Abstract
The present study aimed to investigate the function and mechanism of microRNA-638 (miR-638) in osteosarcoma. MiR-638 expression change in patients with osteosarcoma was detected by reverse transcription-quantitative polymerase chain reaction. Expression of miR-638 was observed to be downregulated in patients with osteosarcoma compared with the control group. In vitro, overexpression of miR-638 induced apoptosis, and inhibited cell proliferation and invasion of osteosarcoma cells. Overexpression of miR-638 induced Bcl-2 associated X and caspase-3 protein expression, and suppressed cyclin D1, phospholipase D1 (PLD1) and vascular endothelial growth factor (VEGF) protein expression in osteosarcoma. The promotion of PLD1 decreased the effects of miR-638 on osteosarcoma cell proliferation. In summary, it was demonstrated that miRNA-638 expression change in patients with osteosarcoma and an in vitro model via PLD1 and VEGF expression and miRNA-638 may be potential clinical indicators of osteosarcoma.
Keywords: microRNA-638, osteosarcoma, phospholipase D1, vascular endothelial growth factor
Introduction
Osteosarcoma accounts for ~0.2% of human solid malignant tumors. The age of onset is generally between 15 and 25 years old (1). Osteosarcoma is more common in males compared with females and frequently occurs in the metaphysis of long bones (1). Of these, the distal and proximal femurs are the most common, followed by the proximal fibula and other limb bones (1). Osteosarcoma may also occur in other bone tissues, including the spine and upper femur (1). The growth invasiveness of osteosarcoma can cause periosteal reaction, which forms a Codman triangle (2). Patients with osteosarcoma are prone to developing early lung metastasis (2). This may be one of the reasons for the 5-year survival rate of patients with osteosarcoma after amputation of only 5–20% (2).
Osteosarcoma primarily occurs in young people. It has a high malignancy grade and severely harms the health of young patients (3). A large body of research has deepened our understanding of osteosarcoma, but its pathogenesis is quite complex (4). It is associated with various genetic factors, including abnormal activation, gene silencing and regulation of pathways (5). Recent research has also indicated that miRNA and lncRNA are involved in the pathogenesis of osteosarcoma (4).
The Human Genome Project was completed in 2003 (6), and the function of RNA in biological processes has been increasingly emphasized (7). MicroRNA (miRNA) was rated as one of the ten major breakthroughs in science and technology by Science in 2002 and 2003 (8). It has become a popular topic in the field of RNA research. At present, almost 1,400 miRNAs have been reported in biological species (8). These species include Drosophila melanogaster, rodents, humans and plants. MiRNAs are closely associated with tissue and organ development, cell proliferation, differentiation and apoptosis. In addition, they are associated with fat metabolism and other activities of animals and plants (4).
Osteosarcoma is a vascular malignant tumor (9). Previous results have demonstrated that vascular endothelial growth factor (VEGF) expression level is associated with microvessel density and metastasis of osteosarcoma (9). A previous study reported that favorable conditions for the survival of malignant tumors are associated with increased microvessel density in osteosarcoma (10). VEGF is the most typical angiogenic factor (10). It can stimulate endothelial cell proliferation, migration and vascular maturation (10). When VEGF is combined with the VEGF receptor, a number of different signaling pathways will be activated (10). Furthermore, nitric oxide, phospholipase Cy and protein kinase C will be released. This can promote angiogenesis (10). Cheng et al (11) observed that miR-638 inhibits cell proliferation in human gastric carcinoma. The present study aimed to investigate the function and mechanism of miRNA-638 in osteosarcoma.
Materials and methods
Clinical samples
Serum (10 ml) of male osteosarcoma patients (n=6; age, 51–64 years) and male healthy volunteers (n=6; age, 55–62 years) was collected at the Department of Physiology, Xuzhou Medical University (Xuzhou, China) from Dec 2015 to Feb 2016 and centrifuged at 8,000 × g for 10 min at 4°C. Serum samples were snap frozen in liquid nitrogen immediately after resection and stored at −80°C. The study protocol was approved by the Medical Ethics Committee of the Xuzhou Hospital of Traditional Chinese Medicine. Written informed consent was obtained from all participants for their inclusion in the present study.
