Abstract
Previous studies have shown that transglutaminase 2 (TG2) induces epithelial to mesenchymal transition (EMT) in various tumors. Several studies have also demonstrated the critical role of microRNAs (miRNAs) in regulating EMT of various types of tumors. However, the relationship between TG2 and miRNAs is not well understood. In the present study, we investigated if miR-205, which is known to inhibit EMT and is commonly regulated by TG2, contributes to TG2-induced EMT of human breast cancer cells. We have analyzed the expression of miR-205 in TG2-expressing and TG2-non-expressing breast cancer cells by quantitative real-time PCR (qRT-PCR) and the expression of TG2 and EMT related markers, such as ZEB1 and Vimentin, by western blotting. We also have studied the regulation of tumor metastasis by miR-205 and TG2 using matrigel invasion assays, intracardiac injection of breast cancer cells into mice and in vivo bioluminescent imaging. MiR-205 was significantly downregulated in high TG2-expressing or TG2-transfected breast cancer cells than in low TG2-expressing or mock-transfected breast cancer cells. Overexpression of miR-205 reduced the bone metastasis of MCF7/TG2-C277S cells that express transamidase-activity deficient TG2 and inhibits the invasiveness of MDA-MB-231 breast cancer cells that express TG2. Bioluminescent imaging showed that intracardiac injection of MCF7/TG2-C277S cells in mice promoted bone tumors, especially in the knee and jaw, but MCF7/TG2-C277S cells ectopically expressing miR-205 did not metastasize. The GTP binding activity, but not transamidase activity, of TG2, induces EMT in breast cancer cells by inhibiting the expression of miR-205 that suppresses EMT by downregulating the expression of ZEB1, an EMT marker. Moreover, in vivo experiments demonstrate that miR-205 down-regulation by TG2 induces bone metastasis of breast cancer cells.
Keywords: MicroRNA, transglutaminase 2, metastasis, breast cancer
Introduction
Breast cancer is the second most commonly diagnosed cancer and the second most common cause of cancer-related deaths in the USA. In 2017, an estimated 252,710 women and 2,470 men were expected to be diagnosed with invasive breast cancer, and an estimated 63,410 women were to be diagnosed with in situ breast carcinoma. Furthermore, 40,610 women and 460 men were expected to die from breast cancer in 2017 [1]. Breast cancer is generally classified by the status of the estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth factor receptor 2 (HER2 or c-erbB2). Patients with triple-negative breast tumors are deficient in ER, PR and HER2 expression and their effective treatment remain a challenge in the clinic because of the lack of ER and HER2 expression which is a target for hormonal therapy or targeted therapy. These patients show worse prognosis because of the lack of effective targeted therapeutics to prevent metastasis [2]. Metastasis is associated with drug resistance and is a significant reason for treatment failure in cancer patients. Therefore, understanding the unknown mechanisms underlying metastasis is critical to overcoming resistance to each anti-cancer drug. Although several mechanisms related to metastasis have been discovered and several biomarkers of Triple-Negative Breast Cancer (TNBC) such as CDCP1, miR-770 and TG2 are known, further in-depth studies are necessary to understand the complex metastatic mechanisms underlying TNBC for development of more effective drugs [3,4].
Transglutaminase 2 (TG2) is an EMT marker protein with diverse functions such as protein cross-linking, post-translational modification of proteins, scaffolding, cell adhesion and cell signaling [5-8]. The most well-known role of TG2 in cancer is depletion of tumor suppressors via protein cross-linking [6]. The crosslinking activity of TG2 activates FAK, AKT and NFκB signaling, which induces EMT in various tumors and promotes resistance to several anti-cancer drugs [9-14]. Moreover, TG2 plays a critical role in EMT induction via cross-talk with several signaling pathways such as Transforming Growth Factor β1 (TGFβ1), Wnt, β-catenin and Nuclear factor-kappaB (NF-κB) [15,16]. Previous clinical studies have shown a correlation between TG2 expression, metastatic cancer and poor survival outcomes in ovarian, breast and colon cancer patients [17,18]. TG2 is also known to mediate motility, invasion, and growth of breast cancer cells [19,20].
