Abstract
Tumor cells overexpress amino acid transporters to meet the increased demand for amino acids. PQ loop repeat‐containing (PQLC)2 is a cationic amino acid transporter that might be involved in cancer progression. Here, we show that upregulation of PQLC2 is critical to gastric cancer (GC) development in vitro and in vivo. Both PQLC2 mRNA and protein were overexpressed in GC tissues, especially of the diffuse type. Overexpression of PQLC2 promoted cell growth, anchorage independence, and tumor formation in nude mice. This was due to activation of MEK/ERK1/2 and PI3K/AKT signaling. Conversely, PQLC2 knockdown caused growth arrest and cell death of cancer cells and suppressed tumor growth in a mouse xenograft model. These results suggest that targeting PQLC2 is an effective strategy for GC treatment.
Keywords: gastric cancer, metastasis, PQLC2, therapeutic target, tumor formation
1. INTRODUCTION
PQ loop repeat‐containing (PQLC)2 is a heptahelical protein with a duplicated PQ loop that functions as an amino acid transporter, mediating the pH‐dependent export of the cationic amino acids arginine, histidine, and lysine from lysosomes.1, 2 PQLC2 is thought to play a role in the lysosomal export of cysteamine‐cysteine mixed disulfide, which is formed following cysteamine treatment of patients with cystinosis, a disease characterized by cysteine accumulation in lysosomes.1 The Saccharomyces cerevisiae homologs Ypq1, Ypq2, and Ypq3 perform an equivalent function in vacuoles and the Schizosaccharomyces pombe homolog Stm1 has been identified as a multicopy suppressor of a ras1 synthetic lethal mutant.3 Ypq3 regulates cell growth and differentiation by modulating the level of cyclic AMP through the G protein Gpa2.3, 4 The defect in the Caenorhabditis elegans homolog lysosomal amino acid transporter 1 leads to delayed embryonic development, highlighting the vital function of these transporters.1
Gastric cancer (GC) is a highly aggressive malignancy that is currently the third most common cause of cancer‐related death worldwide, as it is typically diagnosed at an advanced stage.5, 6 Gastric cancer is the most frequently diagnosed cancer in East Asian countries,7, 8 especially in Japan and Korea. Up to 45% of patients who undergo curative resection experience local or distant recurrence.6, 9 In North America and Europe, approximately 65% of patients have incurable GC or distant metastasis at the time of initial diagnosis. Chemotherapy is effective only in a small subset of GC patients, with advanced cases often showing resistance.9, 10, 11 To improve the prognosis of high‐risk patients, it is important to identify predictive biomarkers and potential therapeutic targets to develop more effective treatment strategies. An ideal candidate target is a protein associated with cell proliferation or survival that is either absent or underexpressed in normal cells but is abundant in cancer cells. As in other solid tumors, agents that block critical inter‐ and intracellular signaling pathways have emerged as a treatment strategy for GC.12, 13, 14 Some agents including trastuzumab and ramucirumab targeting HER‐215 and vascular endothelial growth factor receptor 2,16 respectively, have shown therapeutic efficacy and a good safety profile, and are now licensed in the USA and Europe as part of the treatment regimen of GC patients. The most commonly used markers in GC patients are cancer antigen (CA)72‐4, carcinoembryonic antigen (CEA), and CA19‐917, 18; epidermal growth factor receptor overexpression has been correlated with more aggressive tumor behavior and a worse prognosis for patients with GC;19, 20 hepatocyte growth factor and the hepatocyte growth factor receptor c‐MET have also been proposed as potential therapeutic targets. In addition, inhibitors of mTOR, c‐MET, insulin‐like growth factor receptor, and fibroblast growth factor receptor signaling are currently being investigated in clinical trials.12, 21, 22, 23 However, most biomarkers identified as therapeutic targets have not yet been sufficiently validated and are still controversial.
We previously reported that the S. pombe PQLC2 homolog Stm1 is associated with the Ras1 gene.3 Given that human Ras GTPases play an essential role in growth regulation and tumorigenesis and that S. pombe Ras1 regulates MAPK signaling in mating, we speculated that PQLC2 plays a role in GC development. This was investigated in the present study using both in vitro and in vivo approaches. Our results suggest that PQLC2 acts as an oncogene in GC and is a potential therapeutic target for the development of antineoplastic drugs.
2. MATERIALS AND METHODS
2.1. Materials
Antibodies against Akt, p‐Akt (S473), p‐c‐Raf (S259), p‐c‐Raf (S338), Erk1/2, and p‐Erk1/2 were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibody for GAPDH was purchased from AbFrontier (Seoul, Korea). Anti‐FLAG and anti‐PQLC2 were purchased from Sigma (St. Louis, MO, USA).
2.2. Cell culture and reagents
HEK293 (human embryonic kidney cell line) was cultured in DMEM (Gibco, Paisley, UK) containing 10% (v/v) heat‐inactivated FBS (WELGENE, Gyeongsangbuk‐do, Korea), 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified incubator containing 5% CO2. Stomach cancer cell lines, SNU1, SNU5, SNU620, SNU216, SNU484, SNU638, SNU668, MKN1, MKN28, MKN45, MKN74, and NCI‐N87, were obtained from the Korea Cell Line Bank (Seoul, Korea). HS746T and AGS cell line were obtained from the ATCC (Rockville, MD, USA). Stomach cancer cell lines were cultured in RPMI‐1640 medium (Gibco) containing 10% (v/v) heat‐inactivated FBS (WELGENE).
