Abstract
EGFR-TKI had become the first-line treatment of metastatic NSCLC and widely used in clinical. It was reported that there was difference in response rate between NSCLC patients with or without EGFR mutation. However, there was no relevant studies about the difference in clinical response among patients with different kinds of EGFR mutation. In this study, we recruited 464 patients with NSCLC between March 2014 and March 2015. Circulating tumor DNA (ctDNA) was isolated from plasma and identified EGFR gene mutations. Demographic characteristics, pathological data, safety and three-year survival were compared in patients with different EGFR gene mutations. The primary objective was progression-free survival (PFS), and secondary objectives included overall response rate (ORR), disease control rate (DCR) and overall survival (OS). Among all the patients, the total mutation rate of EGFR gene was 45.04%, major occurred in 19 exon (40.19%) and 21 exon (48.80%), respectively. There was great difference in gender, smoking status, TNM stage among patients with 19 exon, 21 exon and other mutation of EGFR (All P < 0.05). The ORR (34.31% vs. 28.57%, 21.74%) and DCR (73.53% vs. 69.05%, 56.52%) in patients with 21 exon mutation was significantly higher than patients with 19 exon or other mutations. After three-year follow-up, the median PFS was 7.9 months in the 21 exon group, 6.4 months in the 19 exon group and 5.1 months in the other mutation group (P < 0.05). And the median OS in patients with EGFR 21 exon mutation was significantly higher than those of patients with EGFR 19 exon or other mutations. In conclusion, applied with chemotherapy and EGFR-TKI, Chinese NSCLC patients with EGFR gene 21 exon mutation could have better clinical response and long-term survival than those with other kinds of mutation.
Keywords: EGFR, gene polymorphism, NSCLC, PFS, ORR, DCR
Introduction
Lung cancer was the most common cancer and was the leading cause of cancer death worldwide [1]. Non-small cell lung cancer (NSCLC) possessed approximately 80-85% in the prevalence of lung cancer case and seriously threaten to human health and lives [2]. Despite the recent advances in early detection screening protocols, improved surgical techniques, radio and chemotherapeutic regimens, the 5-year survival rate of patient with NSCLC was below 15% [3]. Over the last decade, the management of NSCLC had undergone significant evolution due to improvement in the understanding of molecular drivers of carcinogenesis. The discovery of oncogenes, such as the epidermal growth factor receptor (EGFR) and the development of medications that specifically target EGFR gene mutation led to the ability to personalize therapy.
Epidermal growth factor receptor (EGFR) was a member of the tyrosine kinase receptor family, which was thought to be involved in the development of cancer. EGFR activating mutations, which were widely known for their prominent role in tumorigenesis and progression, were found in 10-15% of patients with NSCLC in Western countries and 30%-40% in Asia, respectively [4]. The two most common mutations, the EGFR exon 19 deletion and the exon 21 L858R substitution, accounted for more than 90% of known EGFR activating mutations [5,6]. The introduction of first-generation EGFR tyrosine kinase inhibitors (TKI) (gefitinib, erlotinib), and second-generation TKIs (afatinib) for the treatment of NSCLC patients with a sensitizing EGFR mutation had demonstrated a high response rate and prolonged progression-free survival (PFS) [7-9]. However, in clinical practice, patients who had the indications for 1st-gen TKI treatment demonstrated significant heterogeneity in treatment responses with a PFS ranging from a few months to several years and about 60% of patients unfortunately acquired drug resistance after a favorable response to EGFR-TKI for 9-13 months on average [5,10-12]. Currently the European Society for Medical Oncology guidelines indicate that EGFR mutation testing is recommended as standard in non-squamous NSCLC [13]. Tumour specimens from curative surgery or bronchial biopsy were regard as the gold standard for testing, but less than one-third of patients are suitable for surgery [14] and bronchial biopsy was impractical for poorly accessible tumours. There were also other cytological samples, such as fine-needle aspirates, bronchial brushings, serum, plasma, circulating tumour cells and pleural effusion samples could be used for EGFR mutation testing [15]. Yanzhen Lai, et al also reported the effect of serum VEGF levels in the early diagnosis and severity assessment of NSCLC [16]. Considering the high convenience and effectiveness of collecting circulating tumor DNA (ctDNA), we evaluated the effect of EGFR gene mutation of ctDNA on efficacy of targeted therapy combined with chemotherapy for Chinese patients with NSCLC.