MicroRNAs (miRNAs) are small, single-stranded non-coding RNAs (about 23 nucleotides long) that play critical roles in the post-transcriptional regulation of gene expression by targeting the complementary mRNA sequence in the 3’-untranslated regions of various target genes [21]. The miRNAs play tumor suppressor or oncogenic roles, and their expression is altered in breast cancer; the miRNAs regulate all the different stages of cancer progression, such as cell proliferation, apoptosis, cell migration, angiogenesis and stem cell maintenance [21,22]. Previous studies showed that the miR-200 family, which includes miR-21 and miR-205, regulates tumor cell proliferation and invasion in various tumors including breast tumors [23,24]. MiR-205 is highly expressed in multiple tissues such as the esophagus, trachea, prostate, thymus and breast tissues [25,26]. The aberrant miR-205 expression is found in many solid tumors. While miR-205 is over-expressed in non-small cell lung cancer [27-29] and endometrial cancer [30], its expression is down-regulated in prostate cancer [31], melanoma [32-34] and breast cancer [24,35]. Especially, miR-205 is highly expressed in the normal ducts of the breast but dramatically reduced in the breast tumor tissues [24,36]. MiR-205 is negatively regulated by HER2, which is one of the biomarkers for worse prognosis in breast cancer [37]. Moreover, the miR-200 family members and miR-205 inhibit epithelial to mesenchymal transition (EMT) via E-cadherin regulation and ZEB1 inhibition [23]. The known tumor suppressive functions of miR-205 in breast cancer include direct repression of several oncogenes such as VEGFA and HER3, as well as suppression of EMT by inhibiting ZEB1 and ZEB2 expression [36,38,39].
It was recently established that GTP binding activity, but not the transamidase activity of TG2 was required for expression of EMT markers in melanoma [40] and breast cancer [41] cells. However, to date, the miRNAs that are regulated by TG2 have not been identified. Therefore, this study aimed to verify if miR-200s or miR-205 are down-regulated by TG2 in breast cancer cells. Moreover, we investigated the EMT induction mechanism by TG2 using the MDA-MB-231 cell line that represents the aggressive TNBC phenotype and shows high TG2 expression.
Material and methods
Cancer cell lines and antibodies
The human breast cancer cells, MCF7 and MDA-MD-231, were purchased from ATCC. The MCF7 and MDA-MB-231 cells were grown in RPMI1640, and L-15 media supplemented with 10% FBS and 1 × antibiotics (Gibco, Waltham, MA, USA), respectively. The anti-TG2 antibody was purchased from Abcam (CUB 7402). The others antibodies for western blot analysis were obtained from Cell Signaling.
Generation of stable transgenic breast cancer cell lines
The control (pcDNA3.1) and TG2-expressing (pcDNA3.1-TG2) plasmid vectors were kindly provided by Dr. Soo-Youl Kim (National Cancer Center, South Korea). The MCF7/mock and MCF7/TG2 cells were established using G418 selection (Gibco, 500 mg/ml). The mutant TG2 constructs (C277S and R580A) were stably expressed in the MCF7 cell line by retroviral transfection and selection against puromycin as previously described [7]. The control shRNA (sc-108080) and TG2 shRNA (sc-37514-V) lentiviral particles were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). We generated MDA-MB-231/mock and MDA-MB-231/shTG2 cells by infecting MDA-MB-231 cells with control-shRNA and TG2-shRNA lentiviruses for 48 h followed by puromycin (Gibco, Waltham, MA, USA) selection. We cloned the scrambled control miRNA clone (CmiR0001-MR04, GeneCopoeia) and the partial length of the miR-205 primary precursor sequence into the pcDNA3.1 vector. We transfected the MCF7/TG2-C277S cells with these constructs and selected the stable clones using G418 (Geneticin). The miRNA inhibitor control clone (CmiR-AN0001-AM04) and the anti-miR-205 vector (HmiR-AN0307-AM04) were also purchased from GeneCopoeia (MD, USA) and selected by hygromycin (Gibco, Waltham, MA, USA).
Western blotting
For western blot analysis, cells were lysed on ice in RIPA buffer (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Fifty micrograms of total protein lysates from all samples were electrophoresed on a NuPAGE (4-20%) Tris-Glycine gel in SDS running buffer (Invitrogen, Carlsbad, CA, USA). The separated proteins were transferred to nitrocellulose membranes using the iBlot transfer apparatus (Invitrogen, Carlsbad, CA, USA). Then, the membranes were blocked in Tris-buffered saline containing 0.5% Tween 20 (TBS-T) and 5% BSA for 1 hr at room temperature followed by incubation with the primary antibodies overnight at 4°C. After the membranes were washed thrice with TBS-T for 10 min each, the blots were incubated with the HRP-conjugated secondary antibody (Bio-Rad, Hercules, CA, USA) in TBS-T containing 1% BSA for 1 hour at room temperature. Then the protein bands were visualized using the G-box Chemi Systems (SynGene, India).
