Abstract
This study investigated optimal pathways for preeclampsia (PE) utilizing the network-based guilt by association (GBA) algorithm. The inference method consisted of four steps: preparing differentially expressed genes (DEGs) between PE patients and normal controls from gene expression data; constructing co-expression network (CEN) for DEGs utilizing Spearman's correlation coefficient (SCC) method; and predicting optimal pathways by network-based GBA algorithm of which the area under the receiver operating characteristics curve (AUROC) was gained for each pathway. There were 351 DEGs and 61,425 edges in the CEN for PE. Subsequently, 53 pathways were obtained with a good classification performance (AUROC >0.5). AUROC for 9 was >0.9 and defined as optimal pathways, especially microRNAs in cancer (AUROC=0.9966), gap junction (AUROC=0.9922), and pathogenic Escherichia coli infection (AUROC=0.9888). Nine optimal pathways were identified through comprehensive analysis of data from PE patients, which might shed new light on uncovering molecular and pathological mechanism of PE.
Keywords: preeclampsia, pathway, co-expression network, guilt by association
Introduction
With the development of high throughput technology and gene data analysis over the past decade, rapid progress has been made in discovering genetic associations of diseases (1,2). Generally, genes do not work individually, but co-operate with each other and actively participate in biological processes systemically. To the best of our knowledge, pathway analysis is the first choice for shedding light on underlying biology of genes in many diseases (3).
In the present study, using pathway annotations and gene expression data, we proposed to predict optimal pathways for PE patients by integrating the guilt by association (GBA) algorithm and network approach, termed with network-based GBA inference method. Co-expression network (CEN) of differentially expressed genes (DEGs) was constructed by the Spearman's correlation coefficient (SCC) method. Pathway data for PE were collected dependent on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and DEGs. Ultimately, the network-based GBA inference method was implemented to predict optimal pathways, of which the area under the receiver operating characteristics curve (AUROC) was obtained for each pathway. The results might provide new insights on uncovering molecular mechanism underlying PE.
Materials and methods
Preparing gene expression data and DEGs
To control the quality gene array E-GEOD-25906 from ArrayExpress database was used. This dataset includes larger number of subjects relatively less affected by other factors. The diagnostic standard with preeclampsia (PE) clinical inclusion criteria of the subjects: women were diagnosed with PE if their systolic blood pressure was at least 140 mmHg, their diastolic blood pressure was at least 90 mmHg and they had proteinuria with an estimated 300 mg of protein or greater excreted in 24 h measured directly or indirectly by protein creatinine ratio. Standard pretreatments were conducted, containing background correction (4), normalization (5), probe match (6) and summarization of expressed values (4). After converting the preprocessed data on probe level into gene symbol measure and removing the duplicated ones, we obtained a total of 19,027 genes in gene expression data.
The lmFit function implemented in Limma was utilized to perform empirical Bayes statistics and false discovery rate (FDR) calibration of the P-values on the data (7–9). Only genes which met to the thresholds of P<0.01 and |log2FoldChange| >2 were defined as DEGs across PE patients and normal controls.
Constructing CEN
In order to illustrate the relationships among DEGs of PE samples, the SCC method was utilized (10). Besides, for an interaction between gene x and y, the SCC was computed as follows:
Note that the absolute SCC value across PE samples and normal controls was denoted as its weight value. The larger of the weight value, the closer of the interaction between two genes was. Next, DEGs and weight values were input into the Cytoscape software to visualize the CEN. Consequently, a CEN with weights was obtained for subsequent analysis.
Recruiting pathway annotation data
Metabolism pathways were recruited from the KEGG pathway database (11). There are 287 pathways covering 6,894 genes in the KEGG pathway database. Subsequently, with an attempt to make these pathways more closely correlated with PE patients, all DEGs were mapped to 287 pathways, and only pathways that had intersections with DEGs were left to the remaining analyses, named as pathway annotation data.