Methods
Study population
A total of 464 NSCLC patients with a treatment plan to begin TKI treatment were enrolled consecutively in our study during March 2014 to March 2015. The diagnosis of NSCLC was made by the pathologist based on histopathological examination. The patients were all newly diagnosed without a history of prior radiotherapy and/or chemotherapy. Exclusion criteria included primary extra pulmonary malignancy, small cell lung cancer, a history of malignant disease, and withdrawal of consent and patient aged less than 18. Pathological staging information on all NSCLC cases was confirmed by manual review of the pathology reports and clinical charts. Nodal status was categorized as no regional lymph nodes affected (N0) or at least one nodal metastasis.
Plasma isolation, DNA extraction and EGFR mutation
Venous blood (3 mL) was collected with anticoagulants and stored in a -70°C freezer until further use. DNA was extracted according to the blood genomic DNA extraction kit instructions (Roche, Diagnostics GmbH, Mannheim, Germany) and stored in a -20°C freezer until further use. Genotyping was performed by detection kit for 29 EGFR mutations (PCR-Fluorescence), which was combined modified amplication refructory mutation system and Taqman probe (DoGene, Shanghai, China).
Study design, endpoints, and treatments
In this study, the primary endpoint was to compare PFS and secondary endpoints included overall response rate (ORR), disease control rate (DCR) and overall survival (OS). The treatment plan of all the patients was designed according to National Comprehensive Cancer Network (NCCN) Guidelines. Patients received vitamin B12, folate, and dexamethasone treatment as premedications for chemotherapy drugs. Dose reductions, delays, and discontinuations due to toxicity were specified by the protocol.
Statistical analysis
For statistical analysis, SPSS v. 16.0 (SPSS Inc., Chicago, IL, USA) was used. Figures were created by GraphPad Prism 5.0 (GraphPad Software, Inc.). Data were expressed as mean ± standard deviation (SD) for numerical data and frequency or percentage (%) for categorical variables. Differences among the groups were analysed using the Kruskal-Wallis test or analysis of variance as appropriate for numerical data, as well as the chi-squared test for categorical values. Multiple logistic regression analysis was performed to evaluate the odds ratio (OR) and associated factors. The median PFS and OS were estimated using the Kaplan-Meier survival analysis. Differences between groups were compared using the stratified log-rank test. Hazard ratio (HR) and 95% CI were also calculated by Cox proportional hazard regression models. Subgroup, including sex (male or female), age (≤ or > 60 years old), smoking (yes or no), histology (SCC or Ad), TNM stage (I+II or III+IV) and EGFR gene mutation (exon 19, 21 or others), was designed to compared the association between every parameter and PFS or OS by univariate Cox proportional hazards model. Only significant variable was included in the multi-variate analysis of survival as covariables when comparing the difference in PFS and OS among patients with three EGFR gene mutation. All P values were two-tailed, and P < 0.05 was considered to be statistically significant.
Results
General data of patients
A total of 464 Chinese patients with NSCLC were recruited in our study between March 2014 and March 2015. There were 421 SCC (squamous cell carcinoma) and 43 Ad (adenocarcinoma) patients collected in our study and the study population (209 men and 255 women) had a mean age of 64.31 ± 9.17 years, ranging from 34 to 83 years at the time of diagnosis. A summary of characteristics including clinical and tumor information of patients in all patients was demonstrated in Table 1.
Table 1.
General data
| Characteristics | Cases |
|---|---|
| Gender | |
| Male | 209 |
| Female | 255 |
| Age (years) | |
| ≤ 60 | 204 |
| > 60 | 260 |
| Smoking status | |
| No | 257 |
| Yes | 207 |
| Primary tumor sites | |
| Upper lobe | 137 |
| Middle and lower lobes | 327 |
| Histology | |
| SCC | 421 |
| Ad | 43 |
| TNM stage | |
| I+II | 225 |
| III+IV | 239 |
Ad, adenocarcinoma; SCC, squamous cell carcinoma.