2.3. Tissue samples and microarray construction
Gastric cancer tissue samples were obtained from 180 consecutive patients who underwent elective surgery for GC at the Chungnam National University Hospital (Daejeon, Korea) between 2000 and 2003. The patients underwent R0 resection with at least a D1 lymph node dissection. Adenocarcinomas from patients’ stomachs were isolated and histologically confirmed. Clinicopathological parameters were assessed according to the Japanese GC Association. Patients provided informed consent for the treatment and use of their tissue for subsequent histological analyses. The experiments were approved beforehand by the institutional review board. Paraffin blocks of the GC samples were processed by H&E staining. Areas representing invasive adenocarcinomas were identified and marked on each donor block. A 2‐mm core from the area of interest was transferred to the master block using the Tissue Microarrayer (Meditech Ind., Seoul, Korea). Two GC cores were arrayed per specimen. Four cores of adjacent normal gastric tissue were also sampled for comparison (Table 1). Additionally, the ST723 tissue microarray consisting of 21 cases of GC (including 5 diffuse cases, with triplicate cores per case) and 3 adjacent normal tissue samples were purchased from US Biomax (Rockville, MD, USA) and used for analysis.
Table 1.
Relationship between PQLC2 expression and clinicopathological features of Korean patients with gastric cancer
| Features | PQLC2 expression in gastric cancer | P value | ||
|---|---|---|---|---|
| Low (91) | High (n = 89) | |||
| No. (%) | No. (%) | |||
| Age | ||||
| <65 y | 124 | 68 (74.7) | 56 (62.9) | .087a |
| ≥65 y | 56 | 23 (25.3) | 33 (37.1) | |
| Gender | ||||
| Male | 127 | 64 (70.3) | 63 (70.8) | .946a |
| Female | 53 | 27 (29.7) | 26 (29.2) | |
| Depth of invasion | ||||
| T1 | 113 | 63 (69.2) | 50 (56.2) | .013b |
| T2‐T4 | 67 | 28 (30.8) | 39 (43.8) | |
| Nodal involvement | ||||
| Negative | 107 | 64 (70.3) | 43 (48.3) | .003a |
| Positive | 73 | 27 (29.7) | 46 (51.7) | |
| Stage | ||||
| I/II | 141 | 79 (86.8) | 62 (69.7) | .005a |
| III/IV | 39 | 12 (13.2) | 27 (30.3) | |
| Borrmann's classification | ||||
| Early | 81 | 44 (48.4) | 34 (38.2) | .025b |
| Type I‐II | 75 | 37 (40.7) | 41 (46.1) | |
| Type III | 16 | 7 (7.7) | 9 (10.1) | |
| Unclassified | 8 | 3 (3.3) | 5 (5.6) | |
| Tumor location | ||||
| Upper | 12 | 4 (4.4) | 8 (9.0) | .212b |
| Middle | 92 | 46 (50.5) | 46 (51.7) | |
| Lower | 75 | 40 (44.0) | 35 (39.3) | |
| Whole | 1 | 1 (0.01) | 0 (0) | |
| Tumor size | ||||
| ≤5 cm | 141 | 77 (84.6) | 64 (71.9) | .039a |
| >5 cm | 39 | 14 (15.4) | 25 (28.1) | |
Calculated by pairwise comparisons from χ2 test.
Calculated by comparisons of 4 groups from linear‐by‐linear associations.
2.4. Immunohistochemistry analysis
Gastric cancer tissue samples were fixed in 10% buffered formalin and embedded in paraffin. The blocks were cut into 3‐μm sections for immunohistochemistry using a rabbit polyclonal anti‐PQCL2 Ab and the rabbit EnVision‐HRP detection system (Dako, Carpinteria, CA, USA). After deparaffinization, antigen retrieval was carried out in 10 mmol/L sodium citrate buffer (pH 6.0) using a pressure cooker at full power for 4 minutes. The sections were then treated with 3% hydrogen peroxide for 10 minutes before overnight incubation in a humid chamber at 4°C with the primary Ab diluted 1:100 in background‐reducing diluent (Dako). A rabbit IgG isotype control without the primary Ab was used as a negative control. The sections were incubated with EnVision reagent for 30 minutes followed by diaminobenzidine for 5 minutes and counterstained with Meyer's hematoxylin. At each step, sections were rinsed several times with TBS with 0.3% Tween‐20.
2.5. Reverse transcription‐PCR and quantitative real‐time PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was synthesized using TOPscript RT DryMIX (Enzynomics, Seoul, Korea).24 Oligonucleotides were synthesized by Bioneer (Daejeon, Korea). The following primers were used for RT‐PCR: PQLC2 forward, 5′‐TCATCAAAGCCTACAAGACGG‐3′; PQLC2 reverse, 5′‐GGTGGACTTCCGGAGGAAGTT‐3′; GAPDH forward, 5′‐CGAGATCCCTCCAAAATCAA‐3′; and GAPDH reverse, 5′‐TCTTGAGGCTGTTGTCATAC‐3′. The PCR products were analyzed using agarose gel electrophoresis. The quantitative real‐time PCR analysis was carried out using the OneStep Multiplex quantitative real‐time PCR Kit according to the manufacturer's instructions (Biofact, Daejeon, Korea). The following primers were used: PQLC2 forward, 5′‐TCTGGAAGAAACTGGGCTCC‐3′; PQLC2 reverse, 5′‐CATGTTGCCCGTCTTGTAGG‐3′.
2.6. Generation of cell lines stably expressing PQLC2
To clone the PQLC2 gene, a 0.8‐kb DNA fragment amplified by PCR using cDNA from human foreskin fibroblast cells was inserted into the BamHI site of pIRES‐EGFP, BamHI/EcoRV sites of p3xflag‐CMV7.1. The following primers were used: forward primer, 5′‐ ATAGGATCCATGGTCTGGAAGAAACTGGGCTC‐3′ for BamHI site; reverse primer, 5′‐CTTGGATCCTCAGCTGGGGAGGAGGGGCTCA‐3′ for BamHI site; 5′‐CTTGATATCTCAGCTGGGGAGGAGGGGCTCA‐3′ for EcoRV. To construct the HEK293 cell lines stably overexpressing PQLC2, the cells were transfected with the pIRES‐EGFP‐PQLC2 or pIRES‐EGFP expression vector by Lipofectamine transfection reagent (Invitrogen). After 48 hours, the cells were passaged at 1:100 ratio in selection medium (DMEM containing 1–2 mg/mL G418). After 3 weeks, the clones showing resistance to G418 and expressing the green fluorescence were isolated. The isolated clones were then expanded in the same selection medium.