Cell lines and transfection
The human osteosarcoma cell line MG63 was purchased from Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China) and grown in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences) at 37°C in a humidified atmosphere of 5% CO2. MiR-638 (5′-GTGAGCGGGCGCGGCAGGGATCGCGGGCGG-3′), anti-miR-638 (5′-AGGCCGCCACCCGCCCGCGATCCCT-3′), negative control mimics (5′-TGACTGTACTGAACTCGACTG-3′) and phospholipase D1 (PLD1) plasmids were supplied by GenePharma Co., Ltd. (Shanghai, China). The MG63 cell line was plated in a 12-well plate and transfected with 100 nM of miR-638, anti-miR-638, negative control mimics and PLD1 plasmid using Lipofectamine 3000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and then incubated for 6 h.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from serum samples or MG63 cells using RNAiso reagent (Takara Bio, Inc., Otsu, Japan) and cDNA was synthesized using the PrimeScript RT reagent kit (Takara Bio, Inc.). qPCR was conducted to quantify relative mRNA expression using SYBR Premix Ex Taq (Takara Bio, Inc.). The primers for miR-638 were: Forward, 5′-GAGAGGATCCTGCCGCAGATCGCTG-3′ and reverse, 5′-GAGTAAGCTTCAGGGAGTCCTCTGCC-3′. The primers for U6 were: Forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The reactions were incubated at 95°C for 5 min, followed by 40 cycles of 95°C for 30 sec and 60°C for 30 sec. The 2−ΔΔCq method was used to analyze the relative expression of target genes (12).
Cell proliferation assay
Cells (1×105) were plated in 96-well culture plates following transfection for 6 h, and incubated for 0, 24, 48 and 72 h at 37°C. Cell proliferation was evaluated with Cell Counting Kit-8 (Beyotime Institute of Biotechnology, Shanghai, China) for 2 h, and examined by measuring absorbance at 450 nm.
Transwell invasion assay
Following transfection for 6 h, cells were plated in 24-well Transwell inserts coated with Matrigel (8-µm; BD Biosciences, San Jose, CA, USA) for cell invasion assays. Cells (1×105 cell/well) were plated in the upper chamber in RPMI-1640 medium and the lower chamber contained RPMI-1640 medium with 10% FBS. The cells on the upper chamber were removed using cotton swabs following 48 h incubation. The cells on the lower surface were fixed with 4% formaldehyde solution for 15 min at room temperature and stained with hematoxylin for 5 min at room temperature. Cells were observed using a light microscope and analyzed using Image Lab 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometry
After transfection for 48 h, cells were stained with PBS containing propidium iodide (50 µg/ml)/Annexin V (BD Biosciences) for 15 min in the dark at room temperature. The percentage of apoptotic cells was measured using a BD FACSCalibur system (FACScan; BD Biosciences) and analyzed using FlowJo version 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Western blotting analysis
Protein was isolated from cells following 48 h of transfection using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) and measured using the BCA method (Beyotime Institute of Biotechnology). Proteins (50 µg) were then subjected to 8–12% SDS-PAGE for separation before being transferred onto a polyvinylidene membrane (EMD Millipore, Billerica, MA, USA). The membrane was blocked with 5% nonfat milk for 1 h at 37°C and incubated with antibodies against Bcl-2-associated X (Bax; cat. no. 5023), cyclin D1 (cat. no. 2978), PLD1 (cat. no. 3832), VEGF (cat. no. 2463) (all dilution 1:2,000) and GAPDH (cat. no. 5174; dilution 1:5,000) (all Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. The membranes were subsequently incubated with a goat anti-rabbit horseradish peroxidase secondary antibody (cat. no. 7074; dilution 1:5,000; Cell Signaling Technology, Inc.) at 37 °C for 1 h and detected using enhanced chemiluminescence reagents (Pierce; Thermo Fisher Scientific, Inc.), and Image-Pro Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Caspase-3 activity
Protein was isolated from cells following 48 h of transfection as described above. A total of 10 µg protein was used to measure the caspase-3 activity using a caspase-3 activity kit (cat. no. C1116; Beyotime Institute of Biotechnology) and the absorbance was measured at 405 nm.
Statistical analysis
Data are presented as the mean ± standard deviation using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical comparisons were performed using a Student's t-test or one-way analysis of variance followed by Dunnett's test. Each experiment was performed a minimum of three times. P<0.05 was considered to indicate a statistically significant difference.
