Abstract
This study explored the diagnostic value of computed tomography (CT) in pulmonary fungal infection to provide a theoretical basis for the clinical diagnosis of pulmonary fungal infections. The clinical data of 82 suspected invasive fungal infection (IFI) patients admitted to the Department of Critical Care Medicine of The Affiliated Hospital of Qingdao University from January 2016 to May 2018 were retrospectively analyzed, and 64 of them were diagnosed with IFI by pathology and sputum culture. The CT results of the 82 patients were compared with the X-ray results in order to analyze the diagnostic value of CT imaging. Taking pathological diagnosis as the gold standard, the number of true-negative, true-positive, false-negative and false-positive results in X-ray diagnosis were 13, 43, 21 and 5, respectively, while those in CT diagnosis were 11, 59, 5 and 7, respectively. The sensitivity, specificity, accuracy, positive coincidence rate, negative coincidence rate, misdiagnosis rate and missed diagnosis rate of CT in IFI were 92.18, 61.11, 85.37, 89.39, 68.75, 38.89 and 7.81%, respectively, while those of X-ray in IFI were 67.19, 72.22, 68.29, 89.58, 38.24, 27.78 and 32.81%, respectively. The sensitivity, accuracy and negative coincidence rate of CT in the diagnosis of IFI were significantly higher than those of X-ray (P<0.05), with a sensitivity of 92.18%, which indicates that CT has a higher diagnostic value in IFI. The results of CT combined with the basic condition of the patients can be used to initially diagnose pulmonary fungal infections, which is of high diagnostic value and can improve clinical treatment.
Keywords: spiral computed tomography, invasive pulmonary fungal infection, diagnosis
Introduction
With the development of health care, the incidence of invasive fungal infections (IFIs) in the high risk and impaired immune-function population has been increasing (1). At present, it is believed that the main risk factors for IFI include neutropenia, malignant hematopathy, bone marrow transplantation, solid organ transplantation, severe burn, glucocorticoids, long-term intensive care, chemotherapy, HIV infection, invasive medical procedures and new immunosuppression agents (2–4). The patients in ICU have the characteristics of long-term bed rest, complicated basic diseases and low immune system, which create the conditions for the breeding and implantation of fungi, leading to an increasing number of pulmonary fungal infections (5–7). The early diagnosis of IFI, especially pulmonary fungal infection, remains a challenge in medical research (8). Invasive pulmonary aspergillosis (IPA) is the most common type in fungal pneumonia (9,10). At present, the diagnosis of IFI relies on complex microbiological methods (11,12), including culture and molecular identification.
Multi-slice spiral computed tomography (CT), a widely used imaging method in recent years, is reported to have achieved good results in the differential diagnosis of complicated pulmonary lesions by many scholars (13). Studies have also shown that CT is an important tool for the early diagnosis of pulmonary infection in immunocompromised hosts (14). The density and spatial resolution of multi-slice spiral CT have been greatly developed and unified. Its thin layer scan not only shows the details and changes of lung tissue more clearly, but also shows clearly all kinds of pathological changes, reconstructs images of any plane, such as axial, sagittal and coronal plane, and provides arbitrary section images by adjusting to different directions, allowing us to better understand the details of the lesion and the space anatomical relationship (15).
The purpose of this study was to investigate the diagnostic value of 64-slice spiral CT in invasive pulmonary fungal infection, and provide a reference for improving the treatment rate and prognosis of patients with pulmonary fungal infection.
Patients and methods
General information
The clinical data of 82 suspected IFI patients treated in the Department of Critical Care Medicine in The Affiliated Hospital of Qingdao University (Qingdao, China) from January 2016 to May 2018 were retrospectively analyzed, including 51 males and 31 females, with an average age of 40.59±10.34 years. There were 21 patients with hematologic tumors, 42 patients treated with immunosuppression agents, 7 patients treated with kidney transplantation and 12 patients treated with solid tumor chemotherapy. The main clinical symptoms were cough and expectoration, and some patients presented with chest pain, hemoptysis, and fever. All patients underwent X-ray and spiral CT examinations during hospitalization. Finally, 64 patients were diagnosed with IFI by pathology and sputum culture. Inclusion criteria: suspected patients with clinical symptoms consistent with IFI. Exclusion criteria: patients with other severe infections; patients with allergies to CT contrast agents; patients with short-term expected death; patients previously using antifungal drugs. The patients and their families agreed to participate in tis study and signed an informed consent. The study was approved by the Ethics Committee of The Affiliated Hospital of Qingdao University. The general information of the patients is shown in Table I.