2.7. Anchorage independence analysis
Cells were trypsinized and resuspended in DMEM/RPMI supplemented with 10% FBS. The cells were counted and plated into top agar at a density of 4000 cells/mL. The top agar consisted of DMEM supplemented with 10% FBS and 0.6% Noble agar (Sigma). The bottom agar was prepared with the same medium supplemented with 2% agar. Weekly, 1 mL medium with 10% FBS was added to the top agar to compensate for evaporation. After 3 weeks of culture, colonies were reacted with the 0.05% crystal violet or the nitroblue tetrazolium assay and photographed.
2.8. Invasion assay
Cell invasion assay was carried out using an insert chamber coated with Matrigel (8‐μm pore size; BD Biosciences, San Jose, CA, USA). Cells (2 × 105) were plated in the top chamber without serum‐free media. The bottom chamber was filled with media supplemented with 20% FBS. After 48 hours, noninvasive cells were removed with a cotton swab. Then the inserts fixed with 4% paraformaldehyde and stained with crystal violet.
2.9. Tumor‐forming assay in nude mice
Female Balb/c nude mice (aged 6 weeks) were procured from Orient‐bio (Seongnam, Korea), housed under aseptic conditions, and handled in accordance with the guidelines of the Laboratory Animal Unit of the Korean Institute of Bioscience and Biotechnology. For the xenograft tumor growth assay, NC and N1 cells (5 × 106 cells) were injected s.c. in the right flank of the mice (n = 10 per group). Tumor size was measured weekly for 5 weeks and volume (V) was calculated according to the formula V = (length × width2)/2.
2.10. Gene knockdown by siRNA and shRNA
The PQLC2 SMART vector lentiviral shRNA constructs had the following sequences: sh‐1, AATGACTTCCTGCCGGGTG; and sh‐2, AGCATCACCAGCGCGAACA (VSC11714, V3SH11240‐229519281, and V3SH11240‐230189578; GE Dharmacon, Lafayette, CO, USA). To confirm the role of PQLC2 in cell proliferation, GC cells were seeded in a 96‐well plate 1 day prior to infection with PQLC2 shRNA lentiviral particles in RPMI‐1640 medium containing 8 μg/mL polybrene for 1 hour at an appropriate multiplicity of infection. The lentiviral solution was replaced with complete culture medium after 12 hours, and cell proliferation was analyzed for 120 hours with the IncuCyte ZOOM system (Essen Bioscience, Ann Arbor, MI, USA). For gene knockdown experiments, cells were seeded in 6‐well plates and grown until 40%‐50% confluence before siRNA transfection (100 pmol) for 72 hours using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer's instructions. The efficacy of siRNA‐mediated knockdown was confirmed by RT‐PCR. At least 3 independent experiments were carried out. The siRNA sequences were as follows: si‐1, 5′‐CCGUGCUGUUGUUCCUCAUTT‐3′; si‐2, 5′‐GGGAUCUCCUACUCUCUGUTT‐3′; and siControl, 5′‐AGCGCUGACAACAGUUUCA‐3′ (ST Pharm., Kyunggido, Korea).
2.11. In vivo antitumor efficacy of PQLC2 silencing in MKN45 cell xenograft model
Animal experiments were carried out according to institutional regulations in a facility that was accredited for laboratory animal care. MKN45 cells (2 × 106 in 200 μL PBS) were s.c. implanted into the abdomens of 8‐week‐old male athymic nu/nu mice (Charles River Japan, Yokohama, Japan). When tumor volumes reached 90‐100 mm3, PQLC2 siRNA (1 × 1010) or control siRNA were administered intratumorally to 8 mice per group on days 1, 3, and 5, with PBS and oligofectamine used as the negative control. Tumor volume was calculated with the formula V = 0.52 × a 2 × b, where “a” and “b” are the smallest and largest external diameters, respectively.25
2.12. Statistical analysis
The χ2 test or linear by linear association was used to assess the correlation between PQLC2 expression and clinicopathological features. Overall survival curves were calculated using the Kaplan‐Meier method based on the length of time between primary surgical treatment and final follow‐up or death. The log‐rank test was used to assess statistical differences between curves. Independent prognostic factors were identified by Cox proportional hazards regression model. P values <.05 were considered statistically significant. All analyses were undertaken using SPSS version 17.0 software program (Cary, NC, USA).