Results
MiR-638 expression change in patients with osteosarcoma
The expression of miR-638 was evaluated in the serum of patients with osteosarcoma and normal controls. As indicated in Fig. 1, the miR-638 serum level was significantly decreased in patients with osteosarcoma compared with the normal controls.
Figure 1.
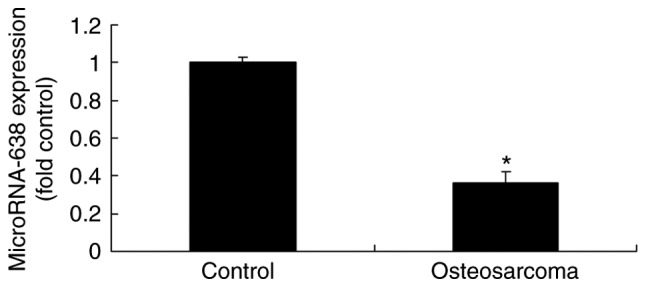
MicroRNA-638 expression in patients with osteosarcoma. Control, healthy volunteers; Osteosarcoma, patients with osteosarcoma. *P<0.01 vs. Control.
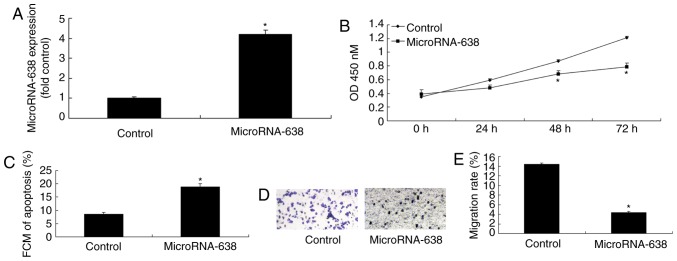
Overexpression of miR-638 induces apoptosis, and inhibits cell proliferation and invasion of osteosarcoma cells
In order to verify the function of miR-638 in osteosarcoma, miR-638 expression was upregulated in osteosarcoma cells using miR-638 mimics. As indicated in Fig. 2, miR-638 expression was significantly upregulated in osteosarcoma cells transfected with miR-638 compared with the control. In addition, overexpression of miR-638 significantly induced apoptosis, and significantly inhibited cell proliferation (at 48 and 72 h) and invasion of osteosarcoma cells compared with the control (Fig. 2).
Figure 2.
Overexpression of microRNA-638 induces apoptosis, and inhibits cell proliferation and invasion of osteosarcoma cells. (A) MicroRNA-638 expression. (B) Cell proliferation. (C) Cell apoptosis. (D and E) Cell invasion (magnification, ×100). *P<0.01 vs. Control. Control, negative control group; MicroRNA-638, overexpression of microRNA-638 group; FCM, flow cytometry; OD, optical density.
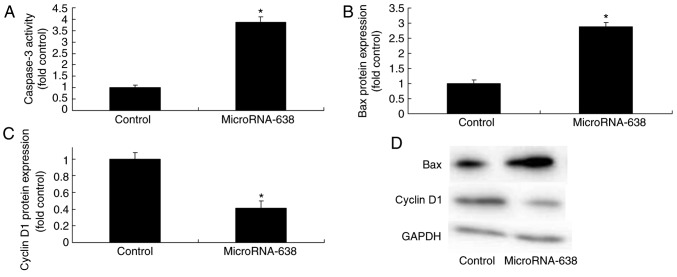
Overexpression of miR-638 affects Bax, caspase-3 and cyclin D1 protein expression in osteosarcoma cells
Overexpression of miR-638 significantly increased caspase-3 activity and Bax protein expression, and significantly decreased cyclin D1 protein expression in osteosarcoma cells compared with the control group (Fig. 3).
Figure 3.
Overexpression of micrsoRNA-638 affects Bax, caspase-3 and cyclin D1 protein expression in osteosarcoma cells. (A) Caspase-3 activity. Relative (B) Bax and (C) cyclin D1 protein expression was evaluated by (D) western blotting analysis in osteosarcoma cells. *P<0.01 vs. Control. Control, negative control group; MicroRNA-638, overexpression of microRNA-638 group; Bax, Bcl-2-associated X protein.