MicroRNA extraction and quantitative RT-PCR
Quantitative RT-PCR was performed on RNA isolated from breast cancer cells. Total RNA was extracted from various breast cancer cell lines using the miRNeasy Mini Kit (Qiagen, Germantown, MD, USA), according to the manufacturer’s instructions. Then, cDNA was synthesized from purified mRNA samples using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster, CA, USA). Quantitative RT-PCR was performed on a LightCycler using SYBR Green (Roche Diagnostics, Switzerland) in the 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster, CA, USA). GAPDH expression was used as an internal control to normalize input cDNA. TaqMan gene expression assays for miR-205 (Hs04231469_s1) and GAPDH (Hs02758991_g1) were purchased from Invitrogen (Carlsbad, CA, USA).
Invasion assay
CytoSelectTM 24-Well Cell Invasion and Migration Assay kits were purchased from Cell Biolabs (CA, USA) and invasion assays were performed according to the manufacturer’s instructions. First, the cells were serum-starved overnight, followed by adding 5 × 105 cells in 300 µl of medium without FBS in the upper chamber of transwell plates, whereas the lower chamber was filled with 500 µl of medium with 10% FBS. We stained the cells that migrated and invaded into the lower chamber with crystal violet.
In vivo metastasis assay using intracardiac injection and bioluminescent imaging
Animal experiments were performed in compliance with RARC guidelines of NIH. Five to six-week-old female athymic NCr-nu/nu mice (NCI, Frederick, MD, USA) were anesthetized, laid on their back, and the cell suspension was injected into the left ventricle of the heart through a transdiaphragmatic access with a 27.5-gauge needle. The baseline luciferase activity of MCF7/Mock/LUC, MCF7/TG2-C277S/LUC and MCF7/TG2-C277S/miR-205/LUC cells was assessed by in vitro bioluminescent imaging using the IVIS Imaging System (Xenogen, Alameda, CA). Then, we added 150 μg/ml D-luciferin (Caliper Life Science, Hopkinton, MA) to the cell culture medium. Then, equal numbers (3 × 105) of MCF7/Mock/LUC, MCF7/TG2-C277S/LUC and MCF7/TG2-C277S/miR-205/LUC cells (n=3 each) in 100 μl were injected into the left ventricle of the individual mice.
In vivo bioluminescent imaging was performed using the IVIS Imaging System as previously described [42]. In brief, 150 mg/kg body weight D-luciferin in D-PBS (Dulbecco’s phosphate-buffered saline) was injected intraperitoneally into mice, 5 min before imaging. Then, the anesthetized mice were imaged dorsally for 3 min and then ventrally for another 3 min in an imaging box. We acquired the images and analyzed the bioluminescent signals using the Living Image software (Xenogen). Serial bioluminescent imaging was performed every week for 10 weeks.
Results
GTP binding activity, but not transamidase activity of TG2 contributes to EMT induction in breast cancer cells
Previous studies showed that the transamidase activity of TG2 contributed to cross-linking between proteins. To determine if the GTP-binding or the transamidase activities of TG2 play a role in the EMT induction in breast cancer cells, we selected the MCF7 and MDA-MB-231 breast cancer cell lines for this study. The TG2-expressing breast cancer cell line, MDA-MB-231, showed higher expression of EMT-related proteins, Vimentin and ZEB1, than the TG2-deficient MCF7 cell line (Figure 1A).
Figure 1.
