Abstract
In the present study, the effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) delivered by chitosan (CS) microspheres on ectopic osteogenesis was investigated in a rat model. rhBMP-2-loaded CS microspheres and blank CS microspheres were prepared. A total of 24 male Sprague Dawley rats were divided into 4 groups with 6 rats in each group: The CS/rhBMP-2 group, the rhBMP-2 group, in which rhBMP-2 was directly implanted (rhBMP-2 dose in either group, 1 mg), the CS blank group and the control group. X-ray was performed at 4 weeks after ectopic osteogenesis surgery and micro-computed tomography (CT) examination was scheduled at 1, 2, 3 and 4 weeks after the surgery to determine ectopic osteogenesis in the different groups. Histological analysis, and determination of alkaline phosphatase (ALP) activity and calcium content were also performed. The mean diameter of the osteoid tissues was 1.1±0.3 cm (range, 0.8–1.4 cm) in the CS/rhBMP-2 group, which was significantly bigger than that in the rhBMP-2 group (0.3±0.1 cm; range, 0.1–0.4 cm) at 4 weeks after the surgery. X-ray analysis and micro-CT scan indicated that the area of high-density tissues and the radionuclide intensity, as well as bone volume in the 3-dimensional reconstruction were greatest in the CS/rhBMP-2 group, followed by those in the rhBMP-2 group. All parameters, including bone mineral density, tissue mineral density, tissue mineral content and bone volume fraction, were significantly higher in the CS/rhBMP-2 group at 3 and 4 weeks after the surgery, compared with those in the rhBMP-2 group. The histological analysis, ALP activity analysis and determination of calcium content revealed that the CS/rhBMP-2 system had the greatest ability to induce osteoblast differentiation. In conclusion, the CS/rhBMP-2 microsphere delivery system significantly enhanced the induction and promotion effects of rhBMP-2 regarding ectopic osteogenesis. The present study enhances the basic data available for future application of the CS/rhBMP-2 microspheres delivery system and provides a deeper understanding of the role of BMP-2 in bone regeneration.
Keywords: recombinant human bone morphogenetic protein-2, chitosan microspheres, ectopic osteogenesis
Introduction
Since bone tissue engineering has provided an efficient tool for bone regeneration, numerous studies have focused on associated effects and the underlying mechanisms (1). Several different growth factors have been indicated to stimulate bone growth (2), collagen synthesis (3) and fracture repair (4), and have thus attracted the attention of scholars regarding their effects on bone regeneration.
Among the factors, bone morphogenetic proteins (BMPs) have been proven to facilitate bone healing without bone tissue transfer (5). Studies have indicated that BMP-2, a protein of BMP family, has important roles in bone generation-associated processes, including osteoblastic differentiation (6), the healing process of segmental bone defects (7) and the capacity of bone marrow stromal cells (BMSCs) to undergo osteogenesis (8), and has been approved by the Food and Drug Administration of the USA for clinical use in the orthopedic and dental fields (9). However, large amounts of BMP-2 are required to achieve clinically significant bone regeneration and BMP-2 is easily inactivated by dilution or interaction with enzymes in the blood if applied alone by intravenous injection (10–12). To enhance the bioavailability of BMP-2, numerous local delivery systems have been assessed (13), among which the Chitosan (CS) delivery system was proven efficient and enhanced the osteogenic activity (14). Certain studies also reported that BMP-2 promotes ectopic osteogenesis (15,16). However, few studies have focused on the effects of BMP-2 delivery systems, e.g. CS-based delivery systems, on processes of ectopic osteogenesis.
To the best of our knowledge, it has remained elusive whether the CS/human recombinant (rh)BMP-2 microsphere delivery system influences the ectopic osteogenesis process. The present study aimed to investigate the effect of rhBMP-2 delivered by CS microspheres on ectopic osteogenesis in rats. It provides basic data for future application of the CS/rhBMP-2 microsphere delivery system and a deeper understanding of the roles of BMP-2 in bone regeneration.
Materials and methods
Preparation of CS blank microspheres and CS/rhBMP-2 microspheres
The preparation of CS blank microspheres and CS/rhBMP-2 microspheres was performed using a procedure modified from a previous study (17), namely 42 ml thiamine pyrophosphate solution was added instead of DS and ZnSO4. In brief, to prepare CS blank microspheres, a certain amount of CS (molecular weight, 50,000-190,000; Aladdin Reagent Co. Ltd., Shanghai, China) was dissolved in 1% (v/v) acetic acid and the pH was adjusted to 5.4 by addition of NaOH solution to obtain a mixture with a CS concentration of 1.52 mg/ml. The mixture was then filtered through a membrane with 0.22 µm pores and 100 ml of the filtrate was stirred for 1 h at room temperature, followed by addition of 42 ml thiamine pyrophosphate solution (0.5 mg/ml). When the nanospheres formed, the solution turned from clear to an emulsion and the reaction was continued until no alteration appeared. After completion of the reaction, the mixture was stirred for another 30 min at room temperature and centrifuged for 15 min at 25,000 × g at room temperature. The precipitate was washed with water and dried under cryogenic conditions with reduced pressure.