Overexpression of miR-638 affects PLD1 and VEGF protein expression of osteosarcoma
To elucidate the mechanism underlying miR-638-mediated suppression of cell proliferation, PLD1 and VEGF protein expression were measured using western blotting analysis. The results demonstrated that overexpression of miR-638 significantly reduced PLD1 and VEGF protein expression in osteosarcoma cells compared with the control group (Fig. 4).
Figure 4.
Overexpression of microRNA-638 affects PLD1 and VEGF protein expression in osteosarcoma cells. (A) Western blotting analysis. (B) Relative VEGF expression. (C) Relative PLD1 expression. *P<0.01 vs. Control. Control, negative control group; MicroRNA-638, overexpression of microRNA-638 group; PLD1, phospholipase D1; VEGF, vascular endothelial growth factor.
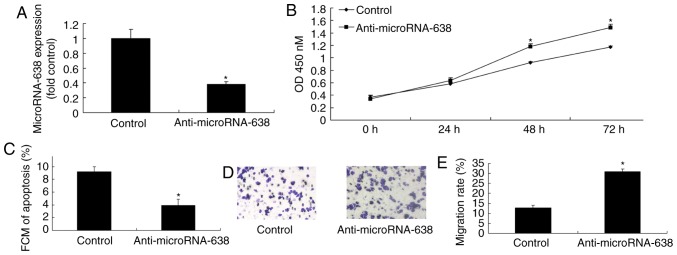
Downregulation of miR-638 inhibits apoptosis, and promotes cell growth and invasion of osteosarcoma cells
To further verify the function of miR-638 in apoptosis of osteosarcoma cells, miR-638 expression was downregulated using anti-miR-638 mimics. The expression of miR-638 was significantly lower in the anti-miR-638 group compared with the control group. In addition, downregulation of miR-638 significantly inhibited apoptosis, and significantly promoted cell proliferation (at 48 and 72 h) and invasion of osteosarcoma cells compared with the control (Fig. 5).
Figure 5.
Downregulation of microRNA-638 inhibited apoptosis, and promoted cell growth and invasion of osteosarcoma cells. (A) MicroRNA-638 expression. (B) Cell proliferation. (C) Cell apoptosis. (D and E) Cell invasion (magnification, ×100). *P<0.01 vs. Control. Control, negative control group; Anti-microRNA-638, downregulation of microRNA-638 group; FCM, flow cytometry; OD, optical density.
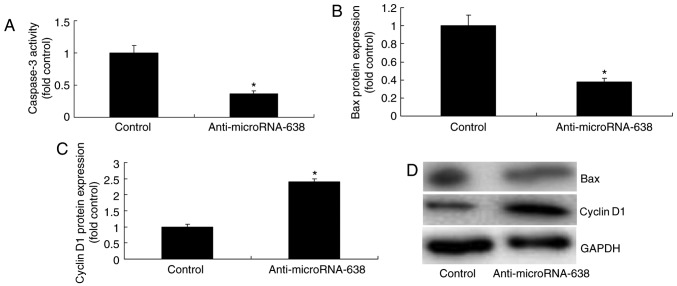
Downregulation of miR-638 affects caspase-3 activity and Bax and cyclin D1 protein expression in osteosarcoma cells
Downregulation of miR-638 significantly reduced caspase-3 activity and Bax protein expression, and significantly increased cyclin D1 protein expression in osteosarcoma cells compared with the control group (Fig. 6).
Figure 6.
Downregulation of microRNA-638 affects Bax, caspase-3 and cyclin D1 protein expression in osteosarcoma cells. (A) Caspase-3 activity. Relative (B) Bax and (C) cyclin D1 protein expression was evaluated by (D) western blotting analysis in osteosarcoma cells. *P<0.01 vs. Control. Control, negative control group; Anti-microRNA-638, downregulation of microRNA-638 group; Bax, Bcl-2-associated X protein.