3. RESULTS
3.1. Overexpression of PQLC2 is associated with clinicopathological parameters in GC
To investigate the association between PQLC2 and GC, PQLC2 expression in GC patients relative to normal tissues was evaluated by immunohistochemistry. PQLC2 was overexpressed in GC; therefore, we examined the relationship between PQLC2 level and clinicopathological parameters such as tumor size, differentiation status, invasion, and tumor grade. Tumors were classified based on staining intensity as follows: 0, no staining; 1, weak; 2, intermediate; and 3, strong. In cases of heterogeneous staining within samples, the higher score was chosen if more than 50% of the cells showed a higher staining intensity. Cases with a score of 0 or 1 were considered as having low PQLC2 expression, and those with a score of 2 or 3 were considered as having high expression (Figure 1A and Table 1). We found that 50.6% (91/180) of patients had low PQLC2 expression whereas 49.5% (89/180) had high expression (Table 1). The latter was significantly associated with tumor depth (P = 0.013), lymph node involvement (P = 0.003), and TNM stage (P = 0.005). High PQLC2 expression was more prevalent among patients showing high (T2‐T4, 67 cases; 58.2%) as compared to low (T1, 113 cases; 44.3%) invasion depth. Overexpression of PQLC2 was also more common in cases with (73 cases; 63.0%) than in those without (107 cases; 40.2%) nodal involvement and was more frequent in high‐grade cancer (stage III‐IV). High PQLC2 expression was also associated with a high proportion of tumors with a size >5 cm (P = 0.039). However, there were no significant associations between PQLC2 level and age, gender, or tumor location. Normal gastric tissue showed no or very weak PQLC2 immunoreactivity. The PQLC2 transcript level was much higher in GC than in normal tissues, as determined by real‐time PCR. PQLC2 expression was elevated in 43.7% (T/N ≥ 2) of high‐grade advanced GC cases (Bormann's grade III) (Figure 1B) and in 35.7% (T/N ≥ 2) of middle‐stage tissues (Bormann's grade I‐II), but the level in early‐stage GC did not differ significantly from that in healthy tissue. Data were further analyzed to determine the relationship between PQLC2 and patient survival. Kaplan‐Meier curves indicated some relevance between high expression of PQLC2 and poor patient survival (P = .043, log‐rank test, Figure 1C). However, multivariate analysis identified only TNM stage and tumor size as independent prognostic factors for GC patients. Respective hazard ratios for TNM stage and tumor size were 4.215 (95% confidence interval, 1.699‐10.460) and 2.237 (95% confidence interval, 1.352‐3.700) (Table 2).
Figure 1.
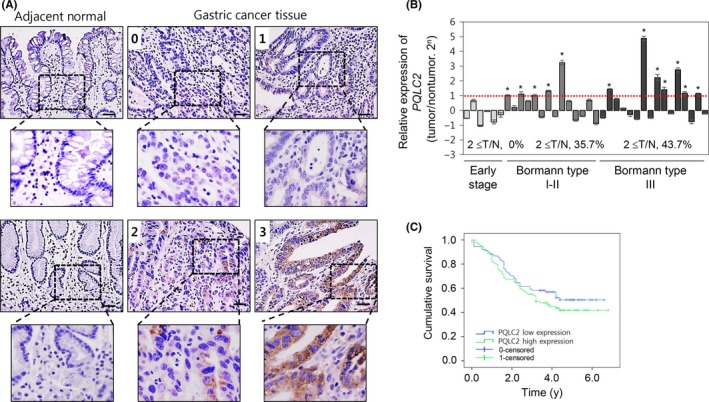
Expression of PQLC2 in gastric cancer (GC) tissues and cells. A, Immunohistochemical analysis of PQLC2 in GC and adjacent normal tissues (Chungnam National University Hospital, Daejeon, Korea). Scale bar = 50 μm. Tumors were classified based on staining intensity: 0, no staining; 1, weak; 2, intermediate; and 3, strong. B, Relative expression levels of PQLC2 in GC and adjacent normal tissues from 34 GC patients, as determined by RT‐PCR (n = 36). GAPDH served as an internal control. According to Bormann's classification, patients were grouped into early stage, BI, BII, and BIII. C, Survival curve of GC patients according to PQLC2 expression. Patients with high PQLC2 expression showed a worse 5‐y survival rate compared with patients with low PQLC2 expression (P = .023, log‐rank test)
Table 2.
Multivariate analyses of the association of prognosis with clinicopathological parameters and PQLC2 expression in patients with gastric cancer
| Variables | Case no. | Multivariate analysis | |
|---|---|---|---|
| HR (95% CI) | P value | ||
| Age (≥65/<60 y) | 124/56 | 0.698 (0.451‐1.080) | .106 |
| Gender (male/female) | 127/53 | 0.705 (0.439‐1.133) | .149 |
| Depth of invasion (T2‐4/T1) | 67/113 | 1.538 (0.717‐3.299) | .268 |
| Nodal involvement (positive/negative) | 73/107 | 0.633 (0.336‐1.194) | .158 |
| Stage (III‐IV/I‐II) | 39/141 | 4.215 (1.699‐10.460) | .002a |
| Tumor size (≥5 cm/<5 cm) | 39/141 | 2.237 (1.352‐3.700) | .002a |
| PQLC2 (high/low) | 89/91 | 0.774 (0.493‐1.216) | .267 |
P < 0.05.
CI, confidence interval; HR, hazard ratio.
Gastric cancer can be divided into intestinal and diffuse types according to the Lauren classification,26 with the latter having a worse prognosis. To investigate the correlation between PQLC2 expression and GC type, we analyzed a GC tissue array that included 24 cases (5 diffuse GC and 3 adjacent normal cases in triplicate). Consistent with results from our patients, high PQLC2 expression was significantly correlated with tumor depth and TNM stage (Tables 1 and 2). Interestingly, PQLC2 was more frequently overexpressed in diffuse‐type (4/5, 80.0%) than in intestinal‐type (7/16, 43.7%) GC. Immunohistochemical analysis showed PQLC2 localization at the cell membrane and in the cytoplasm of tumor cells (Figure 2A; F6). Among 180 Korean patients, only 16 were diagnosed with diffuse‐type GC (Table S1) and high incidence of PQLC2 overexpression in diffuse‐type GC was further certified in another set of 9 Korean GC tissue samples. PQLC2 mRNA level was upregulated in 6/9 (66.7%) diffuse‐type GC tissue samples relative to adjacent normal tissue (Figure 2B,C). These data suggest that PQLC2 contributes to the progression of GC, especially in the diffuse subtype. Although these data indicate that PQLC2 might contribute to the progression of GC, especially in diffusible subtypes, this could not be confirmed due to the limited number of samples.
Figure 2.
