Abstract
Electrospinning using biocompatible polymer scaffolds, seeded with or without stem cells, is considered a promising technique for producing fibrous scaffolds with therapeutic possibilities for ischemic heart disease. However, no optimal scaffolds for treating ischemic heart disease have been identified thus far. In the present study, it was evaluated whether electrospun silk fibroin (SF)-blended poly(L-lactic acid-co-ε-caprolactone) [P(LLA-CL)] scaffolds that were seeded with cluster of differentiation 117 (c-kit)+ bone marrow (BM) cells may serve a protective role in cardiac remodeling following myocardial infarction (MI). Mechanical characteristics and cytocompatibility were compared between SF/P(LLA-CL) and P(LLA-CL) electrospun nanofibrous scaffolds in vitro. It was observed that MI led to a significant increase of the c-kit+ BM cell subpopulation in mice. Magnetic activated cell sorting was performed to harvest the c-kit+ cell population from the BM of mice following MI. c-kit+ BM cells were seeded on SF/P(LLA-CL) and P(LLA-CL) electrospun nanofibrous scaffolds. Results indicated that SF/P(LLA-CL) electrospun nanofibrous scaffolds were superior to P(LLA-CL) electrospun nanofibrous scaffolds in improving c-kit+ BM cell proliferation. Additionally, compared with pure SF/P(LLA-CL) electrospun nanofibrous scaffolds, SF/P(LLA-CL) scaffolds seeded with c-kit+ BM cells resulted in lower levels of MI markers and reduced infarct size, leading to greater global heart function improvement in vivo. The findings of the present study indicated that SF/P(LLA-CL) electrospun nanofibrous scaffolds seeded with c-kit+ BM cells exert a protective effect against MI and may be a promising approach for cardiac regeneration after ischemic heart disease.
Keywords: electrospinning, silk fibroin/poly(L-lactic acid-co-ε-caprolactone) scaffold, myocardial infarction, c-kit+ bone marrow cells
Introduction
Myocardial infarction (MI), commonly known as heart attack, can result in the loss of cardiomyocytes, cardiac dysfunction and heart failure when the unimpaired heart tissue is unable to compensate for the reduced cardiac output (1,2). For this reason, MI is a considered a leading cause of morbidity worldwide (3). To date, cell transplantation-based regenerative therapy, including bone marrow (BM)-derived populations, provide a promising approach for MI (4). Cluster of differentiation (CD)117 (c-kit), a receptor tyrosine kinase, is an important cell surface marker used to identify certain types of hematopoietic progenitors in the BM. Multipotent stem cells derived from the BM or myocardium can express c-kit (5). Previous findings indicate that transplantation of c-kit+ BM precursor cells may lead to recovery of cardiac function following MI. Although it is clear that c-kit+ stem cells can exert a beneficial effect on cardiac repair, the underlying mechanism remains unknown. Notably, previous studies have suggested that the low rates of cell engraftment and poor cell survival may hinder BM-derived stem cell therapy from restoring heart function (6,7).
To overcome the problem of poor cell survival, cells should be subjected to an environment similar to the cardiac extracellular matrix (ECM), which may improve their survival and growth (8,9). To date, biomaterial scaffolds have provided a promising approach for cell retention and survival. Cardiac tissue scaffolds are designed primarily on natural and synthetic biomaterials (10). Electrospinning has become a popular method for tissue engineering due to its ability to provide a micron-range network and its ability to mimic the in vivo ECM structure (11–14). Electrospun poly(L-lactic acid-co-ε-caprolactone) [P(LLA-CL)] scaffolds are a copolymer of L-lactic acid and caprolactone that support the viability and growth of a number of cell types (15). However, multiple studies have also indicated that P(LLA-CL) scaffolds have inadequate cell affinity due to the absence of recognition sites for cell adhesion (16–18). Silk fibroin (SF) has been widely used in tissue and cell engineering, including in the construction of artificial blood vessels, bone and nerves, due to its unique advantages (good biocompatibility, good oxygen permeability and controllable morphology) (19,20). Thus, the blending bioactive SF with the beneficial properties of P(LLA-CL) to produce a new material may support c-kit+ BM stem cell growth.
Nanofibrous scaffolds in tissue engineering have attracted interest, predominantly due to their structural similarity to the natural ECM. Previous studies reported that blending P(LLA-CL) and SF created electrospun fibrous structures, which resulted in scaffolds with good mechanical and biological properties (21,22). However, the function of electrospun SF/P(LLA-CL) nanofibrous scaffolds in the protection of impaired heart tissue remains unknown.
In the present study, the contact angle and physical property of SF/P(LLA-CL) and P(LLA-CL) scaffolds were compared for the purpose of collecting accurate data using controlled concentrations of SF and P(LLA-CL). The present study also assisted to elucidate the accuracy of previously published studies (22,23). The present study explored the proliferating potential of c-kit+ BM cells seeded on SF/P(LLA-CL) and P(LLA-CL) electrospun nanofibrous scaffolds in vitro. The aim of the present study was to clarify if electrospun SF/P(LLA-CL) nanofibrous scaffolds seeded with c-kit+ BM cells were superior to pure SF/P(LLA-CL) electrospun nanofibrous scaffolds with regard to their effectiveness on cardiac repair following MI in vivo. To the best of our knowledge, the present study is the first to demonstrate the effects of the combination of SF and P(LLA-CL) created electrospun nanofibrous scaffolds with cardiac repair function compared with P(LLA-CL) scaffolds. The present findings may be useful for providing a novel treatment of MI.
Materials and methods
Preparation of electrospun scaffolds
The electrospun scaffolds used in the present study were prepared according to procedures described previously (21). Raw silk (Zhejiang Jiaxin Silk Co., Ltd., Zhejiang, China) was degummed three times using a 0.5% (w/w) Na2CO3 (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) solution at 100°C for 30 min and subsequently washed three times. Degummed silk was dissolved in a ternary solvent system of CaCl2/H2O/ethanol (mole ratio: 1/8/2) for 1 h at 70°C. The SF solution was dialyzed using a cellulose tubular membrane (250–257 µm, Sigma-Aldrich; Merck KGaA) in distilled water for 3 days at room temperature. The SF solution was subsequently filtered and lyophilized to obtain regenerated SF sponges. SF blended with P(LLA-CL) (25:75) and pure P(LLA-CL) were dissolved in 1,1,1,3,3,3-hexafluoro-2-opropanol solvents (Daikin Industries Ltd., Osaka, Japan). Subsequently, the blending solutions were stirred at room temperature for 6 h and the prepared solutions were loaded into a 2-ml syringe with a blunt-end needle. The syringe was loaded in a syringe pump (Cole-Parmer Instrument, LLC, Vernon Hills, IL USA). The flow rate was set to 1 ml/h. Electrospinning was performed using a high-voltage power supply (BGG6-358; Bmei Co., Ltd., Beijing, China) and a voltage of 12 kV was applied across the needle and ground collector. The distance between the tip of the needle and the collector was 11 cm. The obtained material films were vacuum-dried for 24 h.
Scanning electron microscope (SEM) analysis
The morphology of the resultant scaffolds was observed with a SEM (Jeol, Ltd., Tokyo, Japan). A total of 100 random fibres observed on SEM images were selected and the mean diameters were subsequently measured using ImageJ software, version 1.8.0 (National Institutes of Health, Bethesda, MD, USA).
