Abstract
Renal cell carcinoma (RCC) is the most commonly diagnosed renal tumor, consisting of ~3% of all malignancies worldwide. The prognosis of RCC can vary widely, and detecting patients at risk of recurrence at an early stage of disease may improve patient outcome. The factors presently used in a clinical setting cannot reliably predict the natural history of the disease. Therefore, there is a requirement to identify novel biomarkers that can aid in predicting patient outcome. Previous studies have indicated that microRNAs (miRNAs/miRs) are potential candidates as prognostic biomarkers for patients suffering from RCC. Consequently, the aims of the present study were to validate the potential of 3 of these miRNAs to predict the prognosis of patients with RCC, and to investigate the stability of endogenous control genes for miRNA studies in RCC tissues. The expression of 7 endogenous controls was measured using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) in formalin-fixed paraffin-embedded tumor and benign tissues from patients suffering from clear cell RCC (ccRCC). The analyses identified RNU48 and U47 as the most stable endogenous controls. The expression of miR-126, miR-21 and miR-10b was analyzed using RT-qPCR in renal tissues from 116 patients diagnosed with ccRCC. All three investigated miRNAs were differentially expressed between malignant and benign tissues. miR-126 and miR-10b were also differentially expressed between grades and stages of ccRCC. In a univariate, but not in a multivariate model, low expression of miR-126 was associated with shorter time to recurrence of the disease. The results of the present study indicate that of the 3 miRNAs investigated, the expression of miR-126 has the strongest potential as a prognostic biomarker for patients suffering from ccRCC.
Keywords: renal cancer, kidney cancer, clear cell renal cell carcinoma, miRNAs, endogenous control
Introduction
Renal cell carcinoma (RCC) is the most common renal tumor accounting for 2–3% of all malignancies worldwide (1). Several histological RCC subtypes have been categorized, and the most frequent subtypes include clear cell RCC (ccRCC), papillary RCC and chromophobe RCC. These subtypes together represent >90% of all diagnosed RCCs (2). Nephrectomy was historically the only treatment option for RCC, with no requirement for advanced prognostication or follow-up. However, since the turn of the century, there have been dramatic changes in the available diagnostics and treatment options (3). There is an increasing incidence of detection of small renal tumors (<40 mm in diameter), primarily due to the increasing use of tomographic radiology, which enables for the improved detection of disease (4,5). Small tumors often grow slowly; however, not all small tumors are indolent in nature (6). Several prognostic factors for RCC are presently used in a clinical setting, including tumor stage, Fuhrman grade, lymph node involvement and histological subtype. However, these factors lack accuracy in predicting the natural history of the disease, particularly in patients with non-metastatic disease at the time of diagnosis (7).
Overall, 20–30% of all patients with RCC present with metastatic disease at the time of diagnosis, and 20–40% develop metastases following nephrectomy (8). The need for prognostic tools in metastatic (m)RCC has also become evident. There are now several available targeted therapies on offer to patients with mRCC in conjunction with metastatic surgery and other ablative therapies of the metastatic lesions. The prognosis of RCC can vary widely, and early detection of recurrence can improve patient outcome, as systemic treatments are more likely to yield a favorable response when the metastatic burden is limited (9). Therefore, there is a requirement to identify molecular biomarkers that can aid in predicting patient outcome for patients with RCC, either alone or in combination with the presently used clinical parameters.
MicroRNAs (miRNAs/miRs) are small non-coding RNA molecules that regulate gene expression at a post-transcriptional level (10). These molecules serve a key role in a diverse range of biological processes important in cancer development, including proliferation, differentiation and apoptosis (11,12). A single miRNA could alter the expression of a large number of target genes, and therefore has the potential to regulate entire disease-specific pathways and signaling cascades (13). Altered miRNA expression has been identified in all human tumors investigated to date, and several diagnostic kits based on miRNA expression profiles are available, including one for the differentiation between histological subtypes of RCC (available from Rosetta Genomics, Ltd., Princeton, NJ, USA). Three of the miRNAs that have previously been suggested as prognostic biomarkers for RCC are miR-21, miR-126 and miR-10b (14–21). miR-21 is an oncogenic miRNA that has been identified to be upregulated in numerous cancer types, including RCC (14,15). The overexpression of miR-21 increases cell proliferation, migration and invasion, as well as inhibiting apoptosis, and the confirmed target genes for miR-21 are tumor protein p53, phosphatase and tensin homolog, and programmed cell death 4 (22–26). The deregulation of miR-126 has also been identified in various types of cancer, where it regulates genes involved in the vascular endothelial growth factor and phosphatidylinositol 3-kinase pathways, and therefore serves an important role in processes including angiogenesis and cell cycle regulation (27–34). The third miRNA, miR-10b, has previously been reported as an oncomiR in specific types of cancer, while it has been reported to exert a tumor suppressor role in others, among them RCC (16,35–38). The overexpression of miR-10b in RCC cell lines inhibits cell proliferation, migration and invasion; however, the exact mechanism has not been completely elucidated (17,38). Even though the expression of these 3 miRNAs have previously been investigated in RCC, their use as prognostic biomarkers for this disease remains debatable. Therefore, further studies are required in order to validate their potential to predict clinical outcome in patients with RCC.
The present study aimed to investigate and validate the value of miR-126, miR-21 and miR-10b expression as prognostic biomarkers in a Swedish cohort of patients with RCC. An additional aim was to identify the most suitable endogenous control gene(s) for miRNA expression studies in ccRCC tissues.