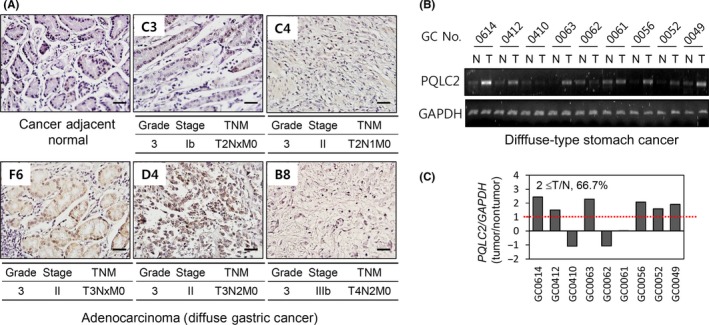
Expression of PQLC2 in diffuse‐type gastric cancer (GC) tissues. A, Immunohistochemical analysis of PQLC2 expression in diffuse‐type GC tissues from an array. Scale bar = 50 μm. B, PQLC2 mRNA levels in paired GC tissues measured by RT‐PCR (n = 9). GAPDH served as an internal control. C, Quantification of relative expression levels using ImageJ software (NIH, Bethesda, MD, USA). N, normal tissue; T, tumor tissue
3.2. Cell proliferation enhanced by PQLC2 in vitro and in vivo
We speculated that the observed overexpression of PQLC2 in GC tissue samples was associated with enhanced tumor cell proliferation. We, therefore, generated HEK293/PQLC2 and/or SNU638/PQLC2 cell lines stably expressing PQLC2 and the corresponding control cell lines Vehicle harboring the pIRES vector. Overexpression of PQLC2 increased cell proliferation, promoted cell invasion, supported anchorage independence, and increased tumor formation in nude mice (Figure 3A‐D). Nine of 10 mice s.c. injected with HEK293/PQLC2 developed tumors after 28 days, compared to only 1 mouse out of 10 following HEK293/Vehicle injection. Thus, PQLC2 overexpression in normal cells stimulates cancer cell growth, suggesting that it acts as an oncogene.
Figure 3.
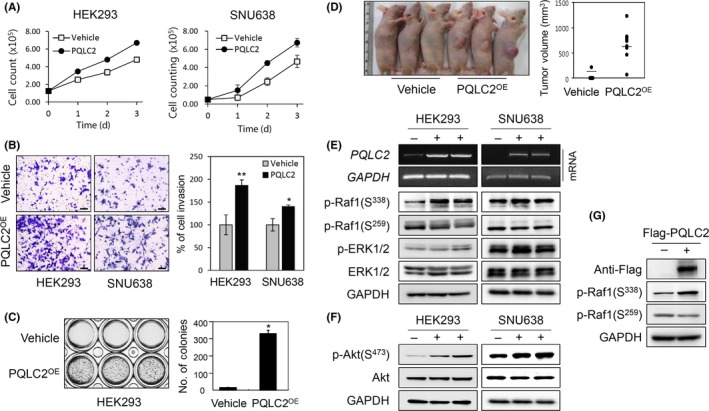
Overexpression of PQLC2 stimulates gastric cancer (GC) cell proliferation and promotes cell growth through the Raf1/ERK and PI3K/AKT signaling pathway. A, Direct cell counting assay was used to analyze the effect of PQLC2 overexpression on cell proliferation. A stable cell line harboring the pIRES‐EGFP vector served as a control. Error bars represent SD (n > 3). B, Invasion assay on PQLC2 overexpressing HEK293 and SNU638 cells. Invasive cells were quantified by determining the optical density at 560 nm after extraction. Results are graphically representation as mean values ± SD. **P < .005. C, Effect of colony formation on PQLC2 overexpressing HEK293 cells (PQLC2OE). Cells stably transfected with empty or PQLC2‐encoding vector were cultured for 3 wk before staining with crystal violet. Number of colonies are graphically representation as mean values ± SD. *P < .01 (Student’s t test). Data are representative of 3 independent experiments. D, Tumor formation was examined in a mouse xenograft model. Vehicle or PQLC2 cells were injected into the right flank of 5‐wk‐old athymic mice; 3 representative animals from each group (n = 10) were photographed 28 d after injection. E,F, PQLC2 overexpression was evaluated by RT‐PCR in HEK293 and SNU638 cell lines. Effect of PQLC2 overexpression on Raf1, ERK1/2, and AKT1/2 phosphorylation related to cell proliferation and cell survival. Total cell lysates (30 μg) were analyzed by immunoblotting. G, Effect of transient PQLC2 overexpression on Raf1 activation. Cells were transfected with 2 μg pCMV‐taq1 empty vector or pCMV‐flag‐ PQLC2 and analyzed by immunoblotting 48 h later
To investigate the mechanism underlying the oncogenic function of PQLC2, we examined the effect of PQLC2 on the activation of signaling pathways associated with cell proliferation and survival, for instance, Raf/MEK/ERK and PI3K/AKT. Overexpression of PQLC2 stimulated Raf1 activity by increasing phosphorylation at the active site serine (S)338 and decreasing that at the inhibitory site S259,27 and by inducing both ERK1/2 and AKT1/2 phosphorylation in PQLC2 overexpression cell lines compared to control (Figure 3E,F). Consistent with these results, Raf1 activity was increased when PQLC2 was transiently overexpressed using a pCMV‐Flag‐PQLC2 construct (Figure 3G).
3.3. Deficiency of PQLC2 suppresses GC cell proliferation in vitro and in vivo
To confirm the role of PQLC2 in GC, we knocked down PQLC2 using lentiviruses expressing shRNAs (sh1 and sh2; Dharmacon) and 2 siRNAs (si‐1 and si‐2; ST Pharm.). Of 14 GC cell lines with varying levels of PQLC2 expression (Figure 4A,B and Table S2), we selected 4, that is, AGS (high protein level), SNU668, MKN1 (intermediate protein level), and SNU638 (low protein level) for these experiments. Knockdown of PQLC2 with sh1 or sh2 suppressed proliferation in MKN1 (55.0%‐74.6%), SNU668 (63.7%‐50.9%), and AGS (60.4%‐50.9%) cells relative to cells transfected with control lentivirus shRNA at 96 hours. In contrast, PQLC2 silencing had little effect in SNU638 cells (6%‐11.1% at 96 hours), which have a very low PQLC2 expression. Furthermore, we observed decreased PQLC2 protein expression in cell lines transfected with lentivirus shRNA (Figure 4C).