Water contact angle determination
The contact angle was measured using a video contact angle analyzer (DataPhysics Instruments GmbH, Filderstadt, Germany). Distilled water was used as the reference liquid, which was dropped randomly in different places of each sample. The contact angle was measured three times from different positions and the mean value was calculated.
Determination of mechanical properties
Mechanical properties were examined using a materials testing machine (H5K-S; Tinius Olsen TMC, Horsham, PA, USA). The elongation speed was set at 10 mm/min. At least 6 rectangular-shaped samples (10×10×0.10 mm) were stretched. Each sample was measured six times.
MI mouse model construction
A total of 36 C57BL/6 mice (male; weight range, 20–25 g) were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (SLACCAS, Shanghai, China). All animals were housed under a 12 h light/dark cycle, with a controlled temperature range (20-24°C) and relative humidity range (40-70%). All animals received free access to food and water. Mice were randomly divided into four groups (each, n= 9) as follows: i) A sham group; ii) an SF/P(LLA-CL) + c-kit+ group; iii) a pure SF/P(LLA-CL) group; and iv) an MI group. The study was approved by the ethics committee of Renji Hospital (Shanghai, China). To induce MI, mice were anesthetized by inhalation of 3% isoflurane in a chamber. A rodent ventilator (model 683, Harvard Apparatus, Inc., Holliston, MA, USA) was used with 1.5% isoflurane during the surgical procedure. Depth of anesthesia was assessed as adequate in the absence of either motor or autonomic responses to nose pinching. Palpebral reflexes were also monitored continuously and the disappearance of palpebral reflexes was considered to mean that animals were fully anesthetized. The mice were kept warm using heat lamps and heating pads. Rectal temperature was monitored and maintained between 36.5 and 37.5°C. The chest was opened with a horizontal incision through the muscle, between the ribs at the third intercostal space, and ischemia was achieved by ligating the anterior descending branch of the left coronary artery (LAD) using a 7-0 nylon suture. Subsequently, the incisions were closed and the wounds were cleaned and disinfected. Sham-operated mice underwent the same procedure, with the exception of the LAD ligation. Clinical observations (respiration, heartbeat and neural reflex) were performed daily in all mice following the operation.
Cell isolation and flow cytometry analysis
BM mononuclear cells were isolated from the BM tissue of mice by density gradient centrifugation 7 days following MI induction. In brief, femurs and tibias were harvested from C57BL/6 mice. The BM was collected by repeated washing of the BM cavity with Hank's balanced salt solution (Biowest, Nuaillé, France), and loaded on Ficoll solution (Dakewe Biotech Co., Ltd., Shenzhen, China). For gradient centrifugation, cells were centrifuged at 400 × g for 20 min at room temperature. Subsequently, the cell layer was isolated and combined with three times the volume of Hanks solution (Biowest, Nuaillé, France). The mixture was centrifuged at 300 × g for 5 min at room temperature and cells were resuspended in PBS containing 1% bovine serum albumin (Biowest, Nuaillé, France). Subsequently, anti-c-kit, anti-sca-1 and anti-CD34 antibodies (BD Biosciences, San Jose, CA, USA) were diluted 1:100 in PBS/1% bovine serum albumin (Biowest). Samples were incubated 30 min in the dark on ice. Finally, cells were washed with PBS and subjected to a BD Accuri C6 flow cytometer. Fluorescence was analyzed with CFlow Plus software, version 1.0.227.4 (BD Biosciences).
c-kit+ BM cell culture and the cell proliferation assay
c-kit+ cells were positively selected using a c-kit+ magnetic activated cell sorting kit (Miltenyi Biotec GmbH, Gladbach, Germany). SF/P(LLA-CL) and P(LLA-CL) electrospun scaffolds were added to the base of 96-well plates. Subsequently, c-kit+ BM cells were cultured in the absence of scaffolds or on the SF/P(LLA-CL) or P(LLA-CL) electrospun nanofibrous scaffolds, respectively, at concentrations of 1×105 cells/well. Cells were cultured in Iscove's modified Dulbecco's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Biowest).
CellTiter 96® AQueous One Solution reagent (Promega Corporation, Madison, WI, USA) was added to the wells according to the manufacturer's instructions. The wells were incubated at 37°C for 4 h for 0, 1, 3, 5 and 7 days. The absorbance (OD 490 nm) was measured using a Biotek Synergy™ HT Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
Electrospun scaffold transplantation
The hearts of mice were exposed for the MI surgery as described above. Following exposure of the hearts, a 7-0 prolene was first sutured in advance in the cardiac scaffolds, then, it was utilized to ligate the left descending artery. Hereafter, the patches were attached to the surface of the left ventricle. The incision was closed and the wound was cleaned and disinfected. The mice were divided into four groups (each, n=9) as follows: i) Sham group; ii) SF/P(LLA-CL) + c-kit+ group; iii) pure SF/P(LLA-CL) group; and iv) MI group.
Survival analysis
Survival analysis was performed for 30 days following transplantation of electrospun scaffolds. The mice exhibited no cardiorespiratory symptoms until they succumbed to MI. Any mice that did not succumb to MI by the end of the experiment were euthanized. Mice were euthanized with carbon dioxide and once unconscious, were subjected to cervical dislocation. Of note, to avoid causing pain, the flow rate of carbon dioxide was displaced at 10–30% of the chamber volume per minute.
Myocardial damage assessment
Myocardial damage was evaluated by measuring the levels of creatine kinase, MB Form (CK-MB) and lactate dehydrogenase (LDH) in the blood. A total of 3 days post-MI induction, blood was collected from the carotid artery and incubated at room temperature for 30 min. Subsequently, the serum was separated by centrifugation (400 × g for 20 min at room temperature) and stored at −70°C. CK-MB (H-197; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and LDH (A020-2; Nanjing Jiancheng Bioengineering Institute) levels were measured using commercial kits according to the manufacturer's instructions.
Echocardiographic studies
Heart function was assessed by transthoracic echocardiography using a Vevo 2100 high-resolution imaging system 7 and 28 days following transplantation. The mice were anesthetized with 3% isoflurane inhalation, and maintained with 1.5% isoflurane during examinations. Mice were placed in the supine position. The fur on the chest was removed using a depilatory cream. Two-dimensional echocardiographic images and M-mode traces were captured from the parasternal short-axis view at the level of the papillary muscles. To evaluate ventricular volume changes, left ventricular end-diastolic volume (EDV) and left ventricular end-systolic volume (ESV) were measured. Furthermore, the ejection fraction (EF) was calculated as an indicator of systolic function.