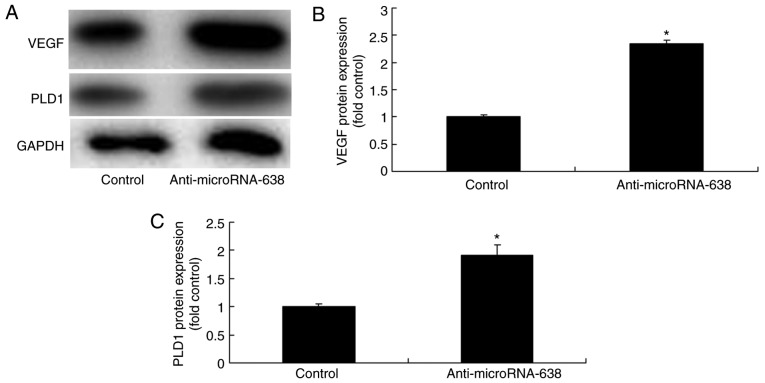
Downregulation of miR-638 affects PLD1 and VEGF protein expression in osteosarcoma cells
It was investigated whether miR-638 downregulation affected PLD1 and VEGF protein expression in osteosarcoma cells. Downregulation of miR-638 significantly increased PLD1 and VEGF protein expression in osteosarcoma cells compared with the control group (Fig. 7).
Figure 7.
Downregulation of microRNA-638 affects PLD1 and VEGF protein expression in osteosarcoma cells. (A) Western blotting analysis. (B) Relative VEGF expression. (C) Relative PLD1 expression. *P<0.01 vs. Control. Control, negative control group; Anti-microRNA-638, downregulation of microRNA-638 group; PLD1, phospholipase D1; VEGF, vascular endothelial growth factor.
Promotion of PLD1 expression induces PLD1 protein expression in osteosarcoma cells following microRNA-638
In order to evaluate the association between miR-638 and PLD1 in human osteosarcoma, PLD1 expression was promoted using PLD1 plasmids. As indicated in Fig. 8, PLD1 and VEGF protein expression were significantly increased in osteosarcoma cells following promotion of microRNA-638 + PLD1 as compared with the miR-638 group.
Figure 8.
Promotion of PLD1 expression induces PLD1 and VEGF protein expression in osteosarcoma cells following microRNA-638 overexpression. (A) Western blotting analysis. (B) Relative VEGF expression. (C) Relative PLD1 expression. *P<0.01 vs. Control; **P<0.01 vs. MicroRNA-638 group. Control, negative control group; MicroRNA-638, overexpression of microRNA-638 group; PLD1, overexpression of microRNA-638 and phospholipase D1 group; VEGF, vascular endothelial growth factor.
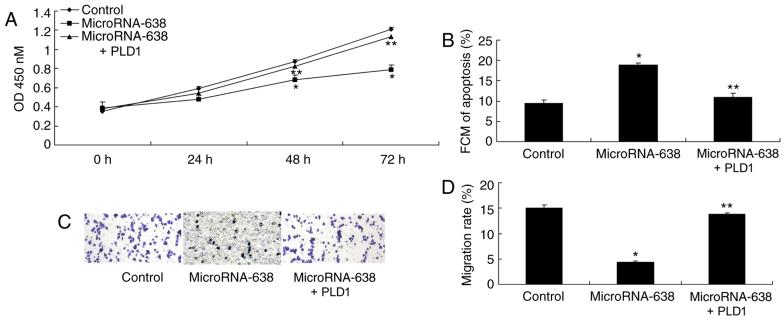
Promotion of PLD1 expression affects cell apoptosis, cell growth and invasion of osteosarcoma cells following miR-638 transfection
Promotion of PLD1 expression significantly increased cell proliferation (at 48 and 72 h) and invasion, and significantly reduced cell apoptosis of osteosarcoma cells following miR-638 transfection, as compared with the miR-638 group (Fig. 9).
Figure 9.
Promotion of PLD1 expression affects cell apoptosis, cell proliferation and invasion of osteosarcoma following microRNA-638 overexpression. (A) Cell proliferation. (B) Cell apoptosis. (C and D) Cell invasion (magnification, ×100). *P<0.01 vs. Control; **P<0.01 vs. MicroRNA-638 sgroup. Control, negative control group; MicroRNA-638, overexpression of microRNA-638 group; PLD1, overexpression of microRNA-638 and phospholipase D1 group; OD, optical density; FCM, flow cytometry.