To prepare the CS/rhBMP-2 microspheres, 100 mg dried CS blank microspheres and 5 mg rhBMP-2 were added to 25 ml double-distilled water, followed by stirring for 30 min at 4°C and subsequent centrifugation for 15 min at 25,000 × g at room temperature. The liquid supernatant was then collected and dried to obtain CS/rhBMP-2 microspheres.
Characterization of CS/rhBMP-2 microspheres
The microsphere morphology and grain diameter were analyzed using a scanning electron microscope (S-4800; Hitachi Ltd., Tokyo, Japan) and a laser diffraction particle size analyzer (N5; Beckman Coulter, Brea, CA, USA). The shape and size of microspheres were determined and the grain diameter was calculated. All experiments were performed in triplicate.
Determination of the entrapment efficiency and drug loading ratio
As described previously (16), the drug loading ratio and entrapment efficiency of CS/rhBMP-2 nanoparticles were calculated by using an ELISA kit (cat. no ELH-BMP2-1; RayBiotech Inc., Norcross, CA, USA). All experiments were performed in triplicate. The optical density of the supernatant was determined at 450 nm according to the protocol for the ELISA kit.
In vitro sustained-release profile and degradation
The measurement of the in vitro sustained-release profile was in accordance with that of a previous study by our group and another study (17,18). In brief, 50 mg CS/rhBMP-2 microspheres were immersed in 2 ml PBS (pH 7.4), followed by agitation in a water bath oscillator. The solution was centrifuged for 15 min (25,000 × g) at room temperature and 100 µl supernatant was taken every 3 days at 6, 12, 18, 24, 48 and 72 h. Each time the supernatant was obtained, 100 µl PBS was added and the solution was agitated in a water bath oscillator. The experiments lasted for 30 days. The content of rhBMP-2 was measured using the ELISA kit as described above.
For measurement of the degradation of the CS/rhBMP-2 microspheres, 50 mg microspheres (weight, m0) was added in a centrifuge tube with 2 ml PBS (pH 7.4), followed by agitation in a water bath. Every 3 days, 3 tubes were randomly selected and the microspheres in each tube were dried for determining the weight (mt). The experiment lasted for 45 days and the in vitro degradation was calculated as D=(m0-mt)/m0 ×100%. All experiments were performed in triplicate.
Animals and treatment
A total of 24 male Sprague Dawley (SD) rats were provided by the Experimental Animal Center of Southern Medical University (Guangzhou, China). The age of all rats was 6 weeks and their weight was 130–160 g. The rats were kept under a 12-h light/dark cycle and at a constant temperature (23-25°C) and relative humidity (70%). All animals were housed in micro-isolator cages with free access to food and water according to the Guide for the Care and Use of Laboratory Animals of China (19). Any effort was made to avoid any unnecessary pain for the animals. The protocol of the present study was approved by the Institutional Animal Care Committee at General Hospital of Southern Theater Command, People's Liberation Army (Guangzhou, China).
All rats were divided into 4 groups with 6 rats in each group: The CS/rhBMP-2 group, which was treated with microspheres loaded with 1 mg rhBMP-2, the rhBMP-2 group in which 1 mg rhBMP-2 was directly implanted, the CS blank group, which was treated with unloaded microspheres of the same size and the control group, which was implanted with a gelatin sponge of the same size. All microspheres were sterilized by irradiation of 3,000 Gy60Co and stored at 4°C prior to the experiments.
Ectopic osteogenesis experiment and measurement
All SD rats were anaesthetized by intraperitoneal injection of 10% chloral hydrate (400 mg/kg). A 1.5-cm incision was made on the left leg of each rat and the muscle bag model of quadriceps femoris was generated. The abovementioned materials (microspheres or rhBMP-2) were implanted into the muscle bag and the wound was then sutured. After the surgery, all rats were intraperitoneally injected with 4×105 units of penicillin every day for 3 days. The hardness of the tissue around the implant was analyzed by palpation every day.
X-ray imaging was performed at 4 weeks after the surgery and micro-computed tomography (CT) examination was performed at 1, 2, 3 and 4 weeks after the surgery to determine the ectopic osteogenesis in the different groups of animals. The grafts were evaluated using a micro-CT apparatus from GE Healthcare (Little Chalfont, UK) with the following parameters: 80 kV, 0.6 mm, 80 µA and exposure time, 3,000 msec. The grafts were evaluated for osteogenesis capacity based on the following morphometric indices: Bone mineral density (BMD), tissue mineral density (TMD), tissue mineral content (TMC) and bone volume fraction (BVF).
Histology
The animals were sacrificed at 4 weeks after the surgery. Tissues around the implants were obtained and fixed in formalin buffer. The samples were subjected to H&E staining and the histologic morphology in each group was observed under an inverted microscope.
Alkaline phosphatase (ALP) activity analysis and determination of the calcium content
The implants were taken out at 4 weeks after the surgery. For each animal, 0.5 g tissue around the implants was obtained, ground and washed with deionized water. Cell Lysis solution (RIPA lysis buffer; Beyotime Institute of Biotechnology, Haimen, China) was added and the mixture was centrifuged at 16,000 × g for 30 min at 4°C. ALP activity was determined using an ALP Detection Kit (cat. no. A059-2; Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China) according to manufacturer's protocols. The calcium content of the tissues was determined using an Atomic absorption spectrophotometer (i7500; Hitachi Ltd.) by using the following formula: Calcium content=calcium content (µg)/sample wet weight (mg).