Table I.
General information [n (%)].
| Clinical data | Cases (n=82) |
|---|---|
| Sex | |
| Male | 51 (62.20) |
| Female | 31 (37.80) |
| Age (years) | |
| ≥40 | 39 (47.56) |
| <40 | 43 (52.44) |
| BMI (kg/m2) | |
| ≥21 | 37 (45.12) |
| <21 | 45 (54.88) |
| Smoking | |
| Yes | 59 (71.95) |
| No | 23 (28.05) |
| Hormone administration | |
| Yes | 48 (58.54) |
| No | 34 (41.46) |
| Severe disease classification | |
| Hematologic tumor | 21 (25.61) |
| Immunosuppression therapy | 42 (51.22) |
| Kidney transplantation | 7 (8.54) |
| Solid tumor chemotherapy | 12 (14.63) |
Detection methods
Sixty-four-slice spiral CT was performed using a CT scanner obtained from GE Healthcare (Chicago, IL, USA). Patients were placed in supine position and scanned with conventional plain scan from lung apex to lung bottom (voltage, 120 kV; current, 200–250 mA; thick section, 5 mm; section interval, 5 mm). If necessary, thinning and reconstruction were performed as needed, and enhanced examination was performed. The lesion location was scanned at a thickness of 2 mm and the section interval was 2 mm. Mediastinal and pulmonary windows were used to observe the size, number, location, shape, density, boundary, surrounding structure and other imaging findings of lesions. The bedside chest radiography (X-ray) was performed by 12A radiographic device (Toshiba Medical Systems Corp., Tokyo, Japan), and chest radiography by GE Revolution XQ/i (GE Healthcare). All patients were examined in prone position and scans were carried out 2–3 times.
Outcome measures
Taking the results of pathology and sputum culture as the gold standard, the imaging features of CT in confirmed patients were analyzed and the sensitivity, specificity, accuracy, positive coincidence rate, negative coincidence rate, misdiagnosis rate and missed diagnosis rate of X-ray and CT in IFI were calculated.
Statistical analysis
SPSS 17.0 statistical software (Shanghai Cabit Information Technology Co., Ltd., Shanghai, China) was used for analysis. t-test was used for the measurement data. P<0.05 was considered to indicate a statistically significant difference.
Results
Spiral CT findings in 64 patients
Among 64 confirmed patients, there were 14 cases of pulmonary mucormycosis, 15 cases of pulmonary Candida infection, 20 cases of pulmonary Aspergillus infection, 13 cases of pulmonary Cryptococcus infection and 2 cases of pulmonary histoplasmosis. Representative CT scans are shown in Figs. 1–5.
Figure 1.
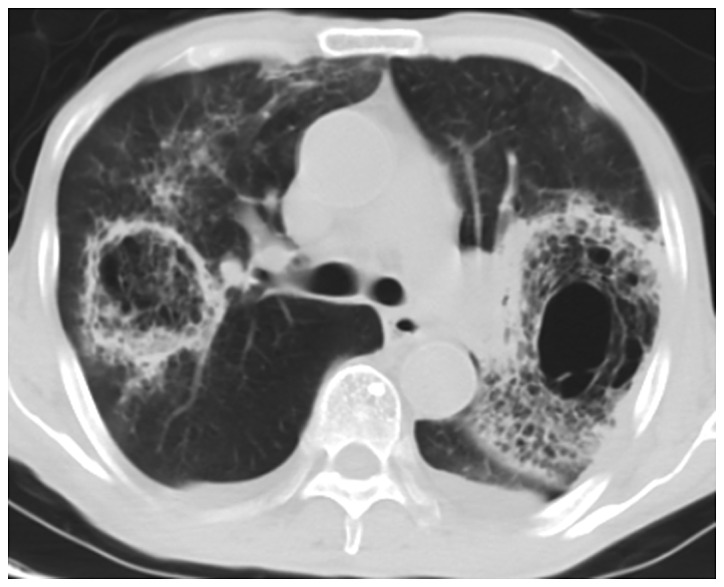
CT image of lung with pulmonary mucormycosis. The bilateral lungs show a symmetrical distribution of glass-like shadows with blurry boundaries and patchy solid shadows. CT, computed tomography.
Figure 5.