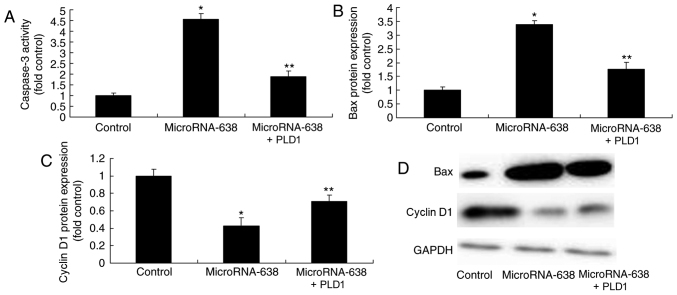
Promotion of PLD1 expression affects caspase-3 activity and Bax and cyclin D1 protein expression in osteosarcoma cells following miR-638 transfection
The promotion of PLD1 expression significantly suppressed caspase-3 activity and Bax protein expression, and significantly increased cyclin D1 protein expression in osteosarcoma cells following miR-638 transfection, as compared with the miR-638 group (Fig. 10).
Figure 10.
Promotion of PLD1 expression affects Bax, caspase-3 and cyclin D1 protein expression in osteosarcoma cells following microRNA-638 overexpression. (A) Caspase-3 activity. Relative (B) Bax and (C) cyclin D1 protein expression was evaluated by (D) western blotting analysis in osteosarcoma cells. *P<0.01 vs. Control; **P<0.01 vs. MicroRNA-638 group. Control, negative control group; MicroRNA-638, overexpression of microRNA-638 group; PLD1, overexpression of microRNA-638 and phospholipase D1 group; Bax, Bcl-2-associated X protein.
Discussion
In recent years, the treatment of osteosarcoma has developed in two aspects. One is the application of comprehensive treatment based on neoadjuvant chemotherapy (13). The other is the development of limb salvage surgery (13), which greatly reduces amputation rate. In the future a surgery-based comprehensive treatment of osteosarcoma should be adopted, which includes surgery, chemotherapy and radiotherapy (14). Early diagnosis, careful preoperative typing, and standardized treatment of osteosarcoma can significantly improve the prognosis of osteosarcoma patients (15).
In the present study, it was demonstrated that miR-638 serum level was downregulated in patients with osteosarcoma compared with the normal group. Cheng et al (11) observed that miR-638 inhibits cell proliferation in human gastric carcinoma. However, in the present study, only six samples were obtained, which was insufficient for this type of investigation. A larger number of clinical samples will be used in a future study.
Osteosarcoma is a type of vascular malignant tumor. Previous research has indicated that VEGF expression level is significantly correlated with microvessel density and metastasis of osteosarcoma (10). However, there is also research suggesting that the correlation may be attributed to a large tumor being dependent on mature blood vessel functionality (16). A previous study reported that the beneficial survival conditions of osteosarcoma were associated with increased microvessel density in osteosarcoma (9). In the present study, it was identified that miR-638 was downregulated in osteosarcoma and in vitro studies indicated that this was associated with increased protein expression of PLD1 and VEGF. Cheng et al (11) identified that the downregulation of miR-638 promotes angiogenesis and growth by targeting VEGF in hepatocellular carcinoma. In the present study, only an osteosarcoma cell line MG63 was used, which was insufficient for this type of study. Additional osteosarcoma cell lines will be used in future studies.
VEGF increased the activity of matrix metalloproteinases (MMPs) and fibrinolytic enzymes and suppressed VEGF degradation of the extracellular matrix by binding with MMPs on the cell membrane (17). VEGF also has an inductive effect on anti-apoptosis factors Bcl-2 and survivin, which can induce the proliferation of vascular endothelium (18). Abnormal proliferation and uncontrolled differentiation of cells are the basic pathological mechanisms of malignant tumors (19). It has been reported that the abnormal proliferation and differentiation of cells are associated with imbalances in the mechanism of apoptosis (18). Research has indicated that the Bcl-2 family serves a key function in regulating apoptosis (18). When the normal apoptosis mechanism is disrupted, tumors may occur. Bcl-2 is an apoptosis suppressor gene and also serves as an important proto-oncogene by acting on the signal transduction pathway of apoptosis (18). Bcl-2 can inhibit cell apoptosis and prolong the survival of cells. Thus, it creates an opportunity for the development of tumors (5). Bcl-2 family proteins are divided into two types: Pro-apoptosis and anti-apoptosis. Bax is a promoting apoptosis member, while Bcl-2 is an anti-apoptosis member (5). Bcl-2 regulates apoptosis and further activates caspase signal cascades by targeting cell mitochondria (5). The present study indicated that the promotion of PLD1 decreased the effects of miR-638 on osteosarcoma cell proliferation. MiR-638 may affect MMPs, particularly MMP-2 and −9, to influence invasion activity. Thus, the protein expression of MMP-2 and −9 will be investigated in future studies.