Materials and methods
Study population
Patients were recruited from the Örebro Kidney Cancer Cohort (OKCC), which consisted of 485 patients consecutively diagnosed with RCC, who received surgical treatment between January 1, 1986 and December 31, 2013 at the Department of Urology, Örebro University Hospital, Örebro, Sweden. The patients were followed through medical records until December 31, 2015 and, in cases where the patient was deceased, the cause of mortality was established by the death certificate from the Swedish Cause of Death Register and classified as mortality from renal cancer or from other causes. The inclusion criteria for the present study were a diagnosis of ccRCC and the undergoing of surgery for RCC between 1986 and 2010. Of the initial 485 patients in the OKCC cohort, 221 patients were excluded from the study (92 had surgery after 2010, 42 had no tumor sample available for histological re-evaluation, 6 had benign tumor at histological re-evaluation, and 81 had other tumor histology than ccRCC), leaving 264 patients matching the inclusion and exclusion criteria. Due to the limited amount of tissue available from these patients, 116 were randomly selected for inclusion in the present study. Of these 116 patients, 69 had malignant and adjacent benign tissue available for nucleic acid extraction, and the remaining 47 patients had only malignant tissue available for extraction (Fig. 1). The selected patient characteristics are listed in Table I. The present study was approved by the ethics committee of the Uppsala and Örebro region, Sweden.
Figure 1.
The Örebro Kidney Cancer Cohort consists of 485 patients undergoing surgery for renal cancer at the University Hospital of Örebro between 1986 and 2013. From this cohort 221 patients were excluded from the current study; 92 were excluded due to undergoing surgery after 2010, 42 did not have tumor tissue available for histological re-evaluation, 6 were diagnosed with a benign renal tumor at re-evaluation, and 81 had histological subtypes of renal cancer other than ccRCC. From the remaining 264 patients, 116 patients were randomly chosen for inclusion in the final study cohort. Of these patients, tumor as well as adjacent benign tissue was available for nucleic acid extraction from 69 patients, and from the remaining 47, only tumor tissue was available. ccRCC, clear cell renal cell carcinoma.
Table I.
Selected patient demographic and clinical characteristics (n=116).
| Characteristics | Value |
|---|---|
| Mean age at diagnosis (range), years | 66.9 (40–93) |
| Sex, n (%) | |
| Male | 62 (53.4) |
| Female | 54 (46.6) |
| Mean BMI at diagnosis (range) | 27.1 (18.1–39.5) |
| Smoking status, n (%) | |
| Yes | 35 (30.2) |
| No | 52 (44.8) |
| Missing | 29 (25.0) |
| Median primary tumor diameter (range), mm | 70 (20–160) |
| AJCC stage | |
| I | 49 (42.2) |
| II | 24 (20.7) |
| III | 26 (22.4) |
| IV | 17 (14.7) |
| Fuhrman grade | |
| I | 4 (3.4) |
| II | 52 (44.8) |
| III | 48 (41.4) |
| IV | 12 (10.3) |
| Metastases at diagnosis | |
| Yes | 14 (12.1) |
| No | 102 (87.9) |
| Radical nephrectomy | |
| Yes | 107 (92.2) |
| No | 9 (7.8) |
| Partial nephrectomy | |
| Yes | 10 (8.6) |
| No | 106 (91.4) |
| Recurrence | |
| Yes | 49 (42.2) |
| No | 67 (57.8) |
| Cause of mortality | |
| Renal cancer | 48 (51.1) |
| Other cause | 46 (48.9) |
| Alive | 22 |
| Median follow-up time (range), months | 63 (0–302) |
BMI, Body Mass Index; AJCC, American Joint Committee on Cancer.
Stability of endogenous control genes
In order to determine which endogenous control genes were the most suitable for miRNA expression studies in ccRCC, a subset of 21 samples were chosen to investigate the stability of 7 previously reported control genes for renal tissues. The samples were chosen to span across all years (1987–2010) and to include various tumor stages and grades (Table II). Malignant and adjacent benign tissues were included in the investigation (n=42).
Table II.
Clinicopathological features of samples included in the selection of endogenous control genes.
| Samplea | Year of diagnosis | Fuhrman grade | AJCC stage |
|---|---|---|---|
| 1 | 1989 | IV | IV |
| 2 | 1990 | II | I |
| 3 | 1991 | III | IV |
| 4 | 1992 | II | II |
| 5 | 1993 | III | IV |
| 6 | 1994 | II | II |
| 7 | 1995 | II | III |
| 8 | 1996 | III | IV |
| 9 | 1997 | II | I |
| 10 | 1998 | IV | III |
| 11 | 1999 | II | I |
| 12 | 2000 | III | III |
| 13 | 2001 | II | I |
| 14 | 2002 | III | III |
| 15 | 2003 | II | I |
| 16 | 2004 | III | IV |
| 17 | 2005 | II | I |
| 18 | 2006 | III | IV |
| 19 | 2007 | II | IV |
| 20 | 2008 | II | I |
| 21 | 2009 | III | II |
For each patient, malignant and adjacent benign tissue were used (n=42). AJCC, American Joint Committee on Cancer.