Infarct size measurement
Infarct size was determined 28 days following transplantation. Mice were anesthetised with carbon dioxide. When the mice were insensible, they were sacrificed using cervical dislocation. Once each assessed mouse was euthanized, the hearts were carefully removed, sectioned into ~2-mm transverse sections and placed in 1% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. The infarcted areas of each slice were measured using ImageJ software, version 1.8.0 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Results were presented as the mean ± standard error of the mean. Each experiment was repeated at least three times. Differences between two groups were analyzed using the Student's t-test. Survival differences were compared using the Kaplan-Meier curve with log-rank analysis and multiple comparisons between groups were performed using Bonferroni's method. One-way analysis of variance with Tukey's post-test was used for multiple comparisons. P<0.05 was used to indicate a statistically significant difference. SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Characteristics of SF/P(LLA-CL) and P(LLA-CL) electrospun scaffolds
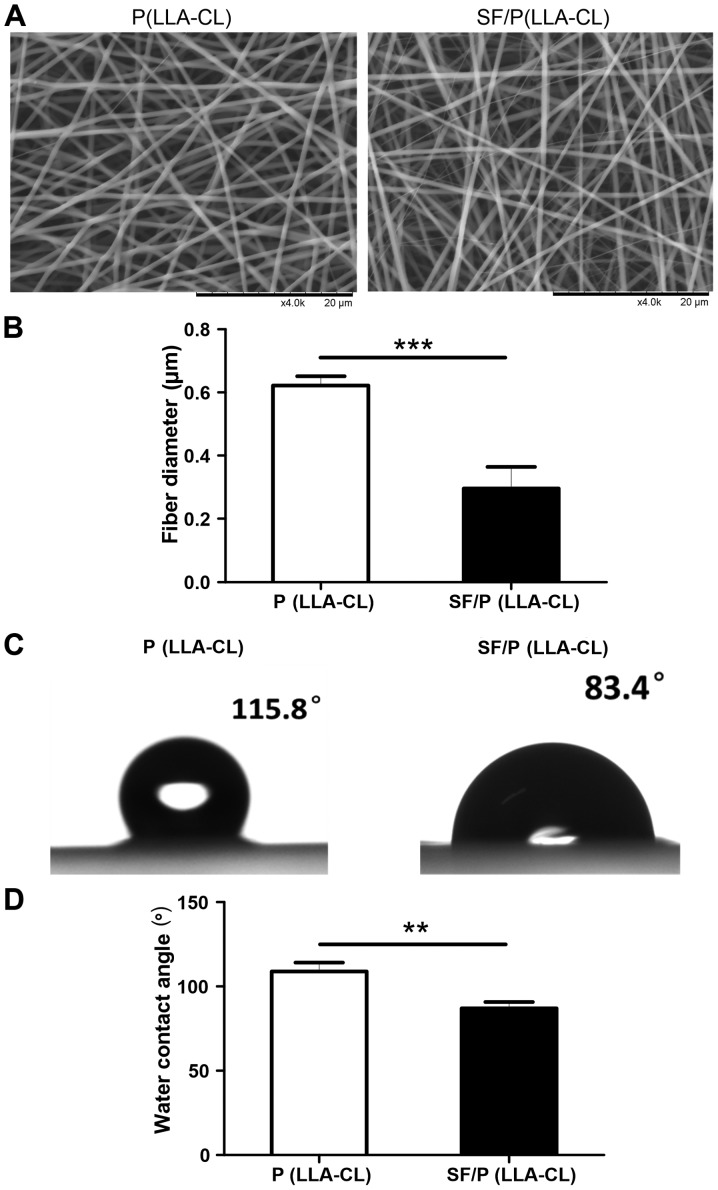
All scaffolds used in the present study were 60–100 µm in thickness and exhibited uniform interconnected pore structure. Furthermore, SEM observation demonstrated that mixing SF with P(LLA-CL) changed the morphology of the electrospun scaffolds. Notably, the diameter of fibers in P(LLA-CL) electrospun scaffolds (0.6217±0.03007 µm) were significantly thicker when compared with those blended with SF (0.2966±0.06811 µm; P<0.001; Fig. 1A and B). To evaluate the surface properties between the two fibrous scaffolds, the wettability was measured using water contact angle analysis (Fig. 1C). The results indicated that the addition of SF enhanced the hydrophilicity of the electrospun sheets when compared with the pure P(LLA-CL) scaffold alone. As indicated in Fig. 1D, the static water contact angle was significantly decreased with the addition of SF content [108.8±5.296° of the P(LLA-CL) group compared with 86.90±3.955° of the SF/P(LLA-CL) group; P<0.01].
Figure 1.
Characteristics of P(LLA-CL) and SF/P(LLA-CL) electrospun nanofibrous scaffolds. (A) Scanning electron microscopic analysis of P(LLA-CL) and SF/P(LLA-CL) electrospun nanofibrous scaffolds (scale bar, 20 µm). (B) Statistical analysis of the fiber diameters of P(LLA-CL) and SF/P(LLA-CL) electrospun nanofibrous scaffolds. (C) Images of contact angle. (D) Statistical analysis of static water contact angle of P(LLA-CL) and SF/P(LLA-CL) electrospun nanofibrous scaffolds. **P<0.01 and ***P<0.001 as indicated. SF, silk fibroin; P(LLA-CL), poly(L-lactic acid-co-ε-caprolactone).
Mechanical properties of electrospun scaffolds
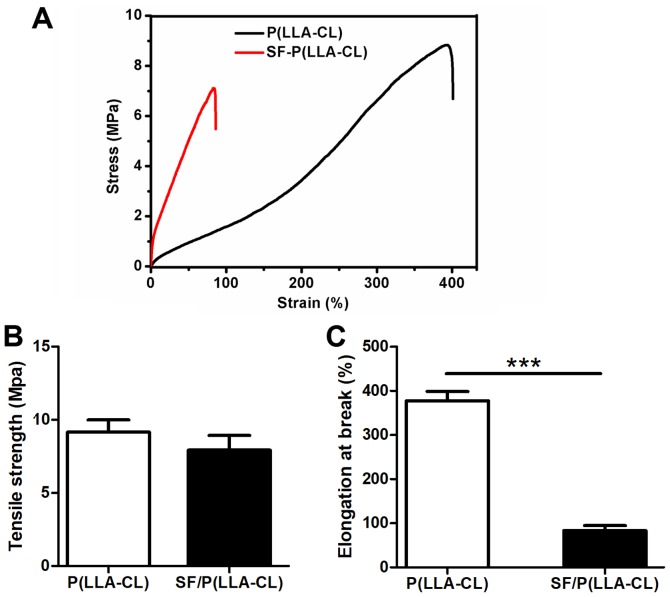
The mechanical properties of P(LLA-CL) and SF/P(LLA-CL) scaffolds were measured using typical tensile stress-strain curves (Fig. 2). The results indicated that the P(LLA-CL) scaffolds transformed from flexible to brittle when blended with SF. However, no significant differences were observed between P(LLA-CL) and SF/P(LLA-CL) electrospun scaffolds, even though tensile strength values of P(LLA-CL) electrospun scaffolds were increased (9.17±0.82 MPa) compared with SF/P(LLA-CL) electrospun scaffolds (7.93±1.00 MPa). In addition, the elongation at break of P(LLA-CL) was significantly longer (377.90±21.19%) compared with SF/P(LLA-CL) (83.15±11.47%; P<0.001).
Figure 2.
Physical property of SF/P(LLA-CL) and P(LLA-CL) scaffolds. (A) Tensile stress vs. strain curve of pure P(LLA-CL) and SF/P(LLA-CL) (25:75 ratio). (B) Tensile strength of pure P(LLA-CL) and SF/P(LLA-CL) (25:75 ratio). (C) Elongation at break of pure P(LLA-CL) and SF/P(LLA-CL) (25:75 ratio). ***P<0.001 as indicated. SF, silk fibroin; P(LLA-CL), poly(L-lactic acid-co-ε-caprolactone).