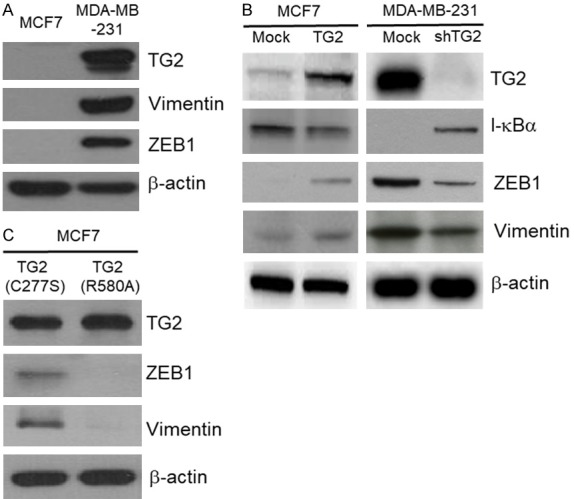
GTP binding activity, but not transamidase activity of TG2 induces EMT. Western blot analysis of TG2, Vimentin and ZEB1 in (A) MCF7 and MDA-MB-231 cells; and (B) mock-transfected MCF7, TG2-transfected MCF7, control shRNA-transfected MDA-MB-231 and TG2 shRNA-transfected MDA-MB-231 cells. (C) Representative immunoblot shows TG2 expression in MCF7 stably transfected with vectors containing transamidase-inactive TG2 (TG2-C277S) and GTP-binding-inactive TG2 (TG2-R580A) constructs.
Next, we analyzed ZEB1 and Vimentin levels in TG2-overexpressing and TG2-knockdown breast cancer cells to determine the role of TG2 in activating EMT. TG2-overexpressing MCF7 cells showed higher ZEB1 and Vimentin levels than the mock-transfected comparator MCF7 cells. In contrast, the TG2 knockdown MDA-MB-231 cells showed decreased EMT signaling (low ZEB1 and Vimentin levels) than the control shRNA-transfected MDA-MB-231 cells (Figure 1B). Furthermore, the catalytically inactive TG2 (TG2-C277S) effectively induced EMT in the MCF7 breast cancer cells, whereas the GTP-binding-deficient TG2 (TG2-R580A) showed decreased ability to induce EMT (Figure 1C). These results suggested that the GTP binding activity of TG2 played a crucial role in induction of EMT in breast cancer cells.
TG2 downregulates miR-205 in breast cancer cells
We performed real-time quantitative RT-PCR in MCF7 and MDA-MB-231 breast cancer cells to detect the expression of miR-205 using U6 small nuclear RNA as an internal control. The MDA-MB-231 cells showed 50% reduced miR-205 expression relative to the MCF7 cells (Figure 2A). TG2 overexpressing MCF7 cells showed a 50% decrease in miR-205 levels than the mock-transfected control MCF7 cells. Moreover, MCF7 cells expressing TG2 deficient in GTP binding activity (R580A) showed higher miR-205 expression than the MCF7 cells expressing TG2 deficient in transamidase activity (C277S), which suggested that GTP binding activity was required for reducing miR-205 expression in MCF7 breast cancer cells (Figure 2B). Furthermore, TG2 knockdown by shRNA in MDA-MB-231 cells restored miR-205 expression (Figure 2C). These data demonstrate that TG2 regulates miR-205 expression in breast cancer cells.
Figure 2.
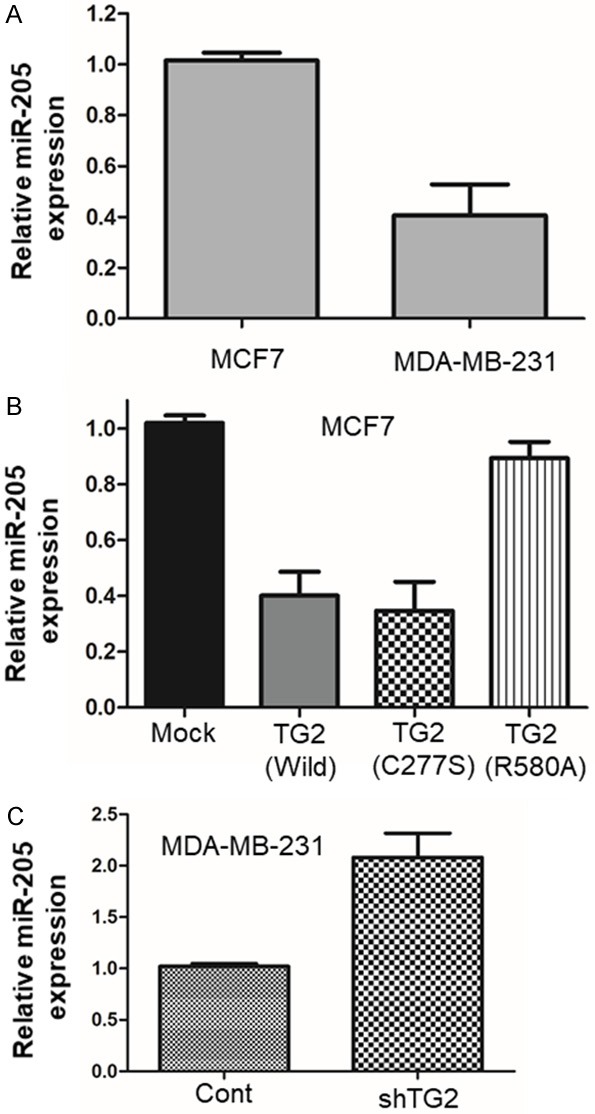
TG2 downregulates miR-205 in breast cancer cells. A. qRT-PCR analysis of miR-205 expression relative to the housekeeping U6 snRNA in the TG2-deficient MCF7 and TG2-positive MDA-MB-231 breast cancer cell lines. B. QRT-PCR analysis of relative expression of miR-205 in MCF7 cells stably transfected with vectors containing the wild-type TG2, transamidase-inactive TG2 (TG2-C277S) and GTP-binding inactive TG2 (TG2-R580A) constructs. C. QRT-PCR analysis of relative expression of miR-205 in MDA-MB-231 cells stably transfected with vectors containing control shRNA and TG2 shRNA (shTG2) constructs.