Statistical analysis
The measurement data are expressed as the mean ± standard deviation. Comparison between two groups was performed using the Student's t-test. Comparison among three or more groups was performed using one-way analysis of variance followed by Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference. All calculations were made using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Characterization of CS/rhBMP-2 microspheres
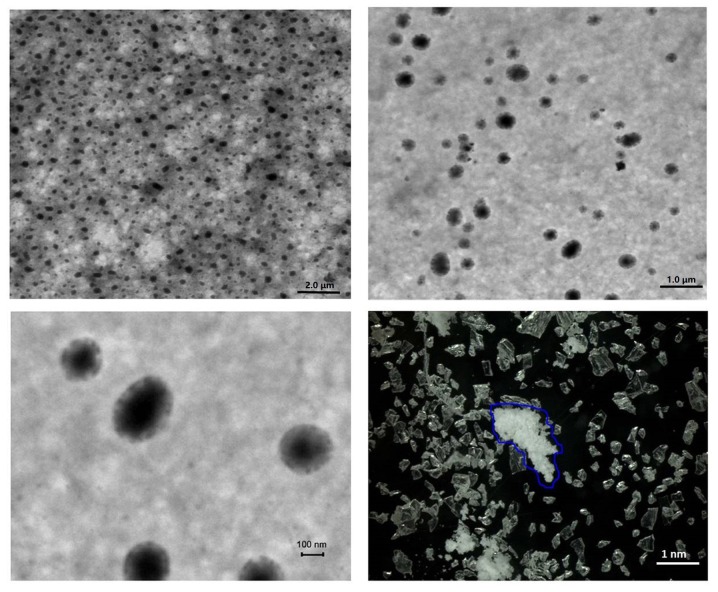
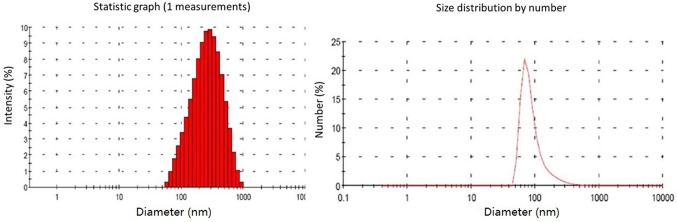
First, the physicochemical properties of the CS/rhBMP-2 microspheres were determined. As presented in Fig. 1, analysis of microsphere morphology indicated that the CS/rhBMP-2 microspheres demonstrated spherical regularity and the surface of the spheres was smooth and unwrinkled, with rhBMP-2 contained in the spheres. The grain diameter analysis indicated that the grain diameter of CS/rhBMP-2 microspheres ranged from 58.8 to 955 nm, with the diameters being mainly distributed within the 142–531 nm range (79.3%; Fig. 2). The mean diameter of the CS/rhBMP-2 microspheres was 230 nm. The entrapment efficiency for CS/rhBMP-2 microspheres was determined to be 66.9±4.6% and the drug loading ratio was 33.4±2.3 µg/mg.
Figure 1.
Morphology of CS/rhBMP-2 microspheres. CS/rhBMP-2 microspheres featured spherical regularization, dispersed evenly, and the surface of the whole sphere was smooth and unwrinkled, with rhBMP-2 in the spheres. The blue line refers to the protein in the nanosphere. rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan.
Figure 2.
Grain diameter analysis by a laser diffraction particle size analyzer. Grain diameters of CS/rhBMP-2 microspheres ranged from 58.8 to 955 nm, mainly distributed within the 142–531 nm range (79.3%). The mean diameter for CS/rhBMP-2 microspheres was 230 nm. rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan.
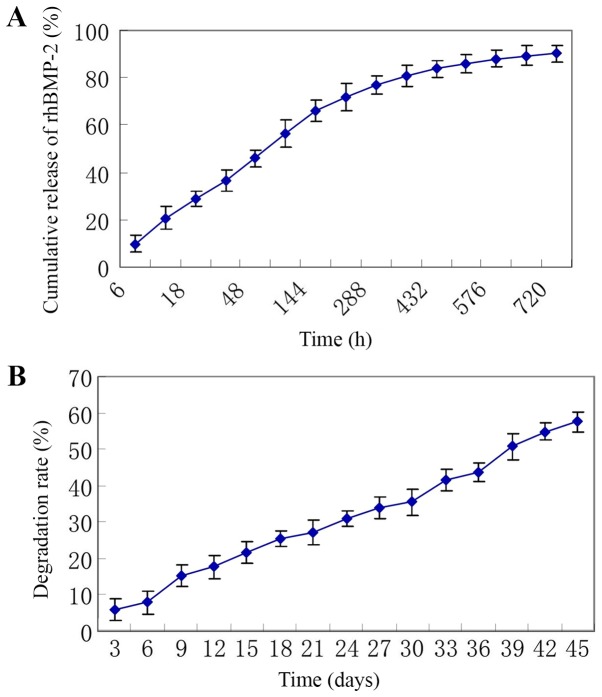
In vitro sustained-release profile and degradation
As presented in Fig. 3A, the release of rhBMP-2 lasted for 30 days and was in consistency with biphasic dynamics. The initial phase was a fast drug release phase with a cumulative drug release rate of 45.9±3.8% at the first 48 h and a cumulative drug release rate of 66.0±4.4% at the first 6 days. The posterior phase was a slow release phase with a cumulative drug release rate of 80.8±4.8% at 15 days and a cumulative drug release rate of 90.1±3.6% at 30 days.