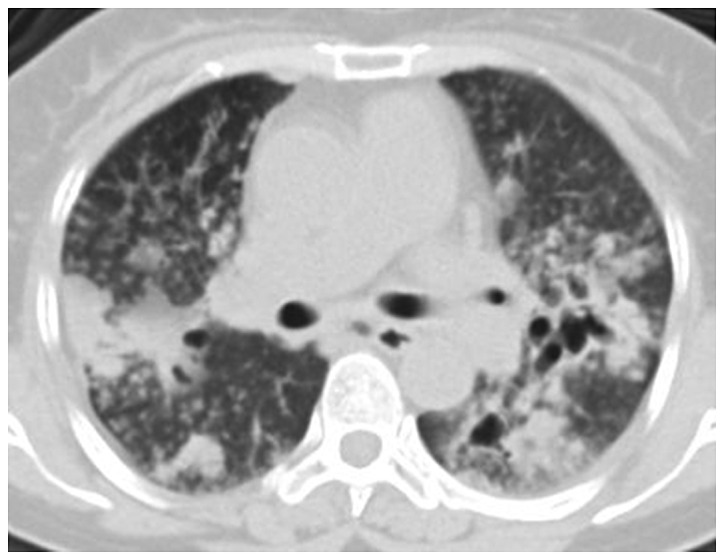
CT image of lung with pulmonary histoplasmosis. There is miliary pulmonary infiltration, cavitation formation and hilar lymphadenectasis, and significant hilar lymph nodes. In part of the lung apex appears a cavity with no obvious specific manifestations. CT, computed tomography.
X-ray and CT diagnosis
Taking pathological diagnosis as the gold standard, the number of true-negative, true-positive, false-negative and false-positive results in X-ray diagnosis were 13, 43, 21 and 5, respectively, while those in CT diagnosis were 11, 59, 5 and 7, respectively (Tables II and III).
Table II.
X-ray diagnosis.
| Pathological diagnosis | |||
|---|---|---|---|
| X-ray diagnosis | Negative | Positive | Total |
| Negative | 13 | 21 | 34 |
| Positive | 5 | 43 | 48 |
| Total | 18 | 64 | 82 |
Table III.
CT diagnosis.
| Pathological diagnosis | |||
|---|---|---|---|
| CT diagnosis | Negative | Positive | Total |
| Negative | 11 | 5 | 16 |
| Positive | 7 | 59 | 66 |
| Total | 18 | 64 | 82 |
CT, computed tomography.
Comparison of diagnostic value of X-ray and CT
The sensitivity, specificity, accuracy, positive coincidence rate, negative coincidence rate, misdiagnosis rate and missed diagnosis rate of X-ray in IFI were 67.19, 72.22, 68.29, 89.58, 38.24, 27.78 and 32.81%, respectively, while those of CT in IFI were 92.18, 61.11, 85.37, 89.39, 68.75, 38.89 and 7.81%, respectively. The sensitivity, accuracy and negative coincidence rate of CT in the diagnosis of IFI were significantly higher than those of X-ray (P<0.05), with a sensitivity of 92.18%, which indicates that CT had a high diagnostic value in IFI (Table IV).
Table IV.
Comparison of the diagnostic value of X-ray and CT.
| Diagnosis | X-ray (n=82) (%) | CT (n=82) (%) | t | P-value |
|---|---|---|---|---|
| Sensitivity | 67.19 | 92.18 | 12.36 | <0.001 |
| Specificity | 72.22 | 61.11 | 0.500 | 0.480 |
| Accuracy | 68.29 | 85.37 | 6.713 | <0.050 |
| Positive coincidence rate | 89.58 | 89.39 | 0.001 | 0.974 |
| Negative coincidence rate | 38.24 | 68.75 | 18.68 | <0.001 |
| Misdiagnosis rate | 27.78 | 38.89 | 0.500 | 0.480 |
| Missed diagnosis rate | 32.81 | 7.81 | 12.36 | <0.001 |
CT, computed tomography.
Discussion
In recent years, with the widespread use of antibiotics, hormones and immunosuppression agents, the number of IFIs has increased (16). However, because of the lack of clinical specificity of IFI, the condition is easily covered up by the primary disease, resulting in delay of the treatment. This is the reason why the highest mortality rate can reach 80% (17). IFI has become one of the leading causes of death in patients with solid organ transplantation and hematological malignancy (18,19). Therefore, the way to make accurate and in time diagnosis of IFI is an urgent issue to be addressed. At present, the gold standard for the diagnosis of IFI is pathological biopsy, but it is not accepted by patients and doctors because of the trauma caused. The sensitivity and specificity of traditional methods, such as respiratory sputum culture are only ~50% (20). Studies have shown that CT plays an important role in the diagnosis of fungal infections (21). CT has also been reported to have higher spatial and density resolution than X-ray, and to make good imaging observation of the basic pathological changes in IFI (22). In the diagnosis of pulmonary fungal infection, CT is expressed as scattered nodules, masses or patchy focis, and other signs, such as halo. Meniscus signs may also appear, and the occurrence of one or both signs at the same time is common (23). It has also been reported that subpleural wedge-shaped consolidation may occur in invasive pulmonary Aspergillus infection imaging (24).