Network-based GBA inference method
All DEGs were mapped to 287 pathways, and the pathways that had intersections with DEGs were left for pathway annotation data. In this work, the network-based GBA inference method was employed to predict pathway functions in the development of PE patients, which combined CEN with the GBA algorithm (12). Taking pathway as our source of functional annotations, a multi-functionality score (MFS) was assigned to each gene i in the CEN (13), Where Numink was the number of genes within pathway group k, whose weighting had the effect of giving contribution to a pathway group.
Where Numink was the number of genes within pathway group k, weighting exerted the action of giving contribution to a pathway group; and Numoutk was the number of genes outside pathway group in the CEN. Where Numink was the number of genes within pathway group k, whose weighting had the effect of giving contribution to a pathway group. In subsequent analysis, we computed the AUROC values for assessing the classification performances between PE samples and normal controls (14). Consequently, the AUROC for each pathway was obtained, and we selected these pathways of AUROC >0.5 as optimal pathways of PE patients.
Results
DEGs and pathway data
As described above, a total of 19,027 genes were identified in E-GEOD-25906 after standard pretreatments. Using the Limma package, we determined 351 DEGs between PE patients and normal controls which satisfied the thresholds of P<0.01 and |log2FoldChange| >2. Significantly, the top five genes in descending order of their P-values were SIAE (P=4.59E-10), TRIM24 (P=7.48E-10), PPP1R12C (P=2.90E-09), TUBA1B (P=3.96E-09), and ENG (P=4.23E-09).
The total 287 pathways (involving 6,894 genes) belonging to metabolism category were collected from the KEGG pathway database. In addition, 351 DEGs of PE patients were mapped to 287 pathways to make these pathways more correlated to PE patients, and we only took the intersections. As a result, 81 pathways including 300 DEGs were reserved as pathway annotation data for subsequent study (Table I), such as Protein processing in endoplasmic reticulum (ID: hsa04141), Ribosome (ID: hsa03010), and Purine metabolism (ID: hsa00230).
Table I.
KEGG pathway annotation data for PE.
| Pathway ID | Pathway name | DEGs |
|---|---|---|
| hsa00010 | Glycolysis/Gluconeogenesis | PGAM1; HK2 |
| hsa00230 | Purine metabolism | POLR2H; RRM1; DCK; PDE8B; HPRT1 |
| hsa00240 | Pyrimidine metabolism | POLR2H; RRM1; DCK |
| hsa00270 | Cysteine and methionine metabolism | MAT2B; GOT1 |
| hsa00350 | Tyrosine metabolism | MIF; GOT1 |
| hsa00360 | Phenylalanine metabolism | MIF; GOT1 |
| hsa00480 | Glutathione metabolism | GCLM; TXNDC12; RRM1 |