Figure 3.
(A) In vitro sustained release profile. The release of rhBMP-2 lasted for 30 days. (B) Degradation analysis indicated that the weight of the microspheres gradually decreased and the degradation rate increased with time. rhBMP-2, recombinant human bone morphogenetic protein-2.
The degradation assay indicated that the weight of the microspheres gradually decreased and the degradation rate increased with time, with a degradation rate of 21.5±3.0% at 15 days, 35.6±3.6% at 30 days and 57.6±2.8% at 45 days (Fig. 3B).
General observations in the ectopic osteogenesis experiment
After the surgery, the rats were returned to their cages with free access to food and water, and the surgical wounds healed well in all animals. After 3 weeks, the area around the implants became hard in the CS/rhBMP-2 group; however, the other groups exhibited no obvious change. After 4 weeks, the area around the implants became hard in the CS/rhBMP-2 group and in the rhBMP-2 group, and osteoid tissues were found in each of these two groups at the area around the implants; however, no such osteoid tissues were present in the CS blank and the control groups.
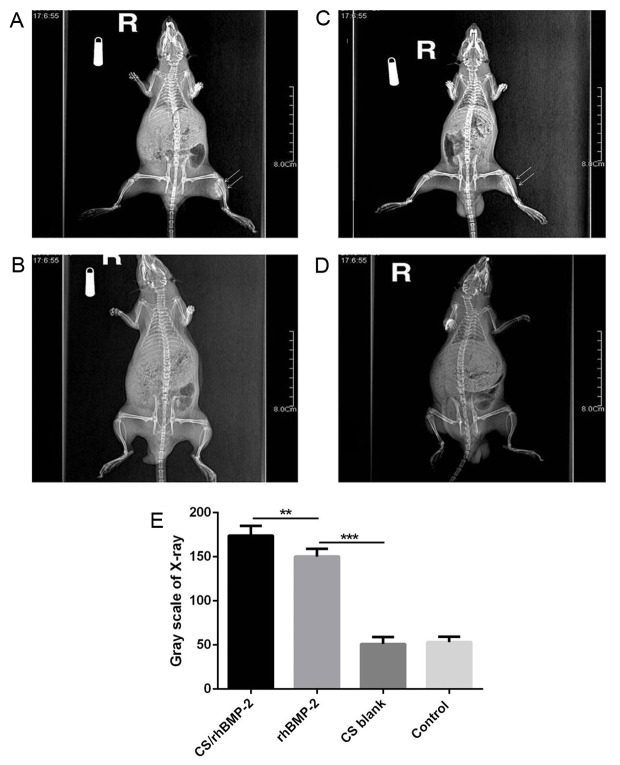
The mean diameter of the osteoid tissues was 1.1±0.3 cm (range, 0.8–1.4 cm) in the CS/rhBMP-2 group, which was significantly bigger than that in the rhBMP-2 group (0.3±0.1; range, 0.1–0.4 cm; P=0.013; Fig. 4). X-ray analysis also demonstrated that the area of high-density tissues was largest in the CS/rhBMP-2 group, followed by the rhBMP-2 group. However, no high-density tissue was observed in the CS blank and the control group (Fig. 5).
Figure 4.
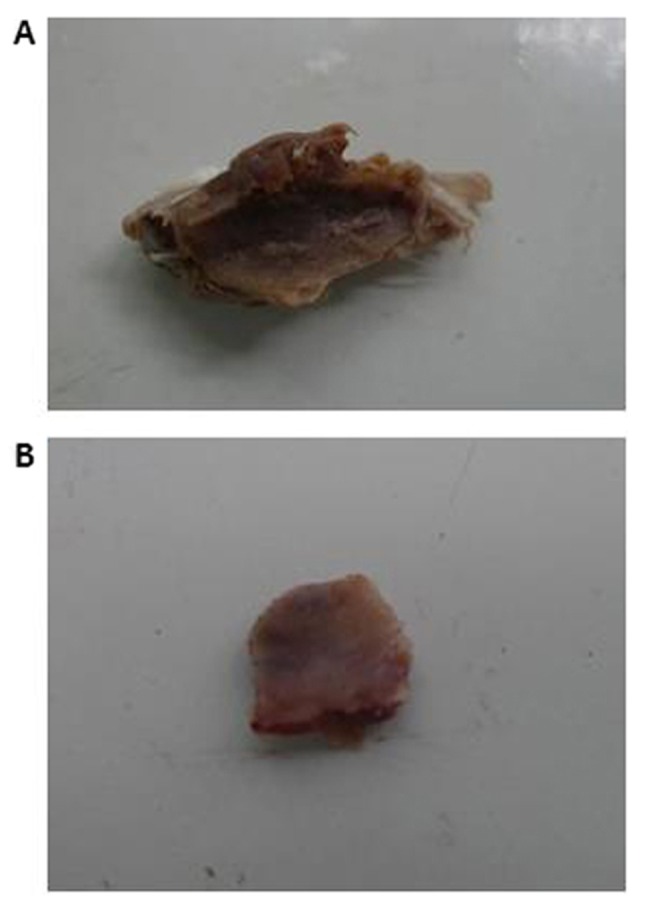
Osteoid tissues from the CS/rhBMP-2 and rhBMP-2 groups. (A) Osteoid tissues from the CS/rhBMP-2 group; (B) osteoid tissues from the rhBMP-2 group. The mean diameter of the osteoid tissues in the CS/rhBMP-2 group (1.1±0.3 cm) was significantly bigger compared with that in the rhBMP-2 group (0.3±0.1 cm). rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan.
