAs in human disease: Emerging mechanisms and therapeutic strategies. Epigenomics. 2015;7:877–879. doi: 10.2217/epi.15.55. [DOI] [PubMed] [Google Scholar]
- 12.Gasri-Plotnitsky L, Ovadia A, Shamalov K, Nizri-Megnaji T, Meir S, Zurer I, Cohen CJ, Ginsberg D. A novel lncRNA, GASL1, inhibits cell proliferation and restricts E2F1 activity. Oncotarget. 2017;8:23775–23786. doi: 10.18632/oncotarget.15864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Shi H, Xi H, Wu X, Cui J, Gao Y, Liang W, Hu C, Liu Y, Li J, et al. Genome-wide lncRNA microarray profiling identifies novel circulating lncRNAs for detection of gastric cancer. Theranostics. 2017;7:213–227. doi: 10.7150/thno.16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Yu B, Li J, Su L, Yan M, Zhu Z, Liu B. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun M, Xia R, Jin F, Xu T, Liu Z, De W, Liu X. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumuor Biol. 2014;35:1065–1073. doi: 10.1007/s13277-013-1142-z. [DOI] [PubMed] [Google Scholar]
- 17.Jin Z, Jiang W, Wang L. Biomarkers for gastric cancer: Progression in early diagnosis and prognosis. Oncol Lett. 2015;9:1502–1508. doi: 10.3892/ol.2015.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn S, Van Vrancken M, Lee M, Ha SY, Lee H, Min BH, Lee JH, Kim JJ, Choi S, Jung SH, et al. Ideal number of biopsy tumor fragments for predicting HER2 status in gastric carcinoma resection specimens. Oncotarget. 2015;6:38372–38380. doi: 10.18632/oncotarget.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Blood biomarkers for brain injury: What are we measuring? Neurosci Biobehav Rev. 2016;68:460–473. doi: 10.1016/j.neubiorev.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.