| hsa00520 | Amino sugar and nucleotide sugar metabolism | HEXB; GNPDA1; HK2 |
| hsa00531 | Glycosaminoglycan degradation | HEXB; GNS |
| hsa00564 | Glycerophospholipid metabolism | PLA2G16; MBOAT1 |
| hsa00650 | Butanoate metabolism | L2HGDH; HMGCS1 |
| hsa00900 | Terpenoid backbone biosynthesis | HMGCS1; PDSS2 |
| hsa01200 | Carbon metabolism | PGAM1; GPT2; GOT1; HK2 |
| hsa01210 | 2-Oxocarboxylic acid metabolism | GPT2; GOT1 |
| hsa01230 | Biosynthesis of amino acids | PGAM1; MAT2B; GPT2; GOT1 |
| hsa02010 | ABC transporters | ABCA7; ABCB6 |
| hsa03008 | Ribosome biogenesis in eukaryotes | WDR75; MPHOSPH10; NVL |
| hsa03010 | Ribosome | RPL7A; MRPS5; RPL18A; RPS2; MRPL14 |
| hsa03013 | RNA transport | TPR; ALYREF; UPF3B; SUMO3 |
| hsa03015 | mRNA surveillance pathway | ALYREF; UPF3B |
| hsa03018 | RNA degradation | BTG1; HSPD1; LSM7 |
| hsa03040 | Spliceosome | SYF2; ALYREF; LSM7 |
| hsa04010 | MAPK signaling pathway | MAP4K3; RRAS2; GNG12 |
| hsa04014 | Ras signaling pathway | RGL2; GNG2; RRAS2; GNG12; PLA2G16 |
| hsa04020 | Calcium signaling pathway | SLC25A5; PHKA2 |
| hsa04062 | Chemokine signaling pathway | GNG2; GNG12 |
| hsa04068 | FoxO signaling pathway | CSNK1E; GABARAPL2; PRKAB2 |
| hsa04141 | Protein processing in endoplasmic reticulum | DNAJC3; OS9; HSP90B1; SSR1; DNAJB11; UGGT2; DNAJB2; SSR4 |
| hsa04142 | Lysosome | GNPTG; CTSC; HEXB; CTSA; GNS |
| hsa04145 | Phagosome | TUBA1B; ACTG1; TUBA1A |
| hsa04151 | PI3K-Akt signaling pathway | JAK1; COL27A1; HSP90B1; GNG2; GNG12 |
| hsa04152 | AMPK signaling pathway | LEP; STRADB; ACACB; PRKAB2 |
| hsa04310 | Wnt signaling pathway | CSNK1E; FZD7 |
| hsa04360 | Axon guidance | SEMA4C; SEMA3B |
| hsa04390 | Hippo signaling pathway | SNAI2; ACTG1; CSNK1E; BMP6; FZD7 |
| hsa04510 | Focal adhesion | PPP1R12C; COL27A1; ACTG1 |
| hsa04520 | Adherens junction | SNAI2; ACTG1; PTPRB |
| hsa04530 | Tight junction | ACTG1; YBX3; RRAS2 |
| hsa04540 | Gap junction | TUBA1B; TUBA1A |
| hsa04550 | Signaling pathways regulating pluripotency of stem cells | JAK1; FZD7 |
| hsa04610 | Complement and coagulation cascades | F13A1; CFB; TFPI |
| hsa04611 | Platelet activation | COL27A1; ACTG1 |
| hsa04614 | Renin-angiotensin system | MME; CTSA; ACE2 |
| hsa04630 | Jak-STAT signaling pathway | JAK1; LEP |
| hsa04640 | Hematopoietic cell lineage | MME; CD24 |
| hsa04710 | Circadian rhythm | CSNK1E; CLOCK; PRKAB2 |
| hsa04713 | Circadian entrainment | GNG2; GNG12 |
| hsa04723 | Retrograde endocannabinoid signaling | GNG2; GNG12 |
| hsa04724 | Glutamatergic synapse | GNG2; GNG12 |
| hsa04725 | Cholinergic synapse | GNG2; GNG12 |
| hsa04726 | Serotonergic synapse | GNG2; GNG12 |
| hsa04727 | GABAergic synapse | GABARAPL2; GNG2; GNG12 |
| hsa04728 | Dopaminergic synapse | GNG2; CLOCK; GNG12 |