Figure 5.
X-ray analysis of the different groups. (A) An area of high-density tissues (arrows) was obvious in the CS/rhBMP-2 group; (B) an area of high-density tissues (arrows) was present in the rhBMP-2 group, but it was smaller than that in the CS/rhBMP-2 group. (C and D) No high-density area was present in the CS blank and the control group. (E) X-ray analysis also indicated that the area of high-density tissues was the greatest in the CS/rhBMP-2 group, followed by the rhBMP-2 group. **P<0.01, ***P<0.001 as indicated. rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan.
Micro-CT scan and 3-dimensional (3D) reconstruction
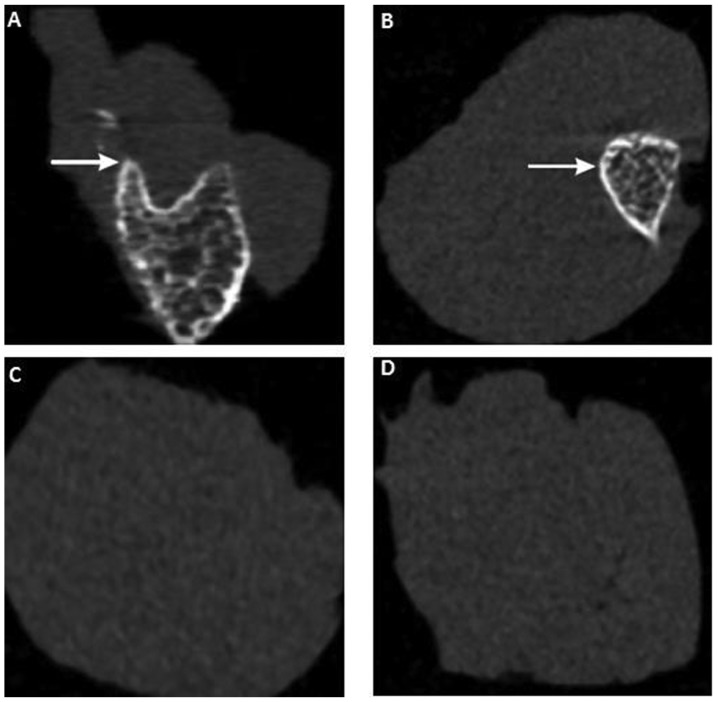
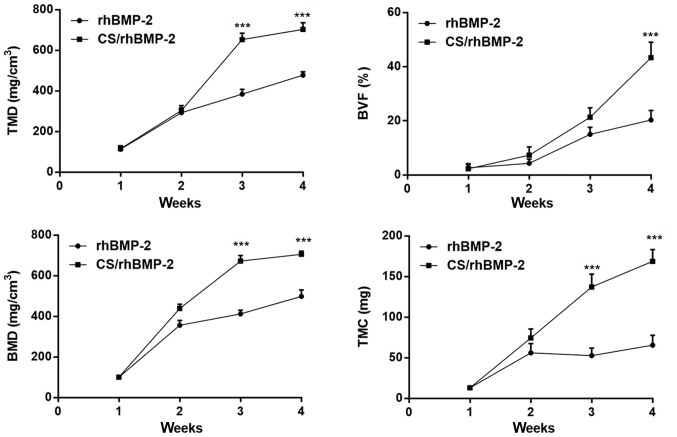
To further investigate the effect of CS/rhBMP-2 on ectopic osteogenesis, micro-CT scan and 3D reconstruction were performed. As presented in Fig. 6, no ectopic osteogenesis was observed in the CS blank and the control group. However, in the CS/rhBMP-2 and rhBMP-2 groups, obvious ectopic osteogenesis was observed with a higher bone density at the edge and a lower bone density at the center. Furthermore, the bone volume in the 3D reconstruction for the CS/rhBMP-2 group was obviously higher than those in the rhBMP-2 group. In addition, analysis of the micro-CT bone parameters revealed that at 3 weeks after surgery, all parameters, namely the TMD, BVF, BMD and TMC, were significantly higher in the CS/rhBMP-2 group compared with those in the rhBMP-2 group (P<0.05; Fig. 7).
Figure 6.
Micro-computed tomography scan for assessment of ectopic osteogenesis in the different groups at 4 weeks after surgery. (A) CS/rhBMP-2 group; (B) rhBMP-2 group; (C) CS blank group; (D) control group. The area of ectopic bone formation is indicated by white colour/arrows. In the CS/rhBMP-2 and rhBMP-2 groups, obvious ectopic osteogenesis was observed with a higher bone density at the edge and a lower bone density at the center. rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan.