In the present study, with pathological diagnosis as the gold standard, the diagnostic value of X-ray and CT in IFI was compared and the imaging features of CT diagnosis were analyzed. The results showed that the sensitivity, accuracy and negative coincidence rate of CT in the diagnosis of IFI were significantly higher than those of X-ray (P<0.05), with a sensitivity of 92.18%, which indicates that CT has a high diagnostic value in IFI. By analyzing the causes of misdiagnosis and missed diagnosis, it has been found that 6 out of 7 cases misdiagnosed with CT are caused by host factors. Although some studies have shown that the diagnosis of fungal infection has a greater dependence on host factors (11), we believe that misdiagnosis can also be caused by excessive interpretation of host factors in the misdiagnosis case analysis. When the causes of misdiagnosis and missed diagnosis of X-ray were analyzed, it was found that most of them were difficult to diagnose because of unclear imaging features. Therefore, we believe that when imaging cannot be judged, sputum culture or serum marker examination should be performed in time to further determine the diagnostic results. For example, when a patient has pulmonary artery embolism and incurable long-term pulmonary abscess, it can be considered as pulmonary mucormycosis infection. In the analysis of the imaging features of CT in this study, it was found that the multiple nodules and meniscus signs appeared more frequently in the collected cases, which coincided with the CT imaging features of IFI described in related literature (22). Some studies have suggested that the CT manifestations of pneumonia caused by different strains have their own characteristics (25). For example, meniscus sign is the characteristic of Aspergillus infection, and multiple nodule and small patch are the characteristics of Cryptococcus infection. In the diagnostic criteria developed by the European Organization for Cancer Therapy and Fungal Research Group, the meniscus sign and the cavity appearing in the consolidation are all characteristic manifestations of pulmonary fungal infection (26).
In conclusion, the diagnostic value of CT in IFI is higher than that of traditional X-ray, and CT has a better enhancement effect in many fungal areas infected with IFI. However, some lesions are difficult to distinguish, so it is necessary to make a comprehensive judgment and diagnosis by combining the clinical data and the medical history of the patients.
Figure 2.
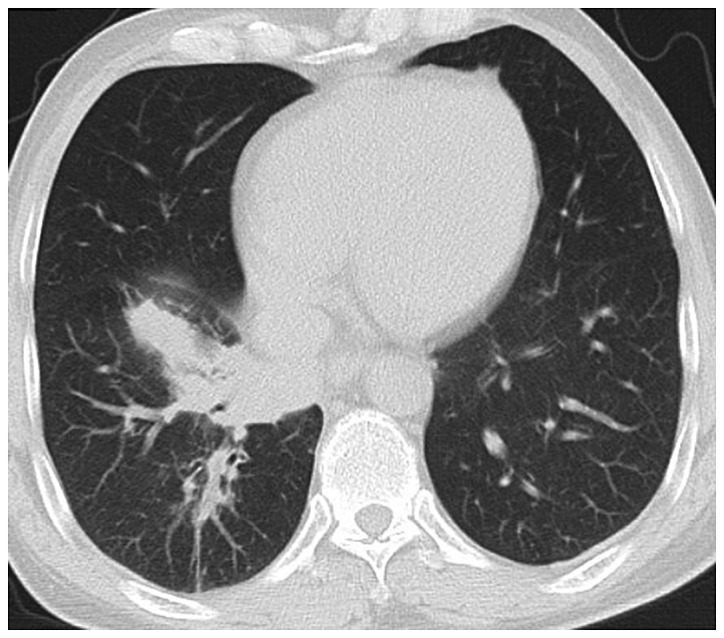
CT image of lung with pulmonary Candida infection. There are many nodules with different sizes and clear boundaries, accompanied by consolidation or tree-in-bud sign or ground-glass nodules, and there are halo signs around some nodules. CT, computed tomography.
Figure 3.