| hsa04810 | Regulation of actin cytoskeleton | PPP1R12C; ACTG1; RRAS2; GNG12 |
| hsa04910 | Insulin signaling pathway | PHKA2; ACACB; HK2; PRKAB2 |
| hsa04913 | Ovarian steroidogenesis | BMP6; HSD17B2 |
| hsa04919 | Thyroid hormone signaling pathway | ACTG1; NCOA2; MED27; RCAN1 |
| hsa04920 | Adipocytokine signaling pathway | LEP; ACACB; PRKAB2 |
| hsa04921 | Oxytocin signaling pathway | PPP1R12C; ACTG1; RCAN1; PRKAB2 |
| hsa04922 | Glucagon signaling pathway | PGAM1; PHKA2; ACACB; PRKAB2 |
| hsa04932 | Non-alcoholic fatty liver disease (NAFLD) | CEBPA; NDUFA12; LEP; PRKAB2 |
| hsa04974 | Protein digestion and absorption | COL27A1; MME; ACE2; KCNN4; COL15A1 |
| hsa05010 | Alzheimer's disease | NDUFA12; MME |
| hsa05012 | Parkinson's disease | NDUFA12; SLC25A5; UBB |
| hsa05016 | Huntington's disease | NDUFA12; SLC25A5; POLR2H |
| hsa05032 | Morphine addiction | GNG2; GNG12; PDE8B |
| hsa05034 | Alcoholism | H2AFY; HIST2H2AC; GNG2; GNG12 |
| hsa05130 | Pathogenic Escherichia coli infection | TUBA1B; ACTG1; TUBA1A |
| hsa05152 | Tuberculosis | JAK1; HSPD1; BCL10 |
| hsa05161 | Hepatitis B | JAK1; LAMTOR5 |
| hsa05164 | Influenza A | DNAJC3; JAK1; ACTG1; KPNA2 |
| hsa05166 | HTLV–I infection | JAK1; SLC25A5; RANBP1; RRAS2; FZD7 |
| hsa05168 | Herpes simplex infection | JAK1; ALYREF; CLOCK |
| hsa05169 | Epstein-Barr virus infection | JAK1; VIM; POLR2H; AKAP8L |
| hsa05200 | Pathways in cancer | CEBPA; TPR; JAK1; HSP90B1; GNG2; GNG12; FZD7 |
| hsa05203 | Viral carcinogenesis | JAK1; RANBP1 |
| hsa05205 | Proteoglycans in cancer | PPP1R12C; ACTG1; RRAS2; FZD7 |
| hsa05206 | MicroRNAs in cancer | FSCN1; VIM |
| hsa05230 | Central carbon metabolism in cancer | PGAM1; HK2 |
| hsa05322 | Systemic lupus erythematosus | H2AFY; HIST2H2AC |
| hsa05410 | Hypertrophic cardiomyopathy (HCM) | ACTG1; PRKAB2 |
CEN
To describe relationships among DEGs clearly, the SCC method was implemented to weight the strength between a pair of genes, and those weighted interactions were input into Cytoscape and visualized as the CEN for PE patients. A total of 351 nodes and 61,425 edges were deposited on the CEN, which suggested that all DEGs were mapped to the network. The edge between KPNA2 and MAT2B (weight=0.9986), FSTL3 and SKIDA1 (weight=0.9984), SSNA1 and PFDN6 (weight=0.9984) had higher weights than the other interactions. Noteworthy, a good liner correlation was uncovered among weights. Additionally, topological centrality analysis on nodes in the CEN of PE was conducted by summing up the nodes it connected directly. We found that the degree distribution for six nodes was not <200, including RDH13 (degree=202), SELENOS (degree=201), PAPPA2 (degree=201), RASSF7 (degree=201), DNAJC3 (degree=200) and PPP1R12C (degree=200).