MI increases the expression of c-kit+ cells in the BM
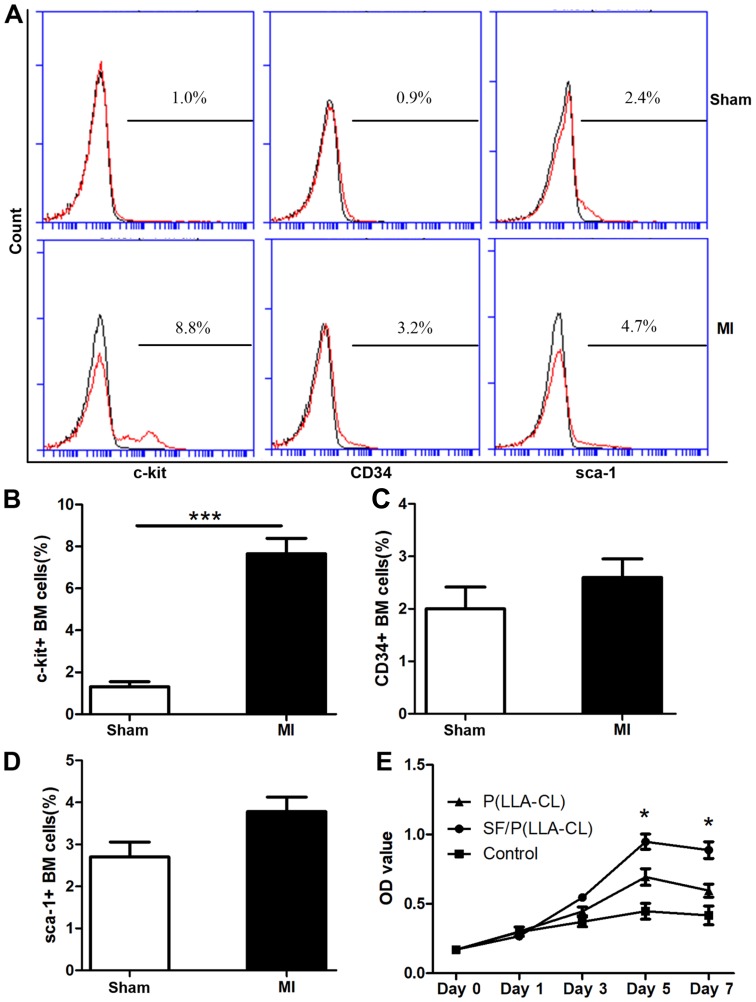
The stem cell surface markers c-kit, CD34 and sca-1 were evaluated in the present study (Fig. 3A). Notably, the c-kit expression levels in BM mononuclear cells were significantly upregulated in response to acute ischemic injury (7.53±0.69%) compared with the sham group (1.65±0.37%; P<0.001; Fig. 3B). Conversely, the CD34 and sca-1 expression levels were not significantly altered (Fig. 3C and D).
Figure 3.
Flow cytometric analysis and proliferation assay. (A) Flow cytometric analysis plots of c-kit+, CD34+ and sca-1+ cells isolated from MI and sham mice. (B-D) Expression analysis of c-kit+, CD34+ and sca-1+ in BM cells. ***P<0.001 as indicated (n=6 for each group). (E) CellTiter 96® AQueous One Solution reagent was used to assess c-kit+ BM cells seeded on P(LLA-CL) or SF/P(LLA-CL) or those cultured without scaffolds at indicated time points. *P<0.05 (n=6 for each group). SF, silk fibroin; P(LLA-CL), poly(L-lactic acid-co-ε-caprolactone); MI, myocardial infarction; BM, bone marrow; CD, cluster of differentiation; CD117, c-kit; sca-1, stem cell antigen-1; OD, optical density.
Effect of SF/P(LLA-CL) nanofibrous scaffolds on the proliferation of c-kit+ BM cells
To evaluate the effect of P(LLA-CL) and SF/P(LLA-CL) electrospun scaffolds on cell proliferation, c-kit+ BM cells were seeded on different scaffolds and the cell proliferation ability was measured using the CellTiter 96® AQueous One Solution reagent on day 0, 1, 3, 5 and 7. The SF/P(LLA-CL) electrospun scaffolds were superior in promoting cell proliferation compared with P(LLA-CL) electrospun scaffolds (P<0.05; day 5 and 7; Fig. 3E). Thus, the SF/P(LLA-CL) electrospun scaffolds were more suitable for promoting proliferation in c-kit+ BM cells.
SF/P(LLA-CL) electrospun scaffolds seeded with c-kit+ BM cells attenuate myocardial damage and improve survival post-MI
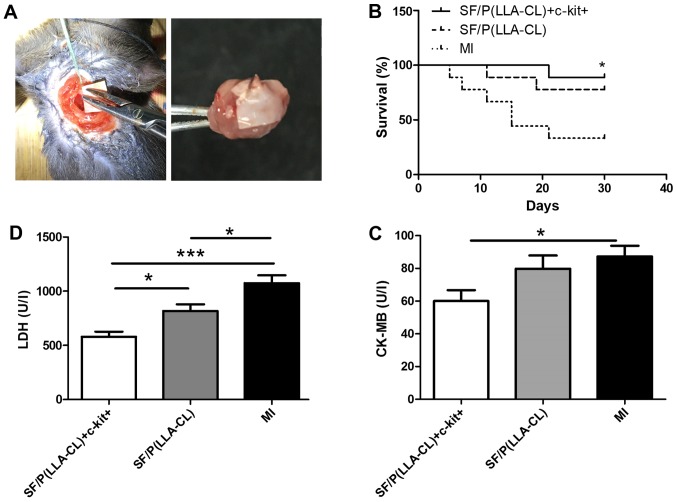
To further assess the role of SF/P(LLA-CL) electrospun scaffolds in cardiac repair, myocardial damage was evaluated by measuring the release of several markers in the serum, including CK-MB and LDH (Fig. 4). Analysis revealed that SF/P(LLA-CL) + c-kit+ treatment significantly decreased the serum levels of CK-MB (P<0.05) and LDH (P<0.001) compared with the MI group. Furthermore, the survival rates were evaluated following MI induction for up to 30 days in SF/P(LLA-CL) + c-kit+, pure SF/P(LLA-CL) and MI groups (Fig. 4). The survival rate of the surgery was consistent with previous research (24–26). The results demonstrated that the SF/P(LLA-CL) + c-kit+ group exhibited significantly improved survival at 30 days post-MI compared with the MI group (P<0.05). However, no significant differences were observed between the pure SF/P(LLA-CL) group and MI group.
Figure 4.
(A) Electrospun scaffold transplantation surgery and representative image of electrospun scaffolds covered the epicardium of the left ventricles when the hearts were collected. (B) Survival curve post-MI in SF/P(LLA-CL) + c-kit+, SF/P(LLA-CL) and MI groups analyzed using the log-rank test. *P<0.05 vs. MI group (n=9 for each group). (C and D) Total LDH and CK-MB in the serum. *P<0.05 and ***P<0.001 as indicated (n=6 for each group). SF, silk fibroin; P(LLA-CL), poly(L-lactic acid-co-ε-caprolactone); MI, myocardial infarction; LDH, lactate dehydrogenase; CK-MB, creatine kinase, MB form.