Nucleic acid extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The patient material in the present study consisted of formalin-fixed paraffin-embedded (FFPE) tissue obtained from patients with ccRCC. Malignant and adjacent benign tissue was marked by two dedicated uro-pathologists (MF and FG) on hematoxylin and eosin slides corresponding with the paraffin blocks prior to punching out two cores (1 mm) of tissue from each area. The tissue cores were deparaffinized according to a standard protocol using xylene and alcohols prior to the extraction of DNA and total RNA (including small RNAs) using the AllPrep DNA/RNA FFPE kit (catalog no. 80234; Qiagen GmbH, Hilden, Germany), according to the manufacturer's protocol. The quality and quantity of the resulting DNA and RNA was assessed using the NanoDrop 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The RNA quality was determined using the 2100 BioAnalyzer system with the RNA 6000 Pico kit (Agilent Technologies, Santa Clara, CA, USA). Total RNA samples were diluted to a concentration of 2 ng/µl prior to RT using the TaqMan® microRNA reverse transcription kit (catalog no. 4366596; Thermo Fisher Scientific, Inc.) and TaqMan® assays specific for the miRNAs and endogenous control genes investigated in the present study. The thermal protocol used for RT was as follows: 16°C for 30 min, 42°C for 30 and 5 min at 85°C. The resulting cDNA was mixed with TaqMan® universal master mix II no AmpErase® UNG (catalog no. 4440040; Thermo Fisher Scientific, Inc.) and TaqMan® small RNA assays specific for each miRNA and endogenous control gene (Table III), and used in qPCRs, according to the manufacturer's protocol. The thermocycling conditions for qPCR were as follows: 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C for 1 min. All reactions were performed on an Applied Biosystems 7900HT real-time PCR system and raw Cq values were determined using SDS software version 2.4 (both Applied Biosystems; Thermo Fisher Scientific, Inc.). A threshold of 0.2 was used for each miRNA.
Table III.
Control genes and miRs investigated in the present study.
| Namea | TaqMan assayb | IDc |
|---|---|---|
| RNU44 | 001094 | NR_002750 |
| RNU24 | 001001 | NR_002447 |
| RNU48 | 001006 | NR_002745 |
| RNU6B | 001093 | NR_002752 |
| U6 | 001973 | NR_004394 |
| U47 | 001223 | AF141346 |
| RPL21 | 001209 | AB061826 |
| miR-126 | 002228 | hsa-miR-126-3p |
| miR-21 | 000397 | hsa-miR-21-5p |
| miR-10b | 002218 | hsa-miR-10b-5p |
Control gene or miR name.
Assay ID from Thermo Fisher Scientific, Inc.
National Center for Biotechnology Information accession no. for the control genes, and miRBase ID for the miRs. miR, microRNA.
Statistical analysis
The stability of the endogenous control genes was evaluated using NormFinder (39). This software application calculates a stability value (SV) defined as the absolute mean + one standard deviation; which is calculated using the intergroup variation values from the candidate control genes. Raw Cq values were used as input. A Wilcoxon signed rank test was performed for the comparison of the expression levels of the endogenous control genes between malignant and adjacent benign samples.
All samples were analyzed in four replicates, and the mean Cq value was calculated and used in subsequent analyses. In order to compare the miRNA expression levels between malignant and adjacent benign tissues, a Wilcoxon test was performed. The relative up- or downregulation (fold change, FC) of miRNAs in malignant tissue compared with adjacent benign tissue was obtained by 2−∆∆Cq method (40), assuming complete efficiency of the qPCR. A Mann-Whitney U test or Kruskal-Wallis test with the ∆Cq values as input values were subsequently used to compare miRNA expression levels between groups of patients. The miRNA expression was divided into high or low expression based on receiver operating characteristic (ROC) curves with disease-specific survival as the endpoint. The Kaplan-Meier method was used to estimate survival probabilities and the log-rank test was used to compare groups. Cox regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of the association between miRNA expression and time to recurrence of the disease and disease-specific survival. Person-time was calculated from the date of cancer diagnosis to the earliest of the following time points: Either mortality from renal cancer or the recurrence of the disease (depending on the endpoint of the analysis), censored at time of mortality from other causes, or the end of follow-up (December 2015). Cox regression models were performed using miRNAs as dichotomous variables, adjusting for primary tumor diameter (<40 vs. >40 mm), American Joint Committee on Cancer (AJCC) stage (I+II vs. III+IV) and Fuhrman grade (I+II vs. III+IV) (41,42). In order to evaluate the discriminating capacities of each miRNA, alone or in combination with other predictive factors, ROC curve analyses were performed with a calculation of the area under the curve (AUC) for disease-specific survival. P<0.05 was considered to indicate a statistically significance. All analyses were performed in SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Endogenous control genes
In order to investigate whether the endogenous control genes used in the present study were stably expressed across all samples, stability tests were performed on 7 endogenous control genes (RNU44, RNU24, RNU48, RNU6B, U6, U47 and RPL21) in 21 paired malignant and adjacent benign renal tissue samples. No significant difference in the expression between malignant and adjacent benign tissue for any of the control genes tested was identified (Table II). The NormFinder software application identified RNU48 (SV, 0.005), U47 (SV, 0.011) and the combination of RNU48 and U47 (SV, 0.006) as the most stably expressed endogenous control genes within these samples (Table IV).
Table IV.
Stability analysis of endogenous control genes with the NormFinder software.
| Control genea | SV | SE | N |
|---|---|---|---|
| RNU44 | 0.016 | 0.006 | 42 |
| RNU24 | 0.013 | 0.005 | 42 |
| RNU48b | 0.005 | 0.004 | 42 |
| RNU6B | 0.014 | 0.004 | 42 |
| U6 | 0.018 | 0.007 | 42 |
| U47b | 0.01 | 0.005 | 42 |
| RPL21 | 0.016 | 0.006 | 42 |
20 degrees of freedom and P≥0.05 for all, using Wilcoxon test.