Cyclin D1 is a key molecule for cells to transform from G1 phase to S phase (20). siRNA targeting Cyclin D1 can interfere with the expression of Cyclin D1 (21). This leads to tumor cell arrest in G1 phase. Inhibition of Cyclin D1 expression can change the distribution pattern of the cell cycle (21). The proportion of G0/1 phase increases, S phase decreases and G1/S increases significantly, indicating cell cycle arrest at G1/S. In the present study, it was identified that overexpression of miR-638 suppressed cyclin D1 protein expression in osteosarcoma. Li et al (22) indicated that miR-638 inhibits cell proliferation and migration through cyclin D1 expression.
In conclusion, the results of the current study indicate that miR-638 may serve a function in tumor growth and metastasis by suppressing osteosarcoma cell proliferation in an in vitro model. The present study demonstrates the importance of miR-638/PLD1/VEGF signaling in osteosarcoma development.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science Youth Foundation of China (grant no. 81500914).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
BS designed the experiment. MX, JS, JC, JW, WQ, ND and CS performed the experiments. BS and MX analyzed the data. BS wrote the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Medical Ethics Committee of the Xuzhou Hospital of Traditional Chinese Medicine and written informed consent was obtained from all participants prior to their inclusion within the study.
Patient consent for publication
Written informed consent was obtained from all participants for the publication of their data.
Competing interests
The authors confirm that they have no competing interests.
References
- 1.Morris CD, Teot LA, Bernstein ML, Marina N, Krailo MD, Villaluna D, Janeway KA, DuBois SG, Gorlick RG, Randall RL. Assessment of extent of surgical resection of primary high-grade osteosarcoma by treating institutions: A report from the children's oncology group. J Surg Oncol. 2016;113:351–354. doi: 10.1002/jso.24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nataraj V, Batra A, Rastogi S, Khan SA, Sharma MC, Vishnubhatla S, Bakhshi S. Developing a prognostic model for patients with localized osteosarcoma treated with uniform chemotherapy protocol without high dose methotrexate: A single-center experience of 237 patients. J Surg Oncol. 2015;112:662–668. doi: 10.1002/jso.24045. [DOI] [PubMed] [Google Scholar]
- 3.Xie L, Liao Y, Shen L, Hu F, Yu S, Zhou Y, Zhang Y, Yang Y, Li D, Ren M, et al. Identification of the miRNA-mRNA regulatory network of small cell osteosarcoma based on RNA-seq. Oncotarget. 2017;8:42525–42536. doi: 10.18632/oncotarget.17208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiwara T, Uotani K, Yoshida A, Morita T, Nezu Y, Kobayashi E, Yoshida A, Uehara T, Omori T, Sugiu K, et al. Clinical significance of circulating miR-25-3p as a novel diagnostic and prognostic biomarker in osteosarcoma. Oncotarget. 2017;8:33375–33392. doi: 10.18632/oncotarget.16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gai P, Sun H, Wang G, Xu Q, Qi X, Zhang Z, Jiang L. miR-22 promotes apoptosis of osteosarcoma cells via inducing cell cycle arrest. Oncol Lett. 2017;13:2354–2358. doi: 10.3892/ol.2017.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Long F, Wan J, Hu Y, He H. MicroRNA-205 acts as a tumor suppressor in osteosarcoma via targeting RUNX2. Oncol Rep. 2016;35:3275–3284. doi: 10.3892/or.2016.4700. [DOI] [PubMed] [Google Scholar]