EGFR mutation detection
By detection kit, 29 kinds of EGFR mutations in all the patients were conducted. Of all patients, 209 subjects were EGFR mutation with total rate being 45.04% (209/464). The EGFR mutation in our study was summarized in Table 2. Among patients with EGFR mutation, 19 exon (-del) and 21 exon (L858R) mutation respectively accounts 84 and 97 patients, over 85% of all types of EGFR mutation.
Table 2.
Characteristic of EGFR gene mutation types in 464 NSCLC patients
| EGFR mutation type | Positive number | Proportion (%) |
|---|---|---|
| 18 exon (G719X) | 6 | 2.87 |
| 19 exon (-del) | 84 | 40.19 |
| 20 exon (T790M) | 15 | 7.18 |
| 20 exon (S768I) | 2 | 0.96 |
| 21 exon (L858R) | 97 | 46.41 |
| 21 exon (L861Q) | 5 | 2.39 |
| Total | 209 | 100.00 |
Demographic characteristics among patients in three groups
According to EGFR mutation types, all the patients were divided into three groups, as 19 exon mutation group (n = 84), 21 exon mutation group (n = 102) and others mutation group (n = 23), including all the patients with other types of EGFR mutation except for 19 and 21 exon. We compared the clinicopathological characteristics and founded that there were great difference in gender, smoking status and TNM stage among these three groups of patients (Table 3). The 19 or 21 exon mutation occured more frequently in female, while the other types of mutation more frequently in male (P < 0.05). Besides, the percentage of 19 and 21 exon mutation in nonsmokers was much higher than those in smokers (19 exon, 52.38% vs. 47.62%; 21 exon, 61.76% vs. 38.23%). However, the percentage of other mutation in nonsmokers was much lower than those in smokers (others, 39.13% vs. 60.87%; P < 0.05). What’s more, there was also a great difference in TNM stage distribution between 21 exon mutation group and other two groups of patients (P < 0.01).
Table 3.
Association of EGFR gene mutation with clinicopathological characteristics in NSCLC patients
| Characteristics | EGFR mutation type | OR (95% CI) | P | ||
|---|---|---|---|---|---|
|
| |||||
| 19 exon 84 | 21 exon 102 | Others 23 | |||
| Gender | |||||
| Male | 35 (41.67%) | 50 (49.02%) | 14 (60.87%) | 1 (Reference) | |
| Female | 49 (58.33%) | 52 (50.98%) | 9 (39.13%) | 2.66 (1.18-4.19) | 0.02 |
| Age (years) | |||||
| ≤ 60 | 37 (44.05%) | 44 (43.14%) | 11(47.83%) | 1 (Reference) | |
| > 60 | 47 (55.95%) | 58 (56.86%) | 12 (52.17%) | 1.10 (0.67-1.54) | 0.12 |
| Smoking status | |||||
| No | 44 (52.38%) | 63 (61.76%) | 9 (39.13%) | 1 (Reference) | |
| Yes | 40 (47.62%) | 39 (38.23%) | 14 (60.87%) | 1.89 (1.22-2.56) | 0.04 |
| Histology | |||||
| SCC | 80 (95.24%) | 95 (93.14%) | 22 (95.65%) | 1 (Reference) | |
| Ad | 4 (4.76%) | 7 (6.86%) | 1 (4.35%) | 0.82(0.55-1.09) | 0.87 |
| TNM stage | |||||
| I+II | 37 (44.05%) | 59 (57.84%) | 10 (43.48%) | 1 (Reference) | |
| III+IV | 47 (55.95%) | 43 (42.16%) | 13 (56.52%) | 2.09 (1.41-2.66) | 0.01 |
Ad, adenocarcinoma; SCC, squamous cell carcinoma; TNM, tumour-node-metastasis staging system.
Progression-free survival
By the deadline of March 31th 2018, median follow-up period was 42.19 months. For all the 209 patients with EGFR mutation, the overall median PFS was 13.5 months (95% CI, 10.4-16.6 months). Smoking status and TNM stage were added to multivariate analysis of PFS as co-variables when comparing the difference in survival among patients with three EGFR mutation (P < 005, Figure 1). In patients with 19 exon, 21 exon and others mutation, median PFS were 15.9 months (95% CI, 13.4-17.4 months), 12.2 months (95% CI, 9.6-14.8 months) and 10.5 months (95% CI, 8.9-12.1 months) respectively. The median PFS was significantly prolonged in 19 exon group compared with other two groups (Figure 1A, P = 0.001, HR 0.77, 95% CI, 0.58 to 0.96). When subgroup analyses were performed by baseline clinical characteristics in forest plots, subgroups including no smoking (95% CI, 1.01 to 1.23) and 19 exon mutation (95% CI, 1.12 to 1.84) could benefit from clinical outcome (P < 005, Figure 1B). No significant difference in median PFS between different gender, age, histology or TNM stage groups.