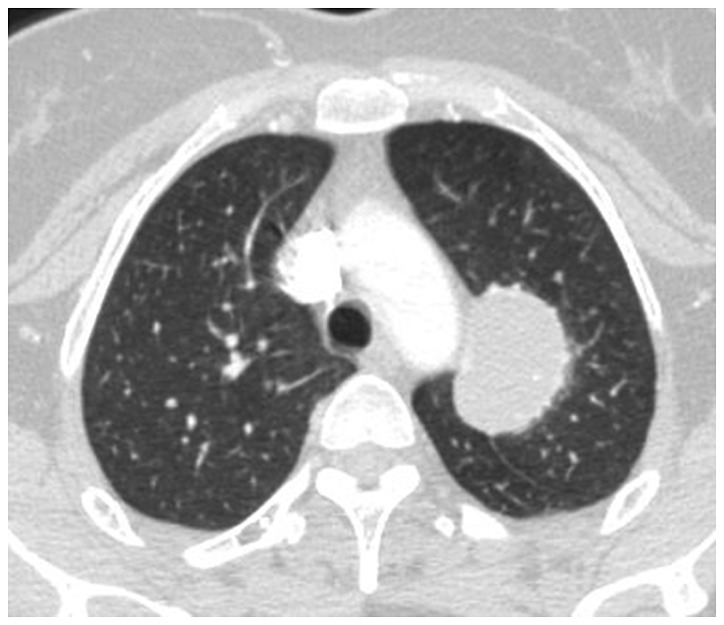
CT image of lung with pulmonary Aspergillus infection. The inside cavity is characterized by the inhomogeneous density of the sphere, rough edge, and the meniscus sign in meniscus radiolucent area of the cavity wall. CT, computed tomography.
Figure 4.
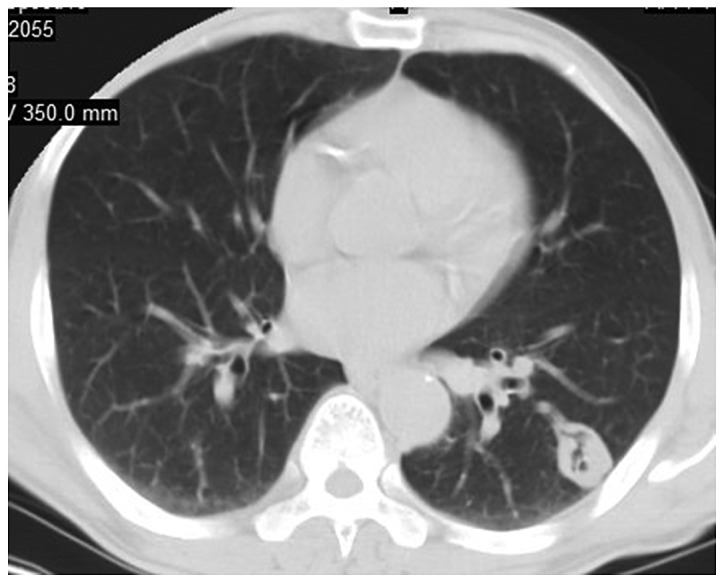
CT image of lung with pulmonary Cryptococcus infection. There are single or multiple nodules or masses with multiple distribution and clear boundaries in most of them, and the lesions are distributed as lobes or segments. CT, computed tomography.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JW, JLi and FY were responsible for the CT result analysis. JLin and CZ recorded and analyzed the general data of patients. JLi, FY and LZ contributed to the statistical analysis. The final version was read and approved by all the authors.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Qingdao, China). Signed written informed consents were obtained from the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J Med Res. 2014;139:195–204. [PMC free article] [PubMed] [Google Scholar]
- 2.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/CMR.13.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011;49(Suppl 1):S7–S12. doi: 10.3109/13693786.2010.505204. [DOI] [PubMed] [Google Scholar]
- 4.Badiee P, Alborzi A, Farhoudi F. A case of Candida mediastinitis after dental extraction. J Infect Dev Ctries. 2011;5:75–78. doi: 10.3855/jidc.1086. [DOI] [PubMed] [Google Scholar]
- 5.Vandewoude KH, Blot SI, Depuydt P, Benoit D, Temmerman W, Colardyn F, Vogelaers D. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care. 2006;10:R31. doi: 10.1186/cc4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, León C, Alvarez-Lerma F, Nolla-Salas J, Iruretagoyena JR, Barcenilla F. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: Risk factors, clinical presentation and outcome. Crit Care. 2005;9:R191–R199. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, Chevrier S, Meunier C, Lebert C, Aupée M, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: A 6-year survey. Clin Infect Dis. 2006;43:577–584. doi: 10.1086/505870. [DOI] [PubMed] [Google Scholar]
- 8.Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis. 2011;52:1144–1155. doi: 10.1093/cid/cir122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno C, Minniti S, Vassanelli A, Pozzi-Mucelli R. Comparison of CT features of Aspergillus and bacterial pneumonia in severely neutropenic patients. J Thorac Imaging. 2007;22:160–165. doi: 10.1097/RTI.0b013e31805f6a42. [DOI] [PubMed] [Google Scholar]
- 10.Escuissato DL, Gasparetto EL, Marchiori E, Rocha GM, Inoue C, Pasquini R, Müller NL. Pulmonary infections after bone marrow transplantation: high-resolution CT findings in 111 patients. AJR Am J Roentgenol. 2005;185:608–615. doi: 10.2214/ajr.185.3.01850608. [DOI] [PubMed] [Google Scholar]