Identified as the two most stable controls and the best combination of two genes with an SV of 0.006. SE, standard error; SV, stability value; N, number of samples.
miRNA expression profiles differentiate between malignant and adjacent benign tissues
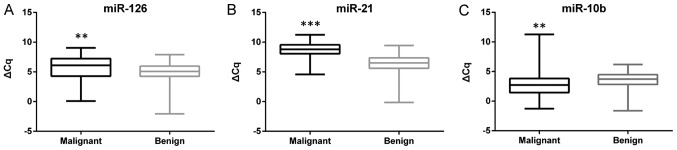
Based on the results from the investigation of endogenous control genes, the miRNA expression values were normalized to the geometric mean of RNU48 and U47. The expression levels of miR-126, miR-21 and miR-10b were investigated in 69 paired samples of malignant and adjacent benign tissues. A difference in the expression between the malignant and benign tissues was evident for all three miRNAs, with miR-126 (P=0.004) and miR-21 (P<0.001) being upregulated, and miR-10b (P<0.001) being downregulated in malignant tissues compared with adjacent benign tissue samples (Fig. 2). Heterogeneity in the up/downregulation of the miRNAs in the samples was also identified. miR-126 was upregulated in 46 of the 69 (66.7%) malignant samples with a median FC of 3.25 (range, 1.05–66.64). A more consistent upregulation was identified for miR-21, which was upregulated in 62 of the 69 (89.8%) samples, with a median FC of 5.95 (range, 1.21–305.57). miR-10b was downregulated in 48 of the 69 (69.6%) malignant samples, with a median FC of 0.37 (range, 0.01–0.98) in the downregulated samples.
Figure 2.
Box-plots of the expression of three miRNAs in malignant and adjacent benign renal tissue. The whiskers indicate minimum and maximum ΔCq values for each tissue type. (A) miR-126 is upregulated, (B) miR-21 is upregulated, and (C) miR-10b is downregulated in malignant renal tissue compared with adjacent benign tissue. **P<0.01 and ***P<0.001. miR/miRNA, microRNA.
miRNA expression profiles as prognostic biomarkers
The expression of miR-126, miR-21 and miR-10b was subsequently investigated in 116 ccRCC tumor specimens from the OKCC. miR-10b downregulation was associated with increasing AJCC stage (I+II vs. III+IV; P<0.001) and Fuhrman grade (I+II vs. III+IV; P<0.001). However, no difference was observed in the expression of miR-10b in the samples of patients presenting with metastatic disease at the time of diagnosis compared with that in patients without metastases (P=0.07). miR-126 downregulation was also more prominent with increasing AJCC stage (I+II vs. III+IV, P<0.001) and Fuhrman grade (I+II vs. III+IV, P<0.001), and a difference in expression was identified in patients with metastases at the time of diagnosis compared with that in patients without metastases (P=0.036). The expression of miR-21 was not associated with any of the clinical variables investigated in the present study, including AJCC stage, Fuhrman grade or metastatic disease at the time of diagnosis.
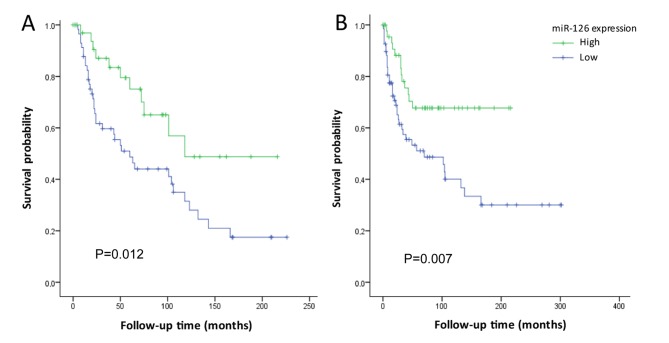
The expression levels of the investigated miRNAs were subsequently divided into low and high expression groups using ROC curves. Disease-specific survival time following diagnosis in the miR-126 low and high groups were 87.8±11.7 and 138.9±17.5 months, respectively (P=0.012; Fig. 3A). Patients with low miR-126 expression also had shorter time to recurrence of disease, compared with that of patients with high miR-126 expression, 124.9±17.9 and 155.4±13.9 months, respectively (P=0.007; Fig. 3B). No differences in the time to recurrence or disease-specific survival were identified between the low and high miR-10b or miR-21 expression groups (P>0.05).
Figure 3.
Kaplan-Meier survival plots of miR-126 expression (low vs. high). (A) Disease-specific survival and (B) recurrence of the disease, among patients diagnosed with clear cell renal cell carcinoma between 1987 and 2010 within the Örebro Kidney Cancer Cohort. miR, microRNA.
In the multivariate analysis, the patients with low miR-126 expression tended to exhibit a shorter recurrence time compared with that of patients with high expression (adjusted HR, 1.79; 95% CI, 0.91–3.52). Patients with low miR-126 expression also tended to exhibit shorter disease-specific survival time (adjusted HR, 1.94; 95% CI, 0.95–3.97) (Table V). Neither miR-10b nor miR-21 expression (low versus high) was associated with recurrence or disease-specific survival times.
Table V.