SF/P(LLA-CL) electrospun scaffolds enhance cardiac repair in vivo
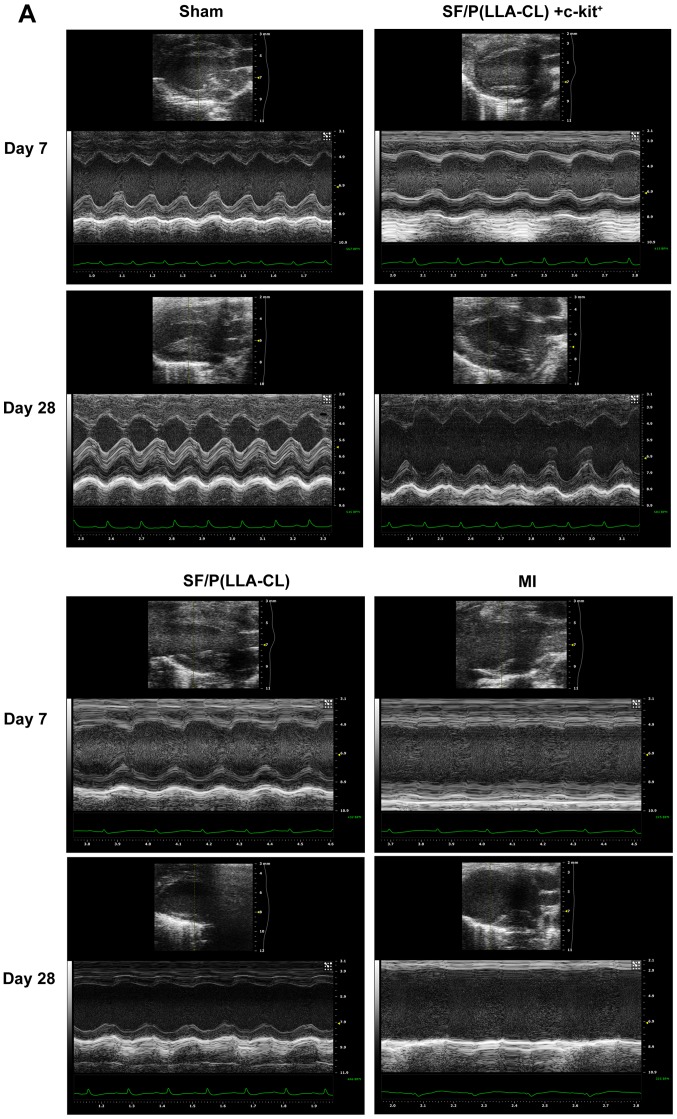
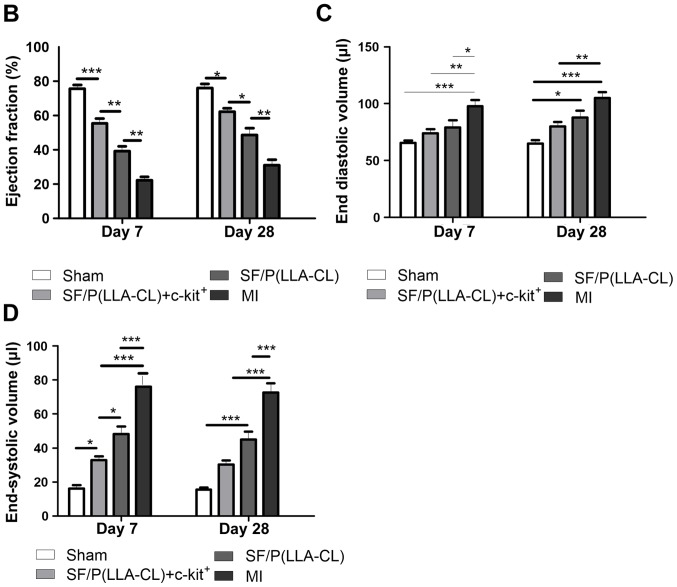
SF/P(LLA-CL) electrospun scaffolds were superior to P(LLA-CL) scaffolds in terms of promoting cell proliferation in vitro. To further assess the role of SF/P(LLA-CL) electrospun scaffolds in cardiac repair, sorted cells were immediately seeded with SF/P(LLA-CL) electrospun scaffolds and cultured for 5 days prior to transplantation. Experimental mice were divided into four groups: i) Sham group; ii) SF/P(LLA-CL) + c-kit+ group; iii) pure SF/P(LLA-CL); and iv) MI group. Images of the surgical procedure and a holistic view of the MI heart with scaffold implantation are presented in Fig. 5A. Echocardiography examinations were performed on days 7 and 28 following transplantation. At 7 days post-transplantation, even though the SF/P(LLA-CL) + c-kit+ electrospun scaffolds group exhibited significantly improved EF values (Fig. 5B), no significant differences were observed between the SF/P(LLA-CL) + c-kit+ electrospun scaffolds group and SF/P(LLA-CL) electrospun scaffolds group regarding EDVs. Furthermore, the SF/P(LLA-CL) + c-kit+ electrospun scaffolds group exhibited significantly improved EDVs (P<0.01) and ESVs (P<0.001) compared with the MI group; (Fig. 5C and D). At 28 days, the reductions in EDV and ESV were associated with increased EF values in the SF/P(LLA-CL) + c-kit+ electrospun scaffolds transplantation group. Mice with SF/P(LLA-CL) + c-kit+ electrospun scaffolds had significantly decreased EDVs (P<0.01) and ESVs (P<0.001) compared with the MI group. However, no significant differences were present between the SF/P(LLA-CL) + c-kit+ electrospun scaffolds and SF/P(LLA-CL) electrospun scaffolds groups regarding EDV and ESV (Fig. 5B, C and D). These findings suggested that SF/P(LLA-CL) + c-kit+ electrospun scaffold transplantation moderately attenuated post-MI left ventricular function. Pure SF/P(LLA-CL) electrospun scaffolds were not as effective as SF/P(LLA-CL) + c-kit+ electrospun scaffolds in protecting cardiac function post-MI.
Figure 5.
Echocardiographic evaluation. (A) M-mode images revealed cardiac function. (B) Ejection fraction, (C) end-diastolic volume and (D) end-systolic volume was indicated. *P<0.05, **P<0.01 and ***P<0.001 as indicated (n=6 for each group). SF, silk fibroin; P(LLA-CL), poly(L-lactic acid-co-ε-caprolactone); MI, myocardial infarction.