Figure 4.
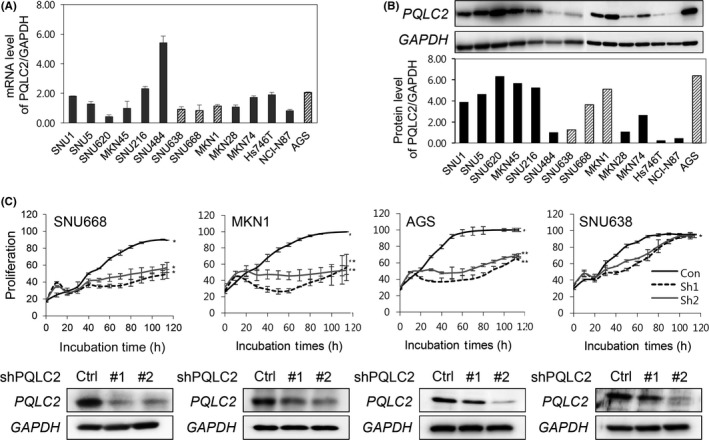
Knockdown of PQLC2 inhibits growth of gastric cancer (GC) cell lines with high PQLC2 expression. A,B, PQLC2 mRNA and protein levels in various GC cell lines. GAPDH served as an internal control for RT‐PCR and western blotting. C, Effect of PQLC2 knockdown on cell proliferation and apoptosis in GC cell lines. Growth rates in 4 cell lines expressing varying levels of PQLC2 were analyzed after treatment of lentivirus expressing PQLC2 or control (Ctrl) shRNA. *P < .01, **P < .05
We examined the signaling pathways that mediate inhibition of cell proliferation following PQLC2 knockdown and found that, in cells transfected with si‐1 and si‐2, AKT1/2 and ERK1/2 phosphorylation was suppressed, suggesting that loss of PQLC2 leads to decreased cell proliferation and survival by inhibition of MEK/ERK1/2 and PI3K/AKT signaling (Figure 5A,B), which is consistent with our observations in the PQLC2 overexpression experiments. Next, a colony forming assay was undertaken with 2 gastric cancer cell lines (MKN1 and MKN45) to confirm the inhibitory effect of PQLC2 knockdown. As a result, inhibition of colony formation was significantly observed in cells treated with PQLC2 siRNA. The potential of PQLC2 as a therapeutic target was evaluated in an in vivo xenograft model of PQLC2 deficiency. Efficient knockdown of the PQLC2 transcript by siPQLC2 (#1) was confirmed by western blot analysis of infected MKN45 cells. The tumor volume of intratumoral injection of siPQLC2 was reduced by approximately 50% compared to tumors injected with control siRNA, without noticeable adverse effects such as weight loss or organ defects. These results suggested that loss of PQLC2 leads to decreased cell growth and induction of cell death by inhibition of MEK/ERK1/2 and PI3K/AKT signaling. However, we need to further investigate PQLC2 mutations and their underlying molecular mechanism in GC.
Figure 5.
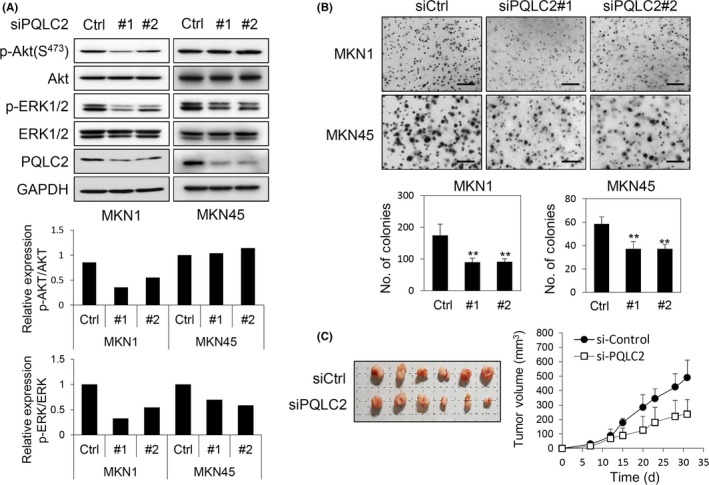
Knockdown of PQLC2 inhibits ERK1/2 and AKT activity to suppress gastric cancer (GC) cell growth and in vivo tumor formation. A, Immunoblot analysis of two GC cell lines with a high level of PQLC2 expression. Cells grown to approximately 40%‐50% confluence were treated with 100 pmol PQLC2 or control (Ctrl) siRNA for 72 h. Relative levels of phospho‐ (p)‐ERK1/2 and p‐AKT1/2 were quantified with ImageJ software (NIH, Bethesda, MD, USA). B, Colony formation assay. Cells were transfected 3 times with 100 pmol siPQLC2 or control siRNA. Scale bar = 500 μm. Number of colonies is shown in the lower panel as mean values ± SD. **P < .005 (Student's t test). Data are representative of 3 independent experiments. C, Downregulation of PQLC2 inhibits the growth of 45 xenograft tumors
4. DISCUSSION
Cationic amino acid transporter PQLC2 plays a role in the cysteine‐depleting mechanism underlying cysteamine therapy of cystinosis; however, the molecular functions of most PQ‐loop family members are unknown, with the exception of cystinosin, a lysosomal cysteine export protein that is defective in cystinosis.28, 29 In this study, we cloned PQLC2 based on its homology to fission yeast Stm1. The stm1 gene of S. pombe was previously cloned by functional complementation in ras1 synthetic lethal mutants, which can grow in the presence or absence of Ras1 protein3; therefore, we investigated the correlation between PQLC2 and cancer development. As expected, PQLC2 was highly expressed in GC tissues (Figure 1 and Table 1), particularly in diffuse‐type GC (although the sample size was small; Figure 2 and Table 3) and in cancer cell lines (Figure 4). The high level of PQLC2 expression was significantly associated with tumor depth (P = .013), lymph node involvement (P = 0.003), and TNM stage (P = .005). These findings indicated that PQLC2 overexpression is closely related with tumor pathophysiology. Although PQLC2 had no independent prognostic significance in multivariate analyses (P = .267 compared to P = .043 by univariate analysis), PQLC2 overexpression could have prognostic value. The lack of this significance might be due to a bias in sample composition, because 78.8% of 180 Korean samples were stage I‐II with tumors smaller than 5 cm. Therefore, we suggest that there is a significant association between PQLC2 and clinicopathological features. Further analyses of more samples are required to correlate the data with prognosis.