Figure 7.
Bone parameters in the CS/rhBMP-2 and rhBMP-2 groups. At 3 weeks after the surgery, the TMD, BVF, BMD and TMC were significantly higher in the CS/rhBMP-2 group compared with those in the rhBMP-2 group. ***P<0.001 vs. the rhBMP-2 group. rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan; BMD, bone mineral density; TMD, tissue mineral density; BVF, bone volume fraction; TMC, tissue mineral content.
Histology
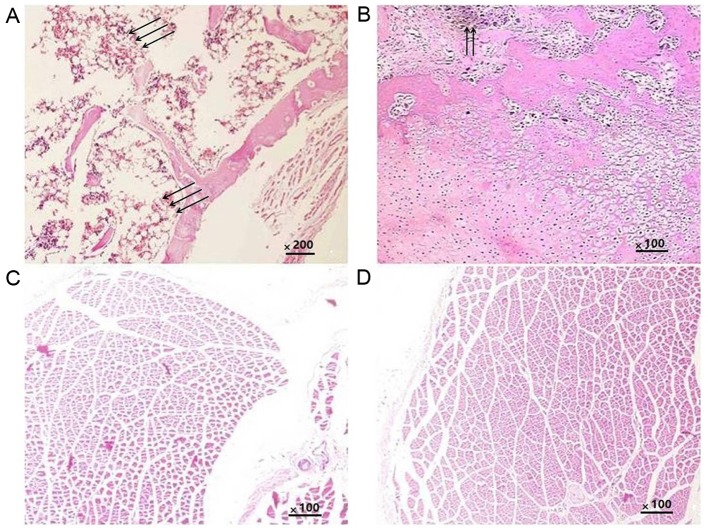
Histological analysis was performed to further confirm the ectopic osteogenesis in the different groups. The results indicated that in all groups, the implants were completely absorbed and no inflammatory or granulomatous tissues were present. In the CS/rhBMP-2 group, mature bone tissue was formed, and the bone cells, bone marrow and bone trabeculae had been converted into mature bone; the formation of a bone marrow cavity and myeloid material between the trabeculae was also observed (Fig. 8A). In the rhBMP-2 group, osteoid tissues were also present (Fig. 8B); however, they were not as mature as those in the CS/rhBMP-2 group. No osteoid tissues were observed in the blank and control groups (Fig. 8C and D).
Figure 8.
Histological analysis of different groups of rats. (A) CS/rhBMP-2 group; (B) rhBMP-2 group; (C) CS blank group; (D) control group (H&E; magnification, ×200). In the CS/rhBMP-2 group, mature bone tissue was formed, the bone cells, bone marrow and bone trabeculae were converted into mature bone, and the formation of a bone marrow cavity and myeloid material between the trabeculae was also observed (arrows). In the rhBMP-2 group, osteoid tissues were also present (arrows); however, they were not as mature as those in the CS/rhBMP-2 group. rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan.
ALP activity and calcium content
At last, the ALP and Ca2+ content was determined in each group. As presented in Table I, the ALP activity and the calcium content were significantly higher in the CS/rhBMP-2 and rhBMP-2 groups compared with those in the other 2 groups (P<0.05). Of note, the ALP activity and calcium content were also significantly higher in the CS/rhBMP-2 group compared with the rhBMP-2 group (P<0.05), indicating that the CS/rhBMP-2 system had the greatest ability to induce osteoblast differentiation.
Table I.
ALP and Ca2+ content in each group (n=6).
| Group | ALP activity (katal/g) | Ca2+ contents (µg/mg) |
|---|---|---|
| CS/rhBMP-2 | 1.94±0.35a–c | 5.20±1.42a–c |
| rhBMP-2 | 1.48±0.56b,c | 3.80±1.40b,c |
| CS blank | 0.20±0.07 | 0.19±0.08 |
| Control | 0.18±0.06 | 0.20±0.08 |
P<0.05 compared with the rhBMP-2 group
P<0.05 compared with the CS blank group
P<0.05 compared with the Control group. rhBMP-2, recombinant human bone morphogenetic protein-2; CS, chitosan; ALP, alkaline phosphatase.
Discussion
In the field of bone tissue engineering, BMP-2 is a growth factor known to enhance osteogenesis. However, the circulation half-life of BMP-2 is rather short and the bone formation does not increase dose-dependently with increasing BMP-2 concentrations. To overcome the insufficiency of direct injection of BMP-2, several novel BMP-2 local delivery systems have been reported, including injectable sonication-induced silk hydrogel (20), polyethylene glycol-coated albumin nanoparticles (21) and CS-based 3D constructs (22). Studies have demonstrated that collagen sponges may be used as an adequate matrix to prolong the duration of BMP-2 residing in the tissue to facilitate bone regeneration (23). Bhakta et al (24) reported that collagen and hyaluronan matrices may be used for delivery of BMP-2, which may help abrogate the adverse clinical effects associated with high-dose growth factor use.