Examination of infarct size
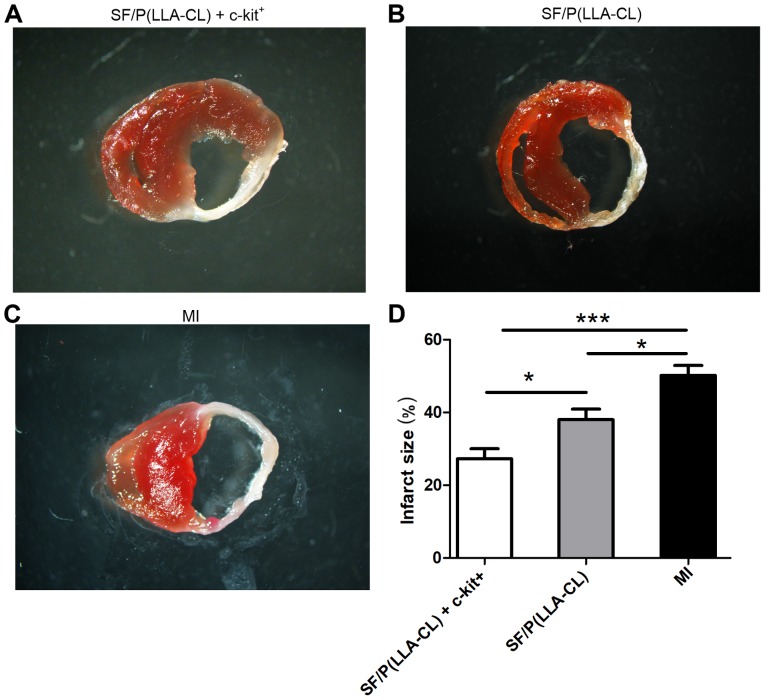
All electrospun sheets covered the epicardium of the left ventricles when the hearts were collected (Fig. 4A). Notably, SF/P(LLA-CL) + c-kit+ (P<0.001) and pure SF/P(LLA-CL) (P<0.05) electrospun scaffold transplantation significantly reduced the infarct size compared with untreated MI (Fig. 6). Furthermore, infarct size in mice treated with SF/P(LLA-CL) + c-kit+ were reduced more compared with the pure SF/P(LLA-CL) treated-mice (P<0.05). Thus, SF/P(LLA-CL) + c-kit+ electrospun scaffolds can reduce infarct volume to a greater extent compared with pure SF/P(LLA-CL) scaffolds following transplantation.
Figure 6.
TTC staining. (A-C) TTC staining of transverse sections. (D) Quantification of infarct size in each group. *P<0.05 and ***P<0.001 as indicated (n=6 for each group). TTC, 2,3,5-triphenyltetrazolium chloride; SF, silk fibroin; P(LLA-CL), poly(L-lactic acid-co-ε-caprolactone); MI, myocardial infarction.
Discussion
In the present study, cardiac SF/P(LLA-CL) electrospun nanofibrous sheets were developed, which served a protective role in cardiac performance when patched onto infarcted hearts in mice. SF/P(LLA-CL) electrospun scaffolds and P(LLA-CL) scaffolds were compared in terms of their mechanical and biotic characteristics in vitro. SF/P(LLA-CL) + c-kit+ scaffolds and pure SF/P(LLA-CL) scaffolds in cardiac repair were also examined following MI in vivo. The results demonstrated that SF/P(LLA-CL) electrospun scaffolds were superior to the P(LLA-CL) electrospun scaffolds in improving c-kit+ BM cell survival and proliferation in vitro. Furthermore, SF/P(LLA-CL) + c-kit+ electrospun scaffolds prevented cardiac rupture, ameliorated myocardial damage and left ventricular remodeling and reduced infarct size post-MI induction in vivo. In conclusion, the data suggested that transplantation of SF/P(LLA-CL) + c-kit+ electrospun scaffolds may be useful in MI treatment.
BM cells are easily accessible and grow rapidly in vitro. Thus, cell therapy using BM-derived stem cells has been considered in cardiac tissue engineering (1,27). Cumulative animal studies have revealed that c-kit+ BM-derived stem cell transplantation can improve heart function following MI (28). However, its therapeutic efficiency remains low due to poor cell survival (2,29). Thus, it is critical to establish a method to improve cell retention and survival rate. In the present study, it was observed that the c-kit+ BM subpopulation was significantly increased in response to MI in mice. This indicated that the c-kit+ BM subpopulation may serve a role post-MI. Additionally, several studies have revealed that the nanofibrous architecture of scaffolds may be beneficial to the proliferation of engrafted cells (30). Therefore, the engraftment of a c-kit+ BM cell population in P(LLA-CL) and SF/P(LLA-CL) scaffolds was investigated and it was analyzed whether these scaffolds enhanced the engrafted c-kit+ cell survival and proliferation in the present study.
Previous studies have used P(LLA-CL) as a scaffold in tissue engineering, primarily due to its excellent mechanical properties and good biocompatibility (6,31). However, P(LLA-CL) polymers have certain disadvantages, including inertness, low bioactive properties and hydrophobicity, which result in limited cell-cell interactions (21,32,33). SF, a natural protein, has been widely used in tissue engineering due to its unique properties, including good biocompatibility, good water vapor permeability and low cost. However, the brittleness of SF may hinder its use for further study of tissue regeneration (34,35). In the present study, SF was blended with P(LLA-CL) to develop a hybrid electrospun nanofibrous scaffold in order to combine the advantages of SF and P(LLA-CL). It was revealed that P(LLA-CL) scaffolds transformed from flexible to brittle when blended with SF. Thus, to maintain the flexibility of the electrospun scaffolds used in the present study, 25:75 ratios of SF and P(LLA-CL), respectively, were used. SEM observation demonstrated that mixing SF with P(LLA-CL) changed the morphology of electrospun scaffolds, and may therefore increase the cell affinity. Furthermore, wettability results indicated that the addition of SF provided electrospun scaffolds with more prominent hydrophilic properties compared with pure P(LLA-CL).
Good mechanical properties of a scaffold are helpful for successful cell transplantation (36). In the present study, it was observed that BM c-kit+ cells exhibited improved proliferation on SF/P(LLA-CL) and P(LLA-CL) electrospun sheets compared with the c-kit+ only group. Notably, cell proliferation was the greatest in the SF/P(LLA-CL) group. The present data demonstrated that the SF/P(LLA-CL) nanofibrous scaffolds exhibited improved cytocompatibility, which is a requirement for any biomaterial used for treatment. In summary, it was revealed that blending SF (25%) with P(LLA-CL) (75%) resulted in scaffolds with favorable mechanical and biological properties. However, the mechanisms by which SF/P(LLA-CL) exerts its effects in stem cell proliferation are not clear and require further investigation. Thus, SF/P(LLA-CL) nanofibrous scaffolds were assessed in vivo in the present study. Providing mechanical support to the infarcted left ventricle area is a unique superiority of electrospun cardiac scaffolds (37). It can prevent paradoxical movement of aneurysms in order to synchronize ventricle movement and consequently prevent heart failure progression (38,39). Notably, good flexibility of the cardiac scaffold is required to withstand repeated left ventricular contractions in vivo. Previous evidence has indicated that one of the biggest drawbacks of SF is its brittleness (21,23). In the present study, 25% SF was blended with 75% P(LLA-CL) to develop a hybrid electrospun nanofibrous scaffold in order to maintain the advantages of SF, including its good biocompatibility, water vapor permeability and cell affinity, in addition to preserving the flexibility of P(LLA-CL). c-kit+ BM cells have been demonstrated to support myocardial regeneration in vitro and in vivo (40). Consequently, c-kit+ cells were seeded on SF/P(LLA-CL) nanofibrous scaffolds prior to transplantation in the present study. A key finding in the present study was that engrafting the SF/P(LLA-CL) nanofibrous scaffold seeded with c-kit+ BM cells at the time of coronary ligation attenuated structural remodeling and improved cardiac function compared with cell-free scaffolds following MI at days 7 and 28 post-transplantation. In the late phase of acute myocardial infraction, left ventricle remodelling is secondary to architectural rearrangements of the surviving myocardium involving myocyte hypertrophy, interstitial fibrosis, thinning and dilation of the infarcted myocardial wall (41). Thus, the 28th day was chosen as the end of the present in vivo experiment due to its match of the late phase of acute MI in humans (42–44). In addition, the present study demonstrated that SF/P(LLA-CL) nanofibrous scaffolds seeded with c-kit+ BM cells prevented cardiac rupture and attenuated myocardial damage in the early post-MI phase. Thus, the ECM-mimicking hybrid electrospun scaffolds seeded with c-kit+ cells may be considered a viable MI treatment option. Although the present results are promising, there are still many questions that require answering. For example, further experiments should be performed to investigate the regeneration of myocardium tissue in response to transplantation of the SF/P(LLA-CL) electrospun scaffold. In addition, more differentiation potential seeds of cells should be evaluated in cardiac tissue engineering.