Figure 1.
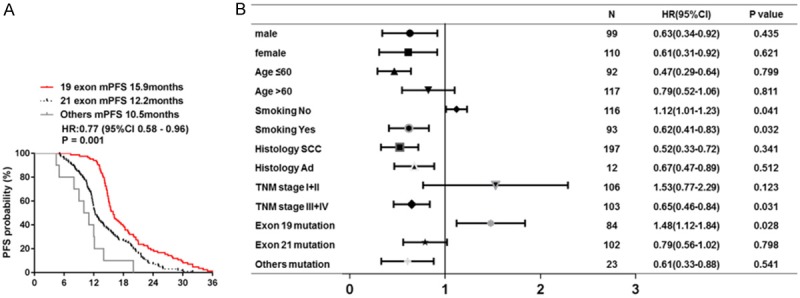
Median PFS and forest plot of hazard ratios for PFS. A. Median PFS in three groups of patients with different EGFR mutation; B. Forest plot of baseline characteristics.
Overall survival
By the deadline of follow-up, 58 events (69.05%) had occurred in 19 exon mutation group, 62 events (60.78%) in 21 exon mutation group and 20 (86.95%) events in others mutation group. Smoking status and TNM stage were added to multivariate analysis of OS as co-variables when comparing the difference in survival among patients with three EGFR mutation (P < 005, Figure 2). Median OS of 209 patients was 27.23 months (95% CI, 23.41-31.01 months). The median OS was 30.4 months in 19 exon mutation group (95% CI, 28.1-32.7 months), 25.9 months in 21 exon mutation group (95% CI, 23.7-28.1 months) and 21.6 months in others mutation group (95% CI, 19.7-23.5 months). Different from EGFR mutation showed obvious tendency to extend median OS (P = 0.001; HR 0.81, 95% CI 0.69 to 0.92, Figure 2A). There was also significant benefit tendency in subgroup OS analysis by baseline smoking status, TNM stages and EGFR mutation (Figure 2B).
Figure 2.
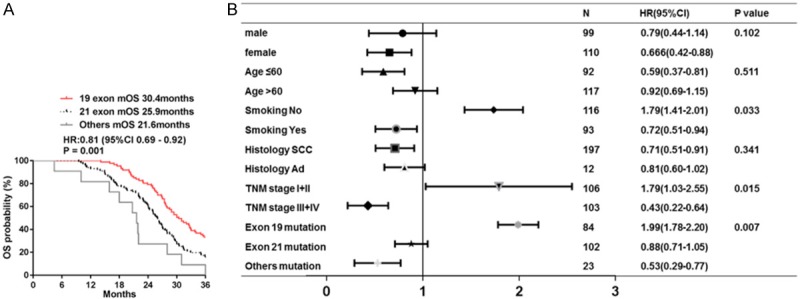
Median OS among three groups with different EGFR mutation. A. Median OS in three groups of patients with different EGFR mutation; B. Forest plot of baseline characteristics.
Response rates
In 19 exon mutation group, there were 32 PR, 34 SD and 18 PD respectively, while in 21 exon mutation group there were 1 CR, 34 PR, 40 SD and 27 PD, in others mutation group 5 PR, 8 SD and 10 PD respectively, which showed significant differences in SD among three groups (Table 4, P < 0.05). There were also great differences in ORR and DCR among patients with different EGFR mutation. The ORR and DCR in both 19 and 21 exon mutation groups was significantly higher than those in others mutation group (all P < 0.05). Besides, compared to patients with 21 exon mutation, patients with 19 exon mutation had significantly higher ORR and DCR (both P < 0.05).
Table 4.