Among the delivery systems, CS-based systems have attracted a large amount of attention. Yilgor et al (25) developed a sequential BMP-2/BMP-7 delivery system with CS-based scaffolds for bone tissue engineering, with which an enhanced ALP activity was achieved. Gan et al (26) designed a CS-based delivery system for BMP-2 and reported that it enhanced bone regeneration. However, whether the CS/rhBMP-2 microspheres delivery system is able to initiate ectopic osteogenesis has remained elusive. The present study aimed to investigate the effect of rhBMP-2 delivered by CS microspheres on ectopic osteogenesis of rats.
First, CS/rhBMP-2 microspheres were successfully prepared and their physicochemical properties were demonstrated. Subsequently, by using several analysis methods, including X-ray analysis, micro-CT scan and 3D reconstruction, histological analysis, ALP activity analysis and calcium content analysis, it was demonstrated that rhBMP induced ectopic osteogenesis in rats, and promoted osteoblast differentiation by enhancing ALP activity and the calcium content. Of note, the CS/rhBMP-2 microsphere delivery system significantly enhanced the induction of osteogenesis compared with that achieved with rhBMP-2 alone.
Several associated studies have focused on BMP-2 in processes of osteogenesis. Lee et al (15) studied the effect of dual treatment of stromal cell-derived factor (SDF)-1 and BMP-2 on ectopic and orthotopic bone formation and identified that BMP-2 induced ectopic and orthotopic bone regeneration; however, SDF-1 treatment did not enhance the effect. Ma et al (27) demonstrated that rhBMP-2 and basic fibroblast growth factor had a synergistic effect on ectopic osteogenesis in mice. Lai et al (28) indicated that application of rhBMP-2 sustained-release nanocapsules significantly promoted ectopic osteogenesis compared with the direct use of rhBMP-2. All of these studies were consistent with the present results.
In conclusion, in the present study, a CS/rhBMP-2 microsphere delivery system was prepared and used to investigate the effect of rhBMP-2 delivered by CS microspheres on ectopic osteogenesis in rats. The results demonstrated that rhBMP induced ectopic osteogenesis in rats, and promoted osteoblast differentiation by enhancing ALP activity and the calcium content. Of note, the CS/rhBMP-2 microsphere delivery system significantly enhanced the ectopic osteogenesis compared with the effects of rhBMP-2 on its own. The present study may provide basic data for the future application of the CS/rhBMP-2 microsphere delivery system and provide a deeper understanding of the roles of BMP-2 in bone regeneration.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Guangzhou Science and Technology project (grant no. 201804010136).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YJX and WW conducted the experiments and analyzed the data. YJX wrote the manuscript and revised the manuscript. HX, QSY and XY conducted the experiments. LHL and JHW collected the data. YZ analyzed the data, revised the manuscript and approved the submission.
Ethics approval and consent to participate
The protocol of the present study was approved by the Institutional Animal Care Committee at General Hospital of Southern Theater Command, PLA (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, Dickinson IC, Choong PF, Schuetz MA, Hutmacher DW. Bone regeneration based on tissue engineering conceptions-a 21st century perspective. Bone Res. 2013;1:216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauth A, Ristevski B, Li R, Schemitsch EH. Growth factors and bone regeneration: How much bone can we expect? Injury. 2011;42:574–579. doi: 10.1016/j.injury.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Fu SZ, Ni PY, Wang BY, Chu B, Zheng L, Luo F, Luo J, Qian Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials. 2012;33:4801–4809. doi: 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233:2937–2948. doi: 10.1002/jcp.26042. [DOI] [PubMed] [Google Scholar]
- 5.Krishnakumar GS, Roffi A, Reale D, Kon E, Filardo G. Clinical application of bone morphogenetic proteins for bone healing: A systematic review. Int Orthop. 2017;41:1073–1083. doi: 10.1007/s00264-017-3471-9. [DOI] [PubMed] [Google Scholar]
- 6.Yan X, Kang D, Pan J, Jiang C, Lin Y, Qi S. Osteoblastic differentiation and cell calcification of adamantinomatous craniopharyngioma induced by bone morphogenetic protein-2. Cancer Biomark. 2017;18:191–198. doi: 10.3233/CBM-161576. [DOI] [PubMed] [Google Scholar]
- 7.Fujita N, Matsushita T, Ishida K, Sasaki K, Kubo S, Matsumoto T, Kurosaka M, Tabata Y, Kuroda R. An analysis of bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein 2 from a biodegradable sponge composed of gelatin and β-tricalcium phosphate. J Tissue Eng Regen Med. 2012;6:291–298. doi: 10.1002/term.432. [DOI] [PubMed] [Google Scholar]