Optimal pathways
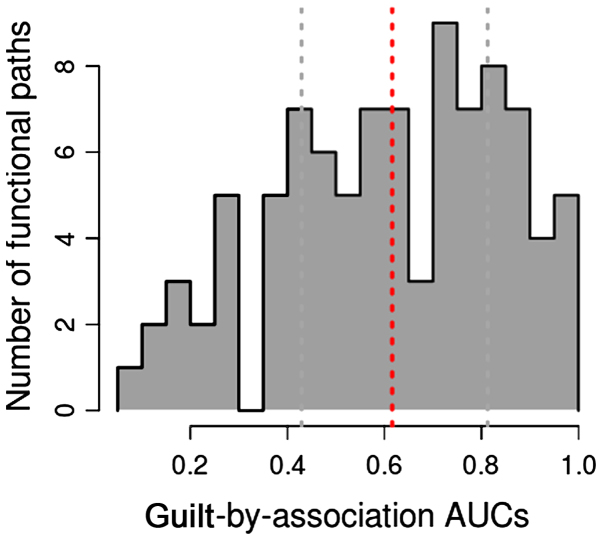
Utilizing pathway annotation data, we identified optimal pathways through gene function inference dependent on the network-based GBA method. During this process, an MFS was produced for each pathway. Importantly, we carried out 3-fold cross-validation on MFS to calculate AUROC for pathways. The AUROC distribution among GO terms is illustrated in Fig. 1. We found that the AUROC for large amount of pathways distributed to the section of 0.4–0.6 and 0.75–0.9. Accordingly, 53 pathways had AUROC >0.5. Furthermore, 9 of 53 pathways with AUROC >0.9 were denoted as optimal pathways, specifically microRNAs in cancer (AUROC=0.9966), gap junction (AUROC=0.9922), pathogenic Escherichia coli infection (AUROC=0.9888), phagosome (AUROC=0.9881), ovarian steroidogenesis (AUROC=0.9821), viral carcinogenesis (AUROC=0.9642), MAPK signaling pathway (AUROC=0.9473), tuberculosis (AUROC=0.9428), and tight junction (AUROC=0.9136).
Figure 1.
The AUROC distribution among GO terms. AUROC for large amount of pathways distributed to the section of 0.4–0.6 and 0.75–0.9.
Discussion
Our results showed that 53 pathways were provided with a good classification performance with AUROC >0.5, 9 of AUROC with >0.9 were defined as optimal pathways, which included microRNAs in cancer, gap junction, pathogenic Escherichia coli infection, phagosome, ovarian steroidogenesis, viral carcinogenesis, MAPK signaling pathway, tuberculosis, and tight junction.
We confirmed that the optimal pathway microRNAs in cancer play a significant role in tumor issues, but the functions for this pathway in PE patients has been reported (15). Furthermore, Bird et al focused on pregnancy endothelial adaptive failure in PE (16). Gap junction implicated modulatory intercellular communication during gestation in accordance with regulation of vascular tone (17). Hence gap junction was closely related to PE patients. Our results showed that 53 pathways had a good classification performance with AUROC >0.5, 9 of AUROC were >0.9 and defined as optimal pathways, which included microRNAs in cancer, gap junction, pathogenic Escherichia coli infection, phagosome, ovarian steroidogenesis, viral carcinogenesis, MAPK signaling pathway, tuberculosis, and tight junction.
BMP6 and HSD17B2 were enriched in ovarian steroidogenesis pathway as one of optimal pathways. From previous studies, hydroxysteroid (17-β) dehydrogenase 1, encoded by HSD17B1, was found to be significantly decreased in PE patients and was identified to be an independent risk factor for PE (18,19), thus, it will be proposed as a potential prognostic factor for PE. Additionally, MAPK signaling pathway has been paid increasing attention by demonstrating it to participate in PE progression as a crucial pathogenesis of PE (20–22).