Cox regression analysis of low versus high miR-126 expression.
| HR (95% CI) | ||
|---|---|---|
| Parameter | Crude model | Adjusted modela |
| Disease recurrence | 2.32 (1.23–4.38) | 1.79 (0.91–3.52) |
| Disease-specific survival | 2.32 (1.18–4.55) | 1.94 (0.95–3.97) |
Adjusted for primary tumor diameter (<40 vs. >40 mm), American Joint Committee on Cancer stage (I+II vs. III+IV) and Fuhrman grade (I+II vs. III+IV). miR, microRNA; HR, hazard ratio; CI, confidence interval.
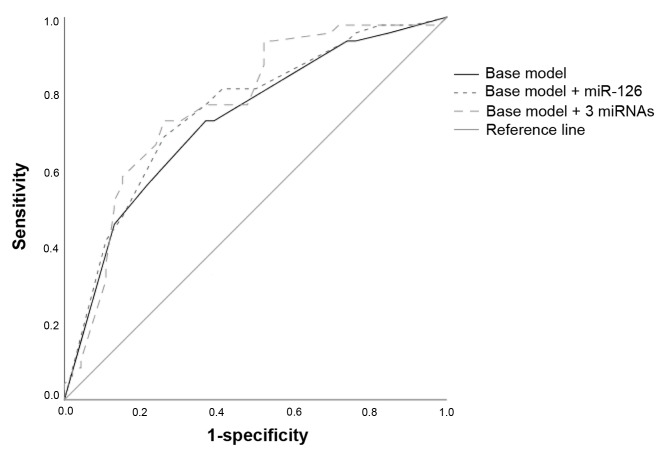
In order to explore the accuracy of miRNA expression and the presently used prognostic factors (Fuhrman grade, AJCC stage and primary tumor diameter) as classifiers of lethal renal cancer, ROC curves were employed. The baseline model, including Fuhrman grade, AJCC stage and primary tumor diameter, yielded an AUC of 0.73 (95% CI, 0.63–0.83). Adding the dichotomized (low versus high) expression of miR-126 yielded an insignificant increase of the AUC (AUC, 0.754; 95% CI, 0.66–0.85; P>0.05). Including the dichotomized expression of all three miRNAs further increased the AUC (AUC, 0.77; 95% CI, 0.67–0.87; P>0.05; Fig. 4); however, this increase was not statistically significant. No single threshold for any measurement of the combined model yielded high sensitivity combined with high specificity.
Figure 4.
Receiver operating characteristics analysis of prognostic factors for renal cell carcinoma. The base model includes American Joint Committee on Cancer stage, Fuhrman grade and primary tumor diameter (>40 vs. <40 mm). Adding dichotomized miR-126 expression (low vs. high) increased the AUC compared to using only the base model (AUC, 0.754 vs. AUC, 0.73; P>0.05). Including the expression of miR-126, miR-21 and miR-10b (high vs. low) also increased the AUC compared to using only the base model (AUC, 0.77 vs. AUC, 0.73; P>0.05). miR, microRNA; AUC, are under the curve.
Subgroup analysis
In order to investigate whether the expression of miR-126, miR-21 or miR-10b may be used as reliable biomarkers for aggressive disease in patients with a primary tumor diameter of ≤40 mm, a subgroup analysis was performed. Patients with a primary tumor diameter of >40 mm and low miR-126 expression exhibited shorter recurrence time (P=0.035) and disease-specific survival time (P=0.028), compared with patients with high expression. However, no difference in the time to disease recurrence or disease-specific mortality were identified between patients with low or high miR-126 expression who had a primary tumor diameter <40 mm. When adjusting for other clinical parameters, no association between miR-126 expression and time to recurrence or disease-specific mortality was observed in patients with a primary tumor diameter of <40 nor >40 mm.
Discussion
Previous studies have investigated the potential role of miRNAs as biomarkers for RCC (14,15,17-21,23,43-50). However, the value of using miRNAs as prognostic biomarkers for RCC is still under debate. The present study selected the miRNAs miR-126, miR-21, and miR-10b, which have previously been suggested as prognostic biomarkers for RCC, and aimed to validate their usefulness as prognostic markers in a Swedish cohort of patients with RCC. The results of the present study demonstrated that all three miRNAs were deregulated in malignant tissues when compared with adjacent benign tissues. However, the present study could only confirm a potential prognostic value for the expression of miR-126, but not for that of miR-21 or miR-10b.
In order to minimize the influence of technical variation and to increase the expression accuracy of biological data, the normalization of gene expression data is required. Due to the low number of target genes investigated, population-based normalization methods commonly used in microarray studies are not suitable for qPCR data, and normalization is instead dependent on the use of endogenous control genes as references for normal expression (51). No universal control gene suitable for all experiments exists, and it has previously been recommended to test the most suitable control gene specific for the experiment performed (52). A total of seven candidate control genes for miRNA expression studies in FFPE tissues from patients with ccRCC were investigated in the present study. The results indicate that RNU48, followed by U47, or the combination of these genes, were the most suitable for normalization of miRNA expression within the present study. Since normalization based on a single control gene could lead to erroneous results, the use of multiple control genes for normalization is preferred (52,53). Therefore, the normalization of the RT-qPCR data in the present study was performed using the geometric mean of RNU48 and U47 expression.