Relationship between EGFR gene mutation type and therapeutic efficacy
| Therapeutic evaluation | EGFR mutation type | P | ||
|---|---|---|---|---|
|
| ||||
| 19 exon | 21 exon | Others | ||
| CR | 0 | 1 | 0 | |
| PR | 32 | 34 | 5 | 0.564 |
| SD | 34 | 40 | 8 | 0.009 |
| PD | 18 | 27 | 10 | 0.167 |
| ORR | 38.10%*,# | 34.31%* | 21.74% | 0.021 |
| DCR | 78.57%*,# | 73.53%* | 56.52% | 0.007 |
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; ORR: objective response rate; DCR: disease control rate.
P < 0.05, vs. Others mutation group;
P < 0.05 vs. 21 exon mutation group.
Discussion
We conducted this prospective cohort study to evaluate the effect of EGFR gene polymorphism on efficacy of chemotherapy combined with targeted therapy for non-small cell lung cancer in Chinese patients. In this study, we detected the EGFR mutation types and compared the clinicopathological characteristics among patients with different EGFR mutations. The overall probability of EGFR mutation was 45.04% and more than 85% mutation happened in 19 exon (-del) and 21 exon (L858R) mutation, respectively accounting for 40.19% and 46.41%. We found there were great difference in gender, smoking status and TNM stage among these three groups of patients. EGFR 19 exon mutation happened significantly more in female and patients with TNM III or IV stages and EGFR 21 exon mutation happened significantly more in male, smoking patients and patients with TNM I or II stages. After three-year follow, the median PFS was significantly prolonged in 19 exon mutation group compared with 21 exon mutation group and others mutation group (15.9 months vs. 12.2 months and 10.5 months). Besides, there was significant benefit tendency in median OS for patients with EGFR 19 exon mutation than other two groups (30.4 months vs. 25.9 months and 21.6 months), which might be associated with the better response rates in patients with 19 exon mutation to targeted therapy focused on EGFR gene. The results of this cohort study revealed that there were significant difference in clinicopathological characteristics among patients with different EGFR mutations and the patients with 19 exon mutation had significantly higher ORR and DCR, which probably led to longer survivals after receiving EGFR-TKI therapy in Chinese patients with NSCLC.
There was increased interest in molecularly targeted drugs for the treatment of patients with NSCLC. Inhibitors of the epidermal growth factor receptor (EGFR) were among the targeted therapies that had been shown to be effective and are currently used in the treatment of advanced NSCLC [17]. EGFR (ErbB-1) was part of the ErbB family of receptors. After combined with Ligands, mainly including transforming growth factor-β (TGF-β) and amphiregulin, activation of EGFR leaded to phosphorylation of the intracellular tyrosine kinase domain of the receptor such as RAS and mitogenactivated protein kinase, which was closely associated with the cell-signaling pathways of cellular proliferation, motility, angiogenesis, invasion, and inhibition of apoptosis [18]. 3 In NSCLC, Over expression of EGFR in NSCLC had been detected and shown to correlate with increases in the rate of proliferation of tumors and incidence of metastases [19]. Given its abundant expression in NSCLC and key role in tumor growth and invasion, inhibition of the EGF receptor attracted attention and had been developed as a therapeutic modality in NSCLC. Nowadays, EGFR-TKI had become the first-line treatment of metastatic NSCLC and widely used in clinical.
Predicting which patients are most likely to benefit from EGFR targeted therapy remained a challenge. The previous studies identified a population that is more likely to respond to anti-EGFR therapy, i.e. never smokers, Asian and female the presence of cutaneous side effects has also been correlated with response rates [6]. Our results revealed that subgroups of patients including no smoking (95% CI, 1.01 to 1.23) and 19 exon mutation (95% CI, 1.12 to 1.84) could benefit from clinical outcome of PFS. No significant difference in median PFS between different gender, age, histology or TNM stage groups. Besides, there was also significant benefit tendency in subgroup OS analysis by baseline smoking status, TNM stages and EGFR mutation.