In conclusion, 9 optimal pathways were disclosed for PE patients by network-based GBA algorithm, which might shed new lights on unraveling the molecular and pathological mechanism of PE. However, validations of these pathways are still not covered, and future studies should be focused on this aspect.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YR, YL and YPL conceived the study, analyzed the data and drafted the manuscript. JZ, XW and WZ performed the experiments, analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31:33–46. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.Glazko GV, Emmert-Streib F. Unite and conquer: Univariate and multivariate approaches for finding differentially expressed gene sets. Bioinformatics. 2009;25:2348–2354. doi: 10.1093/bioinformatics/btp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15–e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Miller JA, Menon V, Goldy J, Kaykas A, Lee CK, Smith KA, Shen EH, Phillips JW, Lein ES, Hawrylycz MJ. Improving reliability and absolute quantification of human brain microarray data by filtering and scaling probes using RNA-Seq. BMC Genomics. 2014;15:154. doi: 10.1186/1471-2164-15-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta S, Satten GA, Benos DJ, Xia J, Heslin MJ, Datta S. An empirical bayes adjustment to increase the sensitivity of detecting differentially expressed genes in microarray experiments. Bioinformatics. 2004;20:235–242. doi: 10.1093/bioinformatics/btg396. [DOI] [PubMed] [Google Scholar]
- 9.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 10.Szmidt E, Kacprzyk J. IEEE International Conference on Intelligent Systems, Is 2010, 7–9 July 2010. University of Westminster; London, UK: 2010. The Spearman rank correlation coefficient between intuitionistic fuzzy sets; pp. 276–280. [Google Scholar]
- 11.Qiu Y-Q. KEGG Pathway Database. In: Dubitzky W, Wolkenhauer O, Cho K-H, Yokota H, editors. Encyclopedia of Systems Biology. Springer New York; New York, NY: 2013. pp. 1068–1069. [DOI] [Google Scholar]
- 12.Mostafavi S, Morris Q. Fast integration of heterogeneous data sources for predicting gene function with limited annotation. Bioinformatics. 2010;26:1759–1765. doi: 10.1093/bioinformatics/btq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillis J, Pavlidis P. The impact of multifunctional genes on ‘guilt by association’ analysis. PLoS One. 2011;6:e17258. doi: 10.1371/journal.pone.0017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Ling CX. Using AUC and accuracy in evaluating learning algorithms. IEEE Trans Knowl Data Eng. 2005;17:299–310. doi: 10.1109/TKDE.2005.50. [DOI] [Google Scholar]
- 15.Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Bird IM, Boeldt DS, Krupp J, Grummer MA, Yi FX, Magness RR. Pregnancy, programming and preeclampsia: Gap junctions at the nexus of pregnancy-induced adaptation of endothelial function and endothelial adaptive failure in PE. Curr Vasc Pharmacol. 2013;11:712–729. doi: 10.2174/1570161111311050009. [DOI] [PubMed] [Google Scholar]
- 17.Ampey BC, Morschauser TJ, Lampe PD, Magness RR. Gap junction regulation of vascular tone: Implications of modulatory intercellular communication during gestation. Adv Exp Med Biol. 2014;814:117–132. doi: 10.1007/978-1-4939-1031-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T, Takizawa T, Hirashima C, Takahashi K, Migita M, et al. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: A novel marker for predicting preeclampsia. Hypertension. 2012;59:265–273. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 19.Ohkuchi A, Ishibashi O, Hirashima C, Takahashi K, Matsubara S, Takizawa T, Suzuki M. Plasma level of hydroxysteroid (17-β) dehydrogenase 1 in the second trimester is an independent risk factor for predicting preeclampsia after adjusting for the effects of mean blood pressure, bilateral notching and plasma level of soluble fms-like tyrosine kinase 1/placental growth factor ratio. Hypertens Res. 2012;35:1152–1158. doi: 10.1038/hr.2012.109. [DOI] [PubMed] [Google Scholar]
- 20.Li FH, Han N, Wang Y, Xu Q. Gadd45a knockdown alleviates oxidative stress through suppressing the p38 MAPK signaling pathway in the pathogenesis of preeclampsia. Placenta. 2018;65:20–28. doi: 10.1016/j.placenta.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Zhao ZM. LncRNA HOXD-AS1 promotes preeclampsia progression via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22:8561–8568. doi: 10.26355/eurrev_201812_16618. [DOI] [PubMed] [Google Scholar]
- 22.D'Oria R, Laviola L, Giorgino F, Unfer V, Bettocchi S, Scioscia M. PKB/Akt and MAPK/ERK phosphorylation is highly induced by inositols: Novel potential insights in endothelial dysfunction in preeclampsia. Pregnancy Hypertens. 2017;10:107–112. doi: 10.1016/j.preghy.2017.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.