In conclusion, in the present study, a new electrospun nanofibrous scaffold was developed. In vitro, it was demonstrated that cultured c-kit+ BM cells with SF/P(LLA-CL) electrospun scaffolds are superior in terms of their proliferative capacity compared with P(LLA-CL) electrospun scaffolds or c-kit+ BM cells alone. Furthermore, the present study provided evidence that the SF/P(LLA-CL) electrospun scaffold serves as an ECM to support c-kit+ BM cells survival and retention in the early phase of MI. In the late phase post-MI, SF/P(LLA-CL) electrospun scaffold are able to support the infarcted left ventricular area and prevent paradoxical movement of aneurysms in order to synchronize ventricle movement, ultimately improving overall heart function and reducing infarct size in a MI mouse model. The present findings suggest that SF/P(LLA-CL) electrospun scaffolds may support the brittle and thinning infarct wall, improve c-kit+ BM cell survival and support cardiomyocyte performance following MI. Thus, the use of SF/P(LLA-CL) electrospun sheets may be a novel approach to MI treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Medicine-Engineering Cross-Research Foundation of Shanghai Jiao Tong University (grant no. YG2014ZD02).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MD performed the histological examination of the hearts and was a major contributor in writing the manuscript. JG performed transthoracic electrocardiography. YM, JW and YX analyzed the data. SX constructed the MI mouse model. XM made the electrospun scaffolds used in the present study and was a major contributor in drafting the manuscript.
Ethics approval and consent to participate
The study was approved by the ethics committee of Renji Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Du M, Schmull S, Zhang W, Wang C, Lian F, Chen Y, Xue S. c-kit(+)AT2R(+) bone marrow mononuclear cell subset is a superior subset for cardiac protection after myocardial infarction. Stem Cells Int. 2016;2016:4913515. doi: 10.1155/2016/4913515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113:539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 4.Lehtinen M, Pätilä T, Kankuri E, Lauerma K, Sinisalo J, Laine M, Kupari M, Vento A, Harjula A, Helsinki BMMC Collaboration Intramyocardial bone marrow mononuclear cell transplantation in ischemic heart failure: Long-term follow-up. J Heart Lung Transplant. 2015;34:899–905. doi: 10.1016/j.healun.2015.01.989. [DOI] [PubMed] [Google Scholar]
- 5.Gude NA, Sussman MA. Chasing c-Kit through the heart: Taking a broader view. Pharmacol Res. 2018;127:110–115. doi: 10.1016/j.phrs.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagostena L, Avitabile D, De Falco E, Orlandi A, Grassi F, Iachininoto MG, Ragone G, Fucile S, Pompilio G, Eusebi F, et al. Electrophysiological properties of mouse bone marrow c-kit+ cells co-cultured onto neonatal cardiac myocytes. Cardiovasc Res. 2005;66:482–492. doi: 10.1016/j.cardiores.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Wu P, Wang H, Wang Y, Du Y, Cheng W, Xu Z, Zhou N, Wang L, Yang Z. Necroptosis induced by Ad-HGF activates endogenous C-Kit+ cardiac stem cells and promotes cardiomyocyte proliferation and angiogenesis in the infarcted aged heart. Cell Physiol Biochem. 2016;40:847–860. doi: 10.1159/000453144. [DOI] [PubMed] [Google Scholar]
- 8.Awada HK, Long DW, Wang Z, Hwang MP, Kim K, Wang Y. A single injection of protein-loaded coacervate-gel significantly improves cardiac function post infarction. Biomaterials. 2017;125:65–80. doi: 10.1016/j.biomaterials.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J Biomed Mater Res A. 2011;99:376–385. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- 10.Godier-Furnemont AF, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, Zhang G, Hudson B, Homma S, Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci USA. 2011;108:7974–7979. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhand C, Ong ST, Dwivedi N, Diaz SM, Venugopal JR, Navaneethan B, Fazil MH, Liu S, Seitz V, Wintermantel E, et al. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials. 2016;104:323–338. doi: 10.1016/j.biomaterials.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Mo Y, Guo R, Zhang Y, Xue W, Cheng B. Controlled dual delivery of angiogenin and curcumin by electrospun nanofibers for skin regeneration. Tissue Eng Part A. 2017;27 doi: 10.1089/ten.tea.2016.0268. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran MP, Kai D, Ghasemi-Mobarakeh L, Ramakrishna S. Electrospun biocomposite nanofibrous patch for cardiac tissue engineering. Biomed Mater. 2011;6:1748–6041. doi: 10.1088/1748-6041/6/5/055001. [DOI] [PubMed] [Google Scholar]
- 14.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res B Appl Biomater. 2011;98:379–386. doi: 10.1002/jbm.b.31862. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Lin M, Xie Q, Sun H, Huang Y, Zhang D, Yu Z, Bi X, Chen J, Wang J, et al. Electrospun silk fibroin/poly(lactide-co-epsilon-caprolactone) nanofibrous scaffolds for bone regeneration. Int J Nanomedicine. 2016;11:1483–1500. doi: 10.2147/IJN.S97445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Ni N, Chen J, Yao Q, Shen B, Zhang Y, Zhu M, Wang Z, Ruan J, Wang J, et al. Electrospun SF/PLCL nanofibrous membrane: A potential scaffold for retinal progenitor cell proliferation and differentiation. Sci Rep. 2015;5:14326. doi: 10.1038/srep14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CY, Zhang KH, Fan CY, Mo XM, Ruan HJ, Li FF. Aligned natural-synthetic polyblend nanofibers for peripheral nerve regeneration. Acta Biomater. 2011;7:634–643. doi: 10.1016/j.actbio.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Yan C, Zhu M, Yao Q, Shao C, Lu W, Wang J, Mo X, Gu P, Fu Y, Fan X. Electrospun nanofibrous SF/P(LLA-CL) membrane: A potential substratum for endothelial keratoplasty. Int J Nanomedicine. 2015;10:3337–3350. doi: 10.2147/IJN.S77706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang P, Wu KC, Zhu Y, Xiang L, Li C, Chen DL, Chen F, Xu G, Wang A, Li M, Jin ZB. A novel Bruch's membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials. 2014;35:9777–9788. doi: 10.1016/j.biomaterials.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Mo X, Huang C, He C, Wang H. Electrospun scaffolds from silk fibroin and their cellular compatibility. J Biomed Mater Res A. 2010;93:976–983. doi: 10.1002/jbm.a.32497. [DOI] [PubMed] [Google Scholar]