- 11.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–622. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 12.Yeo SF, Wong B. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin Microbiol Rev. 2002;15:465–484. doi: 10.1128/CMR.15.3.465-484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma SH, Xu K, Xiao ZW, Wu M, Sun ZY, Wang ZX, Hu ZG, Dai X, Han MJ, Li YG. Peripheral lung cancer: Relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and cyclin D1 expression. Clin Imaging. 2007;31:165–177. doi: 10.1016/j.clinimag.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Caillot D, Couaillier JF, Bernard A, Casasnovas O, Denning DW, Mannone L, Lopez J, Couillault G, Piard F, Vagner O, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs T, Kachelriess M, Kalender WA. Technical advances in multi-slice spiral CT. Eur J Radiol. 2000;36:69–73. doi: 10.1016/S0720-048X(00)00269-2. [DOI] [PubMed] [Google Scholar]
- 16.Yang WL, Cao J, Chen BY, Xie W, Dong LX, Wu YQ, Li JN, Hu ZD. A preliminary study on the measurement of (1,3)-β-D-glucan in bronchoalveolar lavage for the diagnosis of pulmonary fungal infections. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:897–900. (In Chinese) [PubMed] [Google Scholar]
- 17.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case - fatality rate: Systematic review of the literature. Clin Infect Dis. 2001;32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 18.Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med. 2006;173:707–717. doi: 10.1164/rccm.200505-727SO. [DOI] [PubMed] [Google Scholar]
- 19.Patterson TF. Advances and challenges in management of invasive mycoses. Lancet. 2005;366:1013–1025. doi: 10.1016/S0140-6736(05)67381-3. [DOI] [PubMed] [Google Scholar]
- 20.Prasad A, Agarwal K, Deepak D, Atwal SS. Pulmonary Aspergillosis: What CT can offer before it is too late! J Clin Diagn Res. 2016;10:TE01–TE05. doi: 10.7860/JCDR/2016/17141.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christe A, Lin MC, Yen AC, Hallett RL, Roychoudhury K, Schmitzberger F, Fleischmann D, Leung AN, Rubin GD, Vock P, et al. CT patterns of fungal pulmonary infections of the lung: Comparison of standard-dose and simulated low-dose CT. Eur J Radiol. 2012;81:2860–2866. doi: 10.1016/j.ejrad.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Kim SH, Choi SH, Sung H, Kim MN, Woo JH, Kim YS, Park SK, Lee JH, Lee KH, et al. Clinical and radiological features of invasive pulmonary aspergillosis in transplant recipients and neutropenic patients. Transpl Infect Dis. 2010;12:309–315. doi: 10.1111/j.1399-3062.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 23.Franquet T, Müller NL, Giménez A, Guembe P, de La Torre J, Bagué S. Spectrum of pulmonary aspergillosis: Histologic, clinical, and radiologic findings. Radiographics. 2001;21:825–837. doi: 10.1148/radiographics.21.4.g01jl03825. [DOI] [PubMed] [Google Scholar]
- 24.Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, Rediers H, Lievens B. Ecology of aspergillosis: Insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb Biotechnol. 2017;10:296–322. doi: 10.1111/1751-7915.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirkazik FB, Akin A, Uzun O, Akpinar MG, Ariyürek MO. CT findings in immunocompromised patients with pulmonary infections. Diagn Interv Radiol. 2008;14:75–82. [PubMed] [Google Scholar]
- 26.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group: Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