At the molecular level, most patients with partial or complete responses to gefitinib or erlotinib harbored specific mutations in the gene that encoded EGFR, located on chromosome 7p12 [20]. Exon 19 mutations, characterized by in-frame deletions of amino-acids 747-750, accounted for 45% of mutations, exon 21 mutations, resulting in L858R substitutions, account for 40-45% of mutations, and the remaining 10% of mutations involve exon 18 and 20 [21-24]. By detection kit, 29 kinds of EGFR mutations were conducted in our study. Of all patients, 209 subjects were EGFR mutation with total rate being 45.04% (209/464). Among patients with EGFR mutation, 19 exon (-del) and 21 exon (L858R) mutation accounted 84 (40.19%) and 97 (46.41%) patients, respectively, more than 85% of the total mutations. These mutations were reported to increase the kinase activity of EGFR in vitro, which leading to the hyperactivation of downstream pro-survival pathways and consequently conferring oncogenic properties on EGFR [25,26].
The EGFR mutation was also demonstrated to be of great association with clinical responses to EGFR-TKI. Compared to wild type receptors, EGFR mutants were more sensitive to inhibition by gefitinib and erlotinib. Overall, the incidence of EGFR mutations in NSCLC among clinical responders to gefitinb or erlotinib is 77%, compared with 7% in NSCLC cases that do not have a CR or PR [5,6]. However there was no relevant studies discussed the difference in clinical response among patients with different EGFR mutation. Our results illuminated that there were great differences in ORR and DCR among patients with different EGFR mutation. The ORR and DCR in both 19 and 21 exon mutation groups was significantly higher than those in others mutation group. Besides, compared to patients with 21 exon mutation, patients with 19 exon mutation had significantly higher ORR and DCR. After three-year follow-up, the median PFS in patients with 19 exon, 21 exon and others EGFR mutation were 15.9 months (95% CI, 13.4-17.4 months), 12.2 months (95% CI, 9.6-14.8 months) and 10.5 months (95% CI, 8.9-12.1 months) respectively. The median PFS was significantly prolonged in 19 exon group compared with other two groups (Figure 1A, P = 0.001, HR 0.77, 95% CI, 0.58 to 0.96). What’s more, the median OS was 30.4 months in 19 exon mutation group (95% CI, 28.1-32.7 months), 25.9 months in 21 exon mutation group (95% CI, 23.7-28.1 months) and 21.6 months in others mutation group (95% CI, 19.7-23.5 months). There was an obvious tendency between EGFR mutations to extend median OS (HR 0.81, 95% CI 0.69 to 0.92).
Interestingly, our study also found a difference in clinicopathological characteristics, mainly including gender, smoking status and TNM stage among these three groups of patients. The 19 or 21 exon mutation occurred more frequently in female, while the other types of mutation more frequently in male. The percentage of 19 and 21 exon mutation in nonsmokers was much higher than those in smokers (19 exon, 52.38% vs. 47.62%; 21 exon, 61.76% vs. 38.23%). And there was a great difference in TNM stage distribution between 21 exon mutation group and other two groups of patients. The other research also reported that the presence of EGFR kinase mutations seem to be highly correlated with clinical characteristics, i.e. female sex, never smokers, Asian descent, adenocarcinoma histology, whereas, in patients with smoking-associated cancers, EGFR gene amplification [27].
Some limitations of this study should also be considered. Firstly, the number of patients with NSCLC in this single-center study was relatively small, especially in patients with other kinds of EGFR mutation except for 19 or 21 exon mutation. Besides, the phamacogenetics was used to predict whether the selected chemotherapy will be really effective and tolerable to the patients combined with EGFR-TKI therapy, which resulted in a lack of consistency in therapeutic schedule. What’s important, the detection of EGFR mutation was performed by detection kit (PCR-Fluorescence), which was combined modified amplication refructory mutation system and Taqman probe from venous blood genomic DNA. There was a shortage of evaluation of EGFR mutation in tumor tissue.
In summary, the overall probability of EGFR mutation in Chinese patients with NSCLC was 45.04% and more than 85% mutation happened in 19 exon (-del) and 21 exon (L858R) mutation, respectively accounting for 40.19% and 46.41%. There were significant difference in gender, smoking status and TNM stage among patients with different EGFR mutation. After receiving target therapy, patients with 19 exon mutation had better clinical response, including higher ORR and DCR, than those in with 21 exon or others mutation groups. As a result, the median PFS and OS in 19 exon mutation group was significantly prolonged compared with 21 exon mutation group and others mutation group after three-year follow-up. Our research demonstrated that there was significant benefit tendency in clinical response and longer survivals for patients with EGFR 19 exon mutation than patients with 21 exon or other kinds of mutation after receiving EGFR-TKI therapy in Chinese patients with NSCLC. The further study about the underlying link between EGFR 19 exon mutation and development of NSCLC in the future might provide a potential strategy for the precision treatment of NSCLC in Chinese population.