- 21.Kuihua Z, Chunyang W, Cunyi F, Xiumei M. Aligned SF/P(LLA-CL)-blended nanofibers encapsulating nerve growth factor for peripheral nerve regeneration. J Biomed Mater Res A. 2014;102:2680–2691. doi: 10.1002/jbm.a.34922. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, Wang H, Huang C, Su Y, Mo X, Ikada Y. Fabrication of silk fibroin blended P(LLA-CL) nanofibrous scaffolds for tissue engineering. J Biomed Mater Res A. 2010;93:984–993. doi: 10.1002/jbm.a.32504. [DOI] [PubMed] [Google Scholar]
- 23.Aznar-Cervantes S, Pagán A, Martínez JG, Bernabeu-Esclapez A, Otero TF, Meseguer-Olmo L, Paredes JI, Cenis JL. Electrospun silk fibroin scaffolds coated with reduced graphene promote neurite outgrowth of PC-12 cells under electrical stimulation. Mater Sci Eng C Mater Biol Appl. 2017;79:315–325. doi: 10.1016/j.msec.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Zhang L, Wang Z, Yano N, Zhao YT, Wei L, Dubielecka-Szczerba P, Liu PY, Zhuang S, Qin G, Zhao TC. Exendin-4 induces myocardial protection through MKK3 and Akt-1 in infarcted hearts. Am J Physiol Cell Physiol. 2016;310:C270–C283. doi: 10.1152/ajpcell.00194.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks AC, DeMartino AM, Brainard RE, Brittian KR, Bhatnagar A, Jones SP. Induction of activating transcription factor 3 limits survival following infarct-induced heart failure in mice. Am J Physiol Heart Circ Physiol. 2015;309:H1326–H1335. doi: 10.1152/ajpheart.00513.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikido T, Oyama J, Shiraki A, Komoda H, Node K. Deletion of apoptosis inhibitor of macrophage (AIM)/CD5L attenuates the inflammatory response and infarct size in acute myocardial infarction. J Am Heart Assoc. 2016;5:002863. doi: 10.1161/JAHA.115.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X, Huang X, Yang Q, Wang L, Sun J, Zhan H, Lin J, Pu Z, Jiang J, Sun Y, et al. Safety and efficacy of intracoronary hypoxia-preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: The CHINA-AMI randomized controlled trial. Int J Cardiol. 2015;184:446–451. doi: 10.1016/j.ijcard.2015.02.084. [DOI] [PubMed] [Google Scholar]
- 28.Bao L, Meng Q, Li Y, Deng S, Yu Z, Liu Z, Zhang L, Fan H. C-Kit positive cardiac stem cells and bone marrow-derived mesenchymal stem cells synergistically enhance angiogenesis and improve cardiac function after myocardial infarction in a paracrine manner. J Card Fail. 2017;23:403–415. doi: 10.1016/j.cardfail.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Q, Cosme JG, Xu T, Miszuk JM, Picciani PH, Fong H, Sun H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Wu T, Zheng Y, El-Hamshary H, Al-Deyab SS, Mo X. Fabrication and characterization of Mg/P(LLA-CL)-blended nanofiber scaffold. J Biomater Sci Polym Ed. 2014;25:1013–1027. doi: 10.1080/09205063.2014.918456. [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, Fu Q, Yoo J, Chen X, Chandra P, Mo X, Song L, Atala A, Zhao W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017;50:154–164. doi: 10.1016/j.actbio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Fluke LM, Restrepo RD, Patel S, Hoagland BD, Krevetski LM, Stephenson JT. Strength and histology of a nanofiber scaffold in rats. J Surg Res. 2016;205:432–439. doi: 10.1016/j.jss.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 34.Johari N, Madaah Hosseini HR, Samadikuchaksaraei A. Optimized composition of nanocomposite scaffolds formed from silk fibroin and nano-TiO2 for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;79:783–792. doi: 10.1016/j.msec.2017.05.105. [DOI] [PubMed] [Google Scholar]
- 35.Naskar D, Ghosh AK, Mandal M, Das P, Nandi SK, Kundu SC. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials. 2017;136:67–85. doi: 10.1016/j.biomaterials.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Yin A, Luo R, Li J, Mo X, Wang Y, Zhang X. Coaxial electrospinning multicomponent functional controlled-release vascular graft: Optimization of graft properties. Colloids Surf B Biointerfaces. 2017;152:432–439. doi: 10.1016/j.colsurfb.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Xu Y, Wang Z, Wen D, Zhang W, Schmull S, Li H, Chen Y, Xue S. Electrospun nanofibrous sheets of collagen/elastin/polycaprolactone improve cardiac repair after myocardial infarction. Am J Transl Res. 2016;8:1678–1694. [PMC free article] [PubMed] [Google Scholar]
- 38.Kai D, Wang QL, Wang HJ, Prabhakaran MP, Zhang Y, Tan YZ, Ramakrishna S. Stem cell-loaded nanofibrous patch promotes the regeneration of infarcted myocardium with functional improvement in rat model. Acta Biomater. 2014;10:2727–2738. doi: 10.1016/j.actbio.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/S0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 41.Hao H, Hu S, Chen H, Bu D, Zhu L, Xu C, Chu F, Huo X, Tang Y, Sun X, et al. Loss of endothelial CXCR7 impairs vascular homeostasis and cardiac remodeling after myocardial infarction: Implications for cardiovascular drug discovery. Circulation. 2017;135:1253–1264. doi: 10.1161/CIRCULATIONAHA.116.023027. [DOI] [PubMed] [Google Scholar]
- 42.Pinet F, Cuvelliez M, Kelder T, Amouyel P, Radonjic M, Bauters C. Integrative network analysis reveals time-dependent molecular events underlying left ventricular remodeling in post-myocardial infarction patients. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1445–1453. doi: 10.1016/j.bbadis.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Olivier A, Girerd N, Michel JB, Ketelslegers JM, Fay R, Vincent J, Bramlage P, Pitt B, Zannad F, Rossignol P, EPHESUS Investigators Combined baseline and one-month changes in big endothelin-1 and brain natriuretic peptide plasma concentrations predict clinical outcomes in patients with left ventricular dysfunction after acute myocardial infarction: Insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Int J Cardiol. 2017;241:344–350. doi: 10.1016/j.ijcard.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Frey A, Saxon VM, Popp S, Lehmann M, Mathes D, Pachel C, Hofmann U, Ertl G, Lesch KP, Frantz S. Early citalopram treatment increases mortality due to left ventricular rupture in mice after myocardial infarction. J Mol Cell Cardiol. 2016;98:28–36. doi: 10.1016/j.yjmcc.2016.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.