- 7.Xu M, Jin H, Xu CX, Sun B, Mao Z, Bi WZ, Wang Y. miR-382 inhibits tumor growth and enhance chemosensitivity in osteosarcoma. Oncotarget. 2014;5:9472–9483. doi: 10.18632/oncotarget.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai N, Qing Y, Cun Y, Zhong Z, Li C, Zhang S, Shan J, Yang X, Dai X, Cheng Y, et al. miR-513a-5p regulates radiosensitivity of osteosarcoma by targeting human apurinic/apyrimidinic endonuclease. Oncotarget. 2016;9:25414–25426. doi: 10.18632/oncotarget.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, Xiao Z, Chen L, Xiong S. Comment on Xu XW et al: Prognostic significance of VEGF expression in osteosarcoma: A meta-analysis. Tumour Biol. 2014;35:6193–6194. doi: 10.1007/s13277-014-2024-8. [DOI] [PubMed] [Google Scholar]
- 10.Ohba T, Cates JM, Cole HA, Slosky DA, Haro H, Ando T, Schwartz HS, Schoenecker JG. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol Cancer Res. 2014;12:1100–1111. doi: 10.1158/1541-7786.MCR-14-0037. [DOI] [PubMed] [Google Scholar]
- 11.Cheng J, Chen Y, Zhao P, Liu X, Dong J, Li J, Huang C, Wu R, Lv Y. Downregulation of miRNA-638 promotes angiogenesis and growth of hepatocellular carcinoma by targeting VEGF. Oncotarget. 2016;7:30702–30711. doi: 10.18632/oncotarget.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Kudawara I, Aoki Y, Ueda T, Araki N, Naka N, Nakanishi H, Matsumine A, Ieguchi M, Mori S, Myoui A, et al. Neoadjuvant and adjuvant chemotherapy with high-dose ifosfamide, doxorubicin, cisplatin and high-dose methotrexate in non-metastatic osteosarcoma of the extremities: A phase II trial in Japan. J Chemother. 2013;25:41–48. doi: 10.1179/1973947812Y.0000000055. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Xu H, He M, Wang Z, Wu Y. Rho GTPase-activating protein 35 rs1052667 polymorphism and osteosarcoma risk and prognosis. Biomed Res Int. 2014;2014:396947. doi: 10.1155/2014/396947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y, Sangiolo D, Marchesi E, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16:98–107. doi: 10.1016/S1470-2045(14)71136-2. [DOI] [PubMed] [Google Scholar]
- 16.Lin CY, Tzeng HE, Li TM, Chen HT, Lee Y, Yang YC, Wang SW, Yang WH, Tang CH. WISP-3 inhibition of miR-452 promotes VEGF-A expression in chondrosarcoma cells and induces endothelial progenitor cells angiogenesis. Oncotarget. 2017;8:39571–39581. doi: 10.18632/oncotarget.17142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Wen P, Luo X, Fang X, Wang Q, Ma F, Lv J. Association of the vascular endothelial growth factor (VEGF) gene single-nucleotide polymorphisms with osteosarcoma susceptibility in a Chinese population. Tumour Biol. 2014;35:3605–3610. doi: 10.1007/s13277-013-1475-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Zhao Y, Zeng B. Enhanced chemosensitivity by simultaneously inhibiting cell cycle progression and promoting apoptosis of drug-resistant osteosarcoma MG63/DXR cells by targeting Cyclin D1 and Bcl-2. Cancer Biomark. 2012;12:155–167. doi: 10.3233/CBM-130305. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Zheng Q, Wu H, Guo X, Li J, Hao S. Rapamycin increases pCREB, Bcl-2, and VEGF-A through ERK under normoxia. Acta Biochim Biophys Sin (Shanghai) 2013;45:259–267. doi: 10.1093/abbs/gmt002. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Ni J, Yi S, Song D, Ding M. Protein inhibitor of activated STAT xalpha depresses cyclin D and cyclin D kinase, and contributes to the inhibition of osteosarcoma cell progression. Mol Med Rep. 2016;13:1645–1652. doi: 10.3892/mmr.2015.4705. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Cui LL, Yuan J, Wang Y, Song S. Clinical significance of the phosphorylation of MAPK and protein expression of cyclin D1 in human osteosarcoma tissues. Mol Med Rep. 2017;15:2303–2307. doi: 10.3892/mmr.2017.6224. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Liu Y, Yi B, Wang G, You X, Zhao X, Summer R, Qin Y, Sun J. MicroRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGF-BB-induced cell proliferation and migration through targeting orphan nuclear receptor NOR1. Cardiovasc Res. 2013;99:185–193. doi: 10.1093/cvr/cvt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.