Conclusions
The overall probability of EGFR mutation in Chinese patients with NSCLC was 45.04% and more than 85% mutation happened in 19 exon (-del) and 21 exon (L858R) mutation, respectively accounting for 40.19% and 46.41%. Patients with 19 exon mutation had better clinical response than those in with 21 exon or others mutation groups after receiving TKI therapy. The median PFS and OS in 19 exon mutation group was significantly prolonged compared with other kinds of mutation group after three-year follow-up.
Acknowledgements
The authors thank the patients and volunteers recruited from Sichuan Provincial People’s Hospital for participating in the research. The work was funded by Science and Technology Research Program of Sichuan (2016FX0092).
Written informed consents were obtained from all participants.
Disclosure of conflict of interest
None.
Abbreviations
- NSCLC
Non-small cell lung cancer
- EGFR
epidermal growth factor receptor
- TKI
tyrosine kinase inhibitors
- PFS
progression-free survival
- ctDNA
circulating tumor DNA
- ORR
overall response rate
- DCR
disease control rate
- OS
overall survival
- NCCN
National Comprehensive Cancer Network
- OR
odds ratio
- Ad
adenocarcinoma
- SCC
squamous cell carcinoma
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5:2892–911. [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 9.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 10.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I, Ludovini V, Magrini E, Gregorc V, Doglioni C, Sidoni A, Tonato M, Franklin WA, Crino L, Bunn PA Jr, Varella-Garcia M. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–55. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Wang X, Jin H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res. 2014;4:411–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Quan Q, Ding L, Hong X, Zhou N, Liang Y, Wu H. Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors. Oncotarget. 2015;6:24904–11. doi: 10.18632/oncotarget.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: reappraisal with a meta-analysis of randomized controlled trials. J. Clin. Oncol. 2004;22:3860–7. doi: 10.1200/JCO.2004.01.153. [DOI] [PubMed] [Google Scholar]
- 15.Smouse JH, Cibas ES, Jänne PA, Joshi VA, Zou KH, Lindeman NI. EGFR mutations are detected comparably in cytologic and surgical pathology specimens of nonsmall cell lung cancer. Cancer. 2009;117:67–72. doi: 10.1002/cncy.20011. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Wang X, Zeng T, Xing S, Dai S, Wang J, Chen S, Li X, Xie Y, Zhu Y, Liu W. Serum VEGF levels in the early diagnosis and severity assessment of non-small cell lung cancer. J Cancer. 2018;9:1538–47. doi: 10.7150/jca.23973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 18.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(Suppl):21–6. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA Jr. Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29(Suppl 4):3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- 20.Testa JR, Siegfried JM. Chromosome abnormalities in human non-small cell lung cancer. Cancer Res. 1992;52(Suppl):2702s–6s. [PubMed] [Google Scholar]
- 21.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 22.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–23. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 23.Janne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J. Clin. Oncol. 2005;23:3227–34. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, Camplese PP, Iarussi T, Mucilli F, Mezzetti A, Cuccurullo F, Sacco R, Buttitta F. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 2005;23:857–65. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Greulich H, Jänne PA, Sellers WR, Meyerson M, Griffin JD. Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 2005;65:8968–74. doi: 10.1158/0008-5472.CAN-05-1829. [DOI] [PubMed] [Google Scholar]
- 26.Prudkin L, Wistuba II. Epidermal growth factor receptor abnormalities in lung cancer. Pathogenetic and clinical implications. Ann Diagn Pathol. 2006;10:306–15. doi: 10.1016/j.anndiagpath.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, Brannigan BW, Sgroi DC, Muir B, Riemenschneider MJ, Iacona RB, Krebs AD, Johnson DH, Giaccone G, Herbst RS, Manegold C, Fukuoka M, Kris MG, Baselga J, Ochs JS, Haber DA. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J. Clin. Oncol. 2005;23:8081–92. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]