- 8.Chao Q, Zhu C, Yu W, Jiang X, Zhang F, Jian S. Bone morphogenetic protein 2 promotes osteogenesis of bone marrow stromal cells in type 2 diabetic rats via the Wnt signaling pathway. Int J Biochem Cell Biol. 2016;80:143–153. doi: 10.1016/j.biocel.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Carreira AC, Lojudice FH, Halcsik E, Navarro RD, Sogayar MC, Granjeiro JM. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J Dent Res. 2014;93:335–345. doi: 10.1177/0022034513518561. [DOI] [PubMed] [Google Scholar]
- 10.Mohajel N, Najafabadi AR, Azadmanesh K, Amini M, Vatanara A, Moazeni E, Rahimi A, Gilani K. Drying of a plasmid containing formulation: Chitosan as a protecting agent. Daru. 2012;20:22. doi: 10.1186/2008-2231-20-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun SH, Lee EJ, Jang TS, Kim HE, Jang JH, Koh YH. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2013;24:773–782. doi: 10.1007/s10856-012-4822-0. [DOI] [PubMed] [Google Scholar]
- 12.Walker DH, Wright NM. Bone morphogenetic proteins and spinal fusion. Neurosurg Focus. 2002;13:e3. doi: 10.3171/foc.2002.13.6.4. [DOI] [PubMed] [Google Scholar]
- 13.Segredo-Morales E, García-García P, Évora C, Delgado A. BMP delivery systems for bone regeneration: Healthy vs osteoporotic population. Review. J Drug Delivery Sci Technol. 2017;42:107–118. doi: 10.1016/j.jddst.2017.05.014. [DOI] [Google Scholar]
- 14.Chung RJ, Ou KL, Tseng WK, Liu HL. Controlled release of BMP-2 by chitosan/γ-PGA polyelectrolyte multilayers coating on titanium alloy promotes osteogenic differentiation in rat bone-marrow mesenchymal stem cells. Surface Coatings Technol. 2016;303:283–288. doi: 10.1016/j.surfcoat.2016.03.081. [DOI] [Google Scholar]
- 15.Lee CH, Jin MU, Jung HM, Lee JT, Kwon TG. Effect of dual treatment with SDF-1 and BMP-2 on ectopic and orthotopic bone formation. PLoS One. 2015;10:e0120051. doi: 10.1371/journal.pone.0120051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudarić L, Cvek SZ, Cvijanović O, Santić V, Marić I, Crncević-Orlić Z, Bobinac D. Expression of the BMP-2, −4 and −7 and their antagonists gremlin, chordin, noggin and follistatin during ectopic osteogenesis. Coll Antropol. 2013;37:1291–1298. [PubMed] [Google Scholar]
- 17.Xia YJ, Xia H, Chen L, Ying QS, Yu X, Li LH, Wang JH, Zhang Y. Efficient delivery of recombinant human bone morphogenetic protein (rhBMP-2) with dextran sulfate-chitosan microspheres. Exp Ther Med. 2018;15:3265–3272. doi: 10.3892/etm.2018.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Özbaş-Turan S, Akbuǧa J, Aral C. Controlled release of interleukin-2 from chitosan microspheres. J Pharm Sci. 2002;91:1245–1251. doi: 10.1002/jps.10122. [DOI] [PubMed] [Google Scholar]
- 19.Yan MA. Guide for the care and use of laboratory animals. Sichuan Animal. 1989;19:29–30. [Google Scholar]
- 20.Zhang W, Kaplan DL, Jiang X, Zhao J, Xu L, Zhu C, Zeng D, Chen J, Zhang Z, Kaplan DL, Jiang X. The use of injectable sonication-induced silk hydrogel for VEGF(165) and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials. 2011;32:9415–9424. doi: 10.1016/j.biomaterials.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Wang G, Lin X, Chatzinikolaidou M, Jennissen HP, Laub M, Uludağ H. Polyethylenimine-coated albumin nanoparticles for BMP-2 delivery. Biotechnol Prog. 2008;24:945–956. doi: 10.1002/btpr.12. [DOI] [PubMed] [Google Scholar]
- 22.Fan J, Park H, Lee MK, Bezouglaia O, Fartash A, Kim J, Aghaloo T, Lee M. Adipose-derived stem cells and BMP-2 delivery in chitosan-based 3D constructs to enhance bone regeneration in a rat mandibular defect model. Tissue Eng Part A. 2014;20:2169–2179. doi: 10.1089/ten.tea.2013.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Bhakta G, Lim ZX, Rai B, Lin T, Hui JH, Prestwich GD, van Wijnen AJ, Nurcombe V, Cool SM. The influence of collagen and hyaluronan matrices on the delivery and bioactivity of bone morphogenetic protein-2 and ectopic bone formation. Acta Biomater. 2013;9:9098–9106. doi: 10.1016/j.actbio.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Yilgor P, Tuzlakoglu K, Rui LR, Hasirci N, Hasirci V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30:3551–3559. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Gan Q, Zhu J, Yuan Y, Honglai Liu, Qian J, Lib Y, Liu C. A dual-delivery system of pH-responsive chitosan-functionalized mesoporous silica nanoparticles bearing BMP-2 and dexamethasone for enhanced bone regeneration. J Materials Chemistry B. 2015;3:2056–2066. doi: 10.1039/C4TB01897D. [DOI] [PubMed] [Google Scholar]
- 27.Ma SY, Feng ZQ, Lai RF, Zhou ZY, Yin ZD. Synergistic effect of RhBMP-2 and bFGF on ectopic osteogenesis in mice. Asian Pac J Trop Med. 2015;8:53–59. doi: 10.1016/S1995-7645(14)60187-5. [DOI] [PubMed] [Google Scholar]
- 28.Lai RF, Li ZJ, Zhou ZY, Feng ZQ, Zhao QT. Effect of rhBMP-2 sustained-release nanocapsules on the ectopic osteogenesis process in Sprague-Dawley rats. Asian Pac J Trop Med. 2013;6:884–888. doi: 10.1016/S1995-7645(13)60157-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.