The deregulation of miR-126 has been identified in a variety of cancer types; however, its role in carcinogenesis remains to be elucidated. The expression of miR-126 has been observed to be downregulated in lung and esophageal cancer (27,28). On the other hand, the same miRNA has been identified to be upregulated in urothelial and gastric cancer (29,30). In RCC, the expression of miR-126 has been reported to serve a role in the molecular classification of histological subtypes (54). The results of the present study demonstrate that miR-126 was upregulated in ccRCC, and that the expression of miR-126 was decreased with increasing grade and tumor stage, which is consistent with previous findings for RCC (15,19,20,49). However, the present study observed a heterogeneity of miR-126 expression in malignant samples, being upregulated in 66.7% and downregulated in 33.3% of the investigated samples. Similar findings were observed by Vergho et al (15), who hypothesized that miR-126 was up/downregulated in different subgroups of patients with RCC. The same authors later reported a downregulation of miR-126 in ccRCC cases with tumor thrombus of the inferior vena cava, but not in cases without tumor thrombus (20). These results suggest that the downregulation of miR-126 in the primary tumor is associated with a more invasive phenotype. This hypothesis is supported by the data of others including those of the present study, demonstrating that patients with metastatic disease at the time of diagnosis have lower miR-126 expression in the primary tumor compared with patients presenting without metastases at diagnosis (15,19,20,48,49). A low miR-126 expression was also associated with early recurrence and shorter disease-specific survival time in the univariate, but not multivariate, analyses, which was also consistent with results obtained from previous studies (15,20,47). A ROC analysis indicated that the addition of miR-126 expression to currently used clinical factors could improve the prognostic accuracy. However, this small increase in AUC may not easily translate to the clinical setting.
An early event in the pathogenesis of RCC is the inactivation of the tumor suppressor Von Hippel-Lindau (VHL), which is a direct target gene of miR-21 (55). As the downregulation of VHL may be caused by the upregulation of miR-21, the deregulation of miR-21 could constitute an early event in renal carcinogenesis. Several studies have demonstrated that miR-21 is overexpressed in renal tumors compared with benign renal tissue (14,15,20,21,44,46,49,50). In the present study, miR-21 was identified to be upregulated in 89.8% of the ccRCC tissues, in line with a study performed by Zaman et al (21), who reported miR-21 upregulation in 89% of the investigated renal tumors. Previous studies have also observed an increased expression of miR-21 in increasing grades and stages of RCC (14,15), and that patients with metastatic disease at the time of diagnosis had a higher expression of miR-21 in their primary tumor (14,15,20,21,49). However, these results could not be confirmed in the present study, as no association between miR-21 expression and tumor grade, stage or patient outcome was identified.
miR-10b is considered to be associated with the metastatic behavior of tumors, partly due to its role as a suppressor of homeobox D10, a transcriptional repressor that inhibits expression of genes involved in cell migration and extracellular matrix remodeling (56). In the present study, miR-10b was observed to be downregulated in ccRCC tissues compared with adjacent benign tissue, a finding supported by previous studies (18,44,46,49). This investigation did not reveal a difference in the expression of miR-10b between patients presenting with or without metastatic disease at the time of diagnosis. However, several studies have reported miR-10b as one of the most downregulated miRNAs in metastatic tissue, with a gradual downregulation of the expression between normal tissue, primary tumor and metastatic tissue (43,48,49). Patients with a high expression of miR-10b have previously been demonstrated to have longer progression-free and disease-free survival times (18,42). This could not be confirmed in the present study, as no association between miR-10b expression and clinical outcome was evident.
The discrepancies between the results of the present study and previous studies may be due to a number of factors, including differences in patient populations, timing and method of tissue sampling, and the use of fresh-frozen or FFPE tissue. Furthermore, the analysis method (microarray, qPCR or next generation sequencing), or choice of endogenous control genes for normalization of qPCR data may also influence the results of a study. A strength of the present study is the investigation of endogenous control genes within the cohort. Numerous studies use constitutively expressed housekeeping genes for normalization, without proper validation of their presumed stability of expression, which may produce erroneous results. If miRNAs are to be used as biomarkers for RCC, a standardized analysis scheme should be optimized for all these variables.
Another strength of the present study is the well-defined cohort used, including complete follow-up of the study participants, with well defined outcome measurements from the Swedish Cause of Death register. Many of the previously performed studies have limitations due to relatively short follow-up periods of the patients. A caveat within this study is that due to the relatively small study population, there was not enough statistical power to investigate the prognostic potential of miRNA expression in subgroups of patients (e.g., metastatic versus non-metastatic disease). Therefore, additional studies are required to further explore the prognostic potential of miRNA expression in subgroups of patients with ccRCC. Furthermore, only three of almost 2,000 known miRNAs were investigated in the present study, which limits the opportunity to identify novel potential prognostic biomarkers for ccRCC.
To conclude, several prognostic factors for RCC are presently being used in a clinical setting; however, these factors lack accuracy in predicting the natural history of the disease, particularly in patients with non-metastatic disease at the time of diagnosis. Therefore, the identification of biomarkers that can aid in predicting patient outcome for patients with RCC, either alone or in combination with currently used clinical parameters are required. The results of the present study confirm that all of the investigated miRNAs are deregulated in malignant tissue compared with adjacent benign tissue. However, only one of the miRNAs investigated, miR-126, exhibited prognostic potential among the included patients.
Acknowledgements
The authors would like to acknowledge Dr Beata Grabowska (Department of Urology, University Hospital of Örebro, Sweden) who helped obtain the clinical information of the patients included in the study.
Funding
This study was supported by the Örebro county research council (grant no. OLL-430521). The funding source had no role in the design of the study, data collection, analyses, interpretation of data or manuscript writing.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JCa, SD, PS, MF, and FG designed the study. MF and FG performed all histological analyses. JCa and JCh performed the laboratory and statistical analyses. JCa, JC, SD, and PS interpreted the results. All authors helped to draft the manuscript and approved the final version.