Table 3.
Upregulation of PQLC2 in diffuse‐type gastric cancer (GC)
| Feature | PQLC2 expression in GC tissue arraya | |||
|---|---|---|---|---|
| Low (n = 10) | High (n = 11) | P value | ||
| No. (%) | No. (%) | |||
| Age | ||||
| <65 y | n = 15 | 7 (70.0) | 8 (72.7) | .527b |
| ≥65 y | n = 6 | 3 (30.0) | 3 (27.3) | |
| Gender | ||||
| Male | n = 14 | 6 (60.0) | 8 (72.7) | .731b |
| Female | n = 7 | 4 (40.0) | 3 (27.3) | |
| Depth of invasion | ||||
| T1‐T2 | n = 3 | 2 (20.0) | 1 (0.9) | .324c |
| T3 | n = 16 | 8 (80.0) | 8 (72.7) | |
| T4 | n = 2 | 0 (0.0) | 2 (18.2) | |
| Stage | ||||
| I/II | n = 10 | 5 (50.0) | 5 (45.5) | .055b |
| III/IV | n = 11 | 5 (50.0) | 6 (54.5) | |
| Histology | ||||
| Intestinal (grade 3) | n = 16 | 9 (90.0) | 7 (63.6) | .172b |
| Diffuse (grade 3) | n = 5 | 1 (10.0) | 4 (36.4) | |
| Lymphatic invasion | ||||
| Nx | n = 8 | 3 (30.0) | 5 (45.5) | .192b |
| N1 | n = 8 | 4 (40.0) | 4 (36.3) | |
| N2‐N3 | n = 5 | 3 (30.0) | 2 (18.2) | |
Array purchased from US Biomax (Rockville, MD, USA).
Calculated by pairwise comparisons from χ2 test.
Calculated by comparisons of groups from linear‐by‐linear associations.
It has already been identified that PQLC2 is a heptahelical protein that functions as an amino acid transporter mediating the export of the cationic amino acids.1, 2 Amino acids are essential for cellular functions and survival. Tumor cells express amino acid transporters at a higher level than normal cells in order to meet the increased demand for amino acids during rapid growth.30 These transporters are therefore potential drug targets for cancer therapy, as their pharmacological blockade is expected to deprive tumor cells of nutrients and lead to growth arrest and death.31 Thus, some amino acid transporters could be promising targets for the treatment of cancer. The amino acid transporter solute carrier family 1 member 5 (SLC1A5), as well as SLC7A5, SLC7A11, and SLC6A14 are overexpressed in cancers,30 involving the oncogene c‐Myc. SLC7A5, which is functionally coupled to the c‐Myc target SLC1A5,32, 33 activates mTOR signaling.34 The two proteins coordinately promote tumor growth. Recent studies have shown that SLC38A9, localized in the lysosomal membrane, maintains mTOR‐mediated amino acid signaling in cells.21, 35, 36 In particular, the cross‐talk between Akt signaling and amino acid transporter, SLC6A19, and SLC38A1 appears to be an important molecular basis for the development of novel diagnostic markers in cancer patients. Dysregulation of AKT signaling is a hallmark of many cancers.37, 38 Consistent with these reports, we found that PQLC2 overexpression promoted cell growth, anchorage independence, and tumorigenesis. Raf1/ERK activity was enhanced, and AKT activity was upregulated in PQLC2‐overexpressing cells (Figure 3). Raf1/ERK and PI3K/AKT signaling pathways are critical signal transduction mechanisms for cell survival, proliferation, metabolism, and motility in response to extracellular stimulation. Aberrant regulation of both pathways frequently occurs in human cancers.39, 40 Here we also found that knockdown of PQLC2 suppressed the growth of GC cell lines in vitro, with significant effect observed in those expressing high levels of PQLC2 protein (MKN1, SNU668, and AGS cells), but not in the cell line expressing low levels of PQLC2 protein (SNU638). These results suggest that the abundance of target protein/mRNA is a critical factor that determines the efficiency of RNAi‐mediated gene silencing in GC cells. The abundance of PQLC2 might be a potential indicator for patient selection in the targeted therapeutic approach to GC. In addition, a mouse xenograft of MKN45 cells deficient in PQLC2 showed the reduced tumor growth in vivo. Thus, our in vitro and in vivo data indicate that PQLC2 is a critical regulator of GC development.
In conclusion, the results of this study indicate that the amino acid carrier PQLC2 might contribute to dysregulation of the cell cycle and tumor progression. Additional clinical studies and elucidation of molecular mechanisms are warranted to confirm that targeting PQLC2 is an effective therapeutic strategy for preventing the progression of GC.
CONFLICTS OF INTEREST
There are no conflicts of interest to declare.
Supporting information
ACKNOWLEDGMENTS
This work was supported by Basic Science Research through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant nos. 2017R1A2B4010695 and OGM0311711) and the KRIBB Initiative of the Korea Research Council of Fundamental Science and Technology.
Jeung Y‐J, Lee K, Lee HJ, et al. Cationic amino acid transporter PQLC2 is a potential therapeutic target in gastric cancer. Cancer Sci. 2019;110:1453–1463. 10.1111/cas.13966
Yun‐Ji Jeung and Kyeong Lee contributed equally to this work.