Ethics approval and consent to participate
The present study was approved by the ethics committee of the Uppsala and Örebro region (reference number 2015/353). The ethics committee considered that no consent from patients included in the study was needed.
Patient consent for publication
The ethics committee considered that no consent from patients included in the study was needed since all data are presented on a group level and the published results cannot be traced back to an individual patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol. 2009;16:432–443. doi: 10.1111/j.1442-2042.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines of nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–411. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 4.Regional Cancer Center: Renal cancer-National quality register report of 2015. https://www.cancercentrum.se/globalassets/cancerdiagnoser/urinvagar/njurcancer/natvp_njurcancer_2017-09-13_final.pdf?v=c90d6e26233847b9801df004ceb97ae2. [Oct 13;2017 ]; (In Swedish)
- 5.Diaz de Leon A, Pedrosa I. Imaging and screening of kidney cancer. Radiol Clin North Am. 2017;55:1235–1250. doi: 10.1016/j.rcl.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azawi NH, Lund L, Fode M. Renal cell carcinomas mass of <4 cm are not always indolent. Urol Ann. 2017;9:234–238. doi: 10.4103/UA.UA_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delahunt B, Srigley JR, Montironi R, Egevad L. Advances in renal neoplasia: Recommendations from the 2012 international society of urological pathology consensus conference. Urology. 2014;83:969–974. doi: 10.1016/j.urology.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Nerich V, Hugues M, Paillard MJ, Borowski L, Nai T, Stein U, Nguyen Tan Hon T, Montcuquet P, Maurina T, Mouillet G, et al. Clinical impact of targeted therapies in patients with metastatic clear-cell renal cell carcinoma. Onco Targets Ther. 2014;7:365–374. doi: 10.2147/OTT.S56370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: A population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 11.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/S0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 13.Backes C, Meese E, Lenhof HP, Keller A. A dictionary on microRNAs and their putative target pathways. Nucleic Acids Res. 2010;38:4476–4486. doi: 10.1093/nar/gkq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faragalla H, Youssef YM, Scorilas A, Khalil B, White NM, Mejia-Guerrero S, Khella H, Jewett MA, Evans A, Lichner Z, et al. The clinical utility of miR-21 as a diagnostic and prognostic marker for renal cell carcinoma. J Mol Diagn. 2012;14:385–392. doi: 10.1016/j.jmoldx.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Vergho D, Kneitz S, Rosenwald A, Scherer C, Spahn M, Burger M, Riedmiller H, Kneitz B. Combination of expression levels of miR-21 and miR-126 is associated with cancer-specific survival in clear-cell renal cell carcinoma. BMC Cancer. 2014;14:25. doi: 10.1186/1471-2407-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritz HKM, Lindgren D, Ljungberg B, Axelson H, Dahlback B. The miR(21/10b) ratio as a prognostic marker in clear cell renal cell carcinoma. Eur J Cancer. 2014;50:1758–1765. doi: 10.1016/S0959-8049(14)50529-1. [DOI] [PubMed] [Google Scholar]
- 17.He C, Zhao X, Jiang H, Zhong Z, Xu R. Demethylation of miR-10b plays a suppressive role in ccRCC cells. Int J Clin Exp Pathol. 2015;8:10595–10604. [PMC free article] [PubMed] [Google Scholar]
- 18.Khella HWZ, Daniel N, Youssef L, Scorilas A, Nofech-Mozes R, Mirham L, Krylov SN, Liandeau E, Krizova A, Finelli A, et al. miR-10b is a prognostic marker in clear cell renal cell carcinoma. J Clin Pathol. 2017;70:854–859. doi: 10.1136/jclinpath-2017-204341. [DOI] [PubMed] [Google Scholar]
- 19.Khella HW, Scorilas A, Mozes R, Mirham L, Lianidou E, Krylov SN, Lee JY, Ordon M, Stewart R, Jewett MA, Yousef GM. Low expression of miR-126 is a prognostic marker for metastatic clear cell renal cell carcinoma. Am J Pathol. 2015;185:693–703. doi: 10.1016/j.ajpath.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Vergho DC, Kneitz S, Kalogirou C, Burger M, Krebs M, Rosenwald A, Spahn M, Löser A, Kocot A, Riedmiller H, Kneitz B. Impact of miR-21, miR-126 and miR-221 as prognostic factors of clear cell renal cell carcinoma with tumor thrombus of the inferior vena cava. PLoS One. 2014;9:e109877. doi: 10.1371/journal.pone.0109877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaman MS, Shahryari V, Deng G, Thamminana S, Saini S, Majid S, Chang I, Hirata H, Ueno K, Yamamura S, et al. Up-regulation of microRNA-21 correlates with lower kidney cancer survival. PLoS One. 2012;7:e31060. doi: 10.1371/annotation/6662579f-3a41-4bce-9298-9d15f6582ef5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang A, Liu Y, Shen Y, Xu Y, Li X. miR-21 modulates cell apoptosis by targeting multiple genes in renal cell carcinoma. Urology. 2011;78:474 e413–479. doi: 10.1016/j.urology.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 27.Liu SG, Qin XG, Zhao BS, Qi B, Yao WJ, Wang TY, Li HC, Wu XN. Differential expression of miRNAs in esophageal cancer tissue. Oncol Lett. 2013;5:1639–1642. doi: 10.3892/ol.2013.