Contributor Information
Jin Man Kim, Email: jinmank@cnu.ac.kr, Email: kjm630929@gmail.com.
Kyung‐Sook Chung, Email: kschung@kribb.re.kr.
REFERENCES
- 1. Jezegou A, Llinares E, Anne C, et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc Natl Acad Sci USA. 2012;109:E3434‐E3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu B, Du H, Rutkowski R, Gartner A, Wang X. LAAT‐1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science. 2012;337:351‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung KS, Won M, Lee SB, et al. Isolation of a novel gene from Schizosaccharomyces pombe: stm1+ encoding a seven‐transmembrane loop protein that may couple with the heterotrimeric Galpha 2 protein, Gpa2. J Biol Chem. 2001;276:40190‐40201. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman CS. Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe . Biochem Soc Trans. 2005;33:257‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 6. De Vita F, Di Martino N, Fabozzi A, et al. Clinical management of advanced gastric cancer: the role of new molecular drugs. World J Gastroenterol. 2014;20:14537‐14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 8. Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483‐4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence‐based approach. J Gastric Cancer. 2014;14:87‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta‐analysis based on aggregate data. J Clin Oncol. 2006;24:2903‐2909. [DOI] [PubMed] [Google Scholar]
- 11. Group G, Oba K, Paoletti X, et al. Role of chemotherapy for advanced/recurrent gastric cancer: an individual‐patient‐data meta‐analysis. Eur J Cancer. 2013;49:1565‐1577. [DOI] [PubMed] [Google Scholar]
- 12. Lordick F, Allum W, Carneiro F, et al. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. 2014;40:692‐700. [DOI] [PubMed] [Google Scholar]
- 13. Schinzari G, Cassano A, Orlandi A, Basso M, Barone C. Targeted therapy in advanced gastric carcinoma: the future is beginning. Curr Med Chem. 2014;21:1026‐1038. [DOI] [PubMed] [Google Scholar]
- 14. Yang W, Raufi A, Klempner SJ. Targeted therapy for gastric cancer: molecular pathways and ongoing investigations. Biochim Biophys Acta. 2014;1846:232‐237. [DOI] [PubMed] [Google Scholar]
- 15. Gunturu KS, Woo Y, Beaubier N, Remotti HE, Saif MW. Gastric cancer and trastuzumab: first biologic therapy in gastric cancer. Ther Adv Med Oncol. 2013;5:143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh AD, Parmar S. Ramucirumab (Cyramza): a breakthrough treatment for gastric cancer. P T. 2015;40:430‐468. [PMC free article] [PubMed] [Google Scholar]
- 17. Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19‐9, CA 72‐4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075‐1084. [DOI] [PubMed] [Google Scholar]
- 18. Cidon EU, Bustamante R. Gastric cancer: tumor markers as predictive factors for preoperative staging. J Gastrointest Cancer. 2011;42:127‐130. [DOI] [PubMed] [Google Scholar]
- 19. Galizia G, Lieto E, Orditura M, et al. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg. 2007;31:1458‐1468. [DOI] [PubMed] [Google Scholar]
- 20. Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti‐epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1454‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang S, Tsun ZY, Wolfson RL, et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347:188‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanat O, O'Neil B, Shahda S. Targeted therapy for advanced gastric cancer: a review of current status and future prospects. World J Gastrointest Oncol. 2015;7:401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawakami H, Okamoto I, Arao T, et al. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget. 2013;4:9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeung YJ, Kim HG, Ahn J, et al. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim Biophys Acta. 2016;1863:2584‐2593. [DOI] [PubMed] [Google Scholar]
- 25. Won KJ, Im JY, Yun CO, et al. Human Noxin is an anti‐apoptotic protein in response to DNA damage of A549 non‐small cell lung carcinoma. Int J Cancer. 2014;134:2595‐2604. [DOI] [PubMed] [Google Scholar]
- 26. Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni‐Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dumaz N, Marais R. Protein kinase A blocks Raf‐1 activity by stimulating 14‐3‐3 binding and blocking Raf‐1 interaction with Ras. J Biol Chem. 2003;278:29819‐29823. [DOI] [PubMed] [Google Scholar]
- 28. Kalatzis V, Cherqui S, Antignac C, Gasnier B. Cystinosin, the protein defective in cystinosis, is a H(+)‐driven lysosomal cystine transporter. EMBO J. 2001;20:5940‐5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Town M, Jean G, Cherqui S, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319‐324. [DOI] [PubMed] [Google Scholar]
- 30. Bhutia YD, Babu E, Ramachandran S, Ganapathy V. Amino acid transporters in cancer and their relevance to “glutamine addiction”: novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782‐1788. [DOI] [PubMed] [Google Scholar]
- 31. Dann SG, Thomas G. The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett. 2006;580:2821‐2829. [DOI] [PubMed] [Google Scholar]
- 32. Gao P, Tchernyshyov I, Chang TC, et al. c‐Myc suppression of miR‐23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782‐18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rebsamen M, Pochini L, Stasyk T, et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rebsamen M, Superti‐Furga G. SLC38A9: A lysosomal amino acid transporter at the core of the amino acid‐sensing machinery that controls MTORC1. Autophagy. 2016;12:1061‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bogatikov E, Munoz C, Pakladok T, et al. Up‐regulation of amino acid transporter SLC6A19 activity and surface protein abundance by PKB/Akt and PIKfyve. Cell Physiol Biochem. 2012;30:1538‐1546. [DOI] [PubMed] [Google Scholar]
- 38. Wang K, Cao F, Fang W, et al. Activation of SNAT1/SLC38A1 in human breast cancer: correlation with p‐Akt overexpression. BMC Cancer. 2013;13:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAf/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249‐279. [DOI] [PubMed] [Google Scholar]
- 40. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