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan P, Yin Z, Li X, Wu W, Zhou B. Meta-analysis of human lung cancer microRNA expression profiling studies comparing cancer tissues with normal tissues. J Exp Clin Cancer Res. 2012;31:54. doi: 10.1186/1756-9966-31-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snowdon J, Boag S, Feilotter H, Izard J, Siemens DR. A pilot study of urinary microRNA as a biomarker for urothelial cancer. Can Urol Assoc J. 2013;7:28–32. doi: 10.5489/cuaj.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otsubo T, Akiyama Y, Hashimoto Y, Shimada S, Goto K, Yuasa Y. MicroRNA-126 inhibits SOX2 expression and contributes to gastric carcinogenesis. PLoS One. 2011;6:e16617. doi: 10.1371/journal.pone.0016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Peng XC, Zheng XL, Wang J, Qin YW. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, Bai Y, Shen Y, Yuan W, Jing Q, Qin Y. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351:157–164. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- 33.Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad A, Sethi S, Chen W, Ali-Fehmi R, Mittal S, Sarkar FH. Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am J Transl Res. 2014;6:384–390. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YY, Li L, Ye ZY, Zhao ZS, Yan ZL. MicroRNA-10b promotes migration and invasion through Hoxd10 in human gastric cancer. World J Surg Oncol. 2015;13:259. doi: 10.1186/s12957-015-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Yan W, Wang Y, Sun G, Luo H, Zhang J, Wang X, You Y, Yang Z, Liu N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9–18. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Chen D, Jin L, Liu J, Su Z, Qi Z, Shi M, Jiang Z, Ni L, Yang S, et al. Oncogenic cAMP responsive element binding protein 1 is overexpressed upon loss of tumor suppressive miR-10b-5p and miR-363-3p in renal cancer. Oncol Rep. 2016;35:1967–1978. doi: 10.3892/or.2016.4579. [DOI] [PubMed] [Google Scholar]
- 39.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Edge SB, Byrd D, Compton C, Fritz AG, Greene FL, Trotti A., III . AJCC cancer staging manual. 7th. Springer-Verlag; New York, NY: 2010. [Google Scholar]
- 42.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Heinzelmann J, Henning B, Sanjmyatav J, Posorski N, Steiner T, Wunderlich H, Gajda MR, Junker K. Specific miRNA signatures are associated with metastasis and poor prognosis in clear cell renal cell carcinoma. World J Urol. 2011;29:367–373. doi: 10.1007/s00345-010-0633-4. [DOI] [PubMed] [Google Scholar]
- 44.Juan D, Alexe G, Antes T, Liu H, Madabhushi A, Delisi C, Ganesan S, Bhanot G, Liou LS. Identification of a microRNA panel for clear-cell kidney cancer. Urology. 2010;75:835–841. doi: 10.1016/j.urology.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 45.Jung M, Mollenkopf HJ, Grimm C, Wagner I, Albrecht M, Waller T, Pilarsky C, Johannsen M, Stephan C, Lehrach H, et al. MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J Cell Mol Med. 2009;13:3918–3928. doi: 10.1111/j.1582-4934.2009.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osanto S, Qin Y, Buermans HP, Berkers J, Lerut E, Goeman JJ, van Poppel H. Genome-wide microRNA expression analysis of clear cell renal cell carcinoma by next generation deep sequencing. PLoS One. 2012;7:e38298. doi: 10.1371/journal.pone.0038298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slaby O, Redova M, Poprach A, Nekvindova J, Iliev R, Radova L, Lakomy R, Svoboda M, Vyzula R. Identification of MicroRNAs associated with early relapse after nephrectomy in renal cell carcinoma patients. Genes Chromosomes Cancer. 2012;51:707–716. doi: 10.1002/gcc.21957. [DOI] [PubMed] [Google Scholar]
- 48.White NM, Khella HW, Grigull J, Adzovic S, Youssef YM, Honey RJ, Stewart R, Pace KT, Bjarnason GA, Jewett MA, et al. miRNA profiling in metastatic renal cell carcinoma reveals a tumour-suppressor effect for miR-215. Br J Cancer. 2011;105:1741–1749. doi: 10.1038/bjc.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wotschofsky Z, Liep J, Meyer HA, Jung M, Wagner I, Disch AC, Schaser KD, Melcher I, Kilic E, Busch J, et al. Identification of metastamirs as metastasis-associated microRNAs in clear cell renal cell carcinomas. Int J Biol Sci. 2012;8:1363–1374. doi: 10.7150/ijbs.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi Z, Fu Y, Zhao S, Zhang X, Ma C. Differential expression of miRNA patterns in renal cell carcinoma and nontumorous tissues. J Cancer Res Clin Oncol. 2010;136:855–862. doi: 10.1007/s00432-009-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: Identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14:844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: Normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309:293–300. doi: 10.1016/S0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- 54.Youssef YM, White NM, Grigull J, Krizova A, Samy C, Mejia-Guerrero S, Evans A, Yousef GM. Accurate molecular classification of kidney cancer subtypes using microRNA signature. Eur Urol. 2011;59:721–730. doi: 10.1016/j.eururo.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Zhang KL, Han L, Chen LY, Shi ZD, Yang M, Ren Y, Chen LC, Zhang JX, Pu PY, Kang CS. Blockage of a miR-21/EGFR regulatory feedback loop augments anti-EGFR therapy in glioblastomas. Cancer Lett. 2014;342:139–149. doi: 10.1016/j.canlet.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 56.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.