MiR-205 overexpression inhibits EMT induction in breast cancer cells by TG2 harboring GTP binding activity
Next, we tested if ectopic expression of miR-205 expression in TG2-transfected cells promotes EMT in breast cancer cells. Therefore, we analyzed miR-205 expression by qRT-PCR in MCF7/TG2-C277S cells that ectopically express miR-205 (Figure 3A, Left). As shown in Figure 3B, ectopic miR-205 expression in MCF7/TG2-C277S cells significantly downregulated ZEB1 and Vimentin expression and concurrently reduced cell invasiveness (Figure 3C, Top). Conversely, knockdown of miR-205 by the miR-205 inhibitor (Figure 3A, Right) upregulated ZEB1 and Vimentin expression accompanied by increased cell invasiveness (Figure 3C, Bottom). These results suggested that TG2 activates ZEB1 and Vimentin expression by downregulating miR-205.
Figure 3.
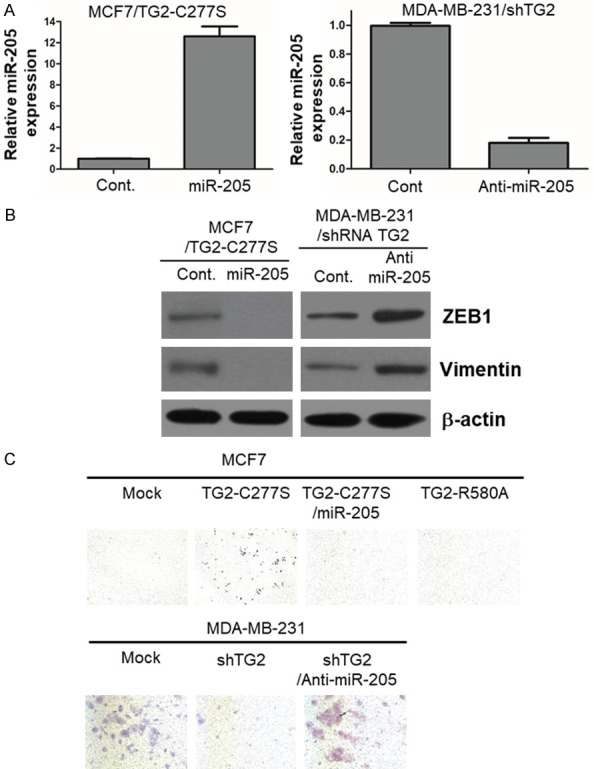
MiR-205 overexpression inhibits EMT induction by transamidase-inactive TG2 that harbors GTP binding activity in breast cancer cells. qRT-PCR analysis of the relative expression of (A) miR-205 and (B) EMT markers ZEB1 and Vimentin in TG2-overexpressing MCF7 (MCF7/TG2-C277S) cells that are stably transfected with a vector containing control and miR-205 constructs, and TG2-knockdown MDA-MB-231 cells (MDA-MB-231/shTG2) that are stably transfected with vector harboring control and anti-miR-205 constructs. (C) Representative photographs show in vitro invasion assay results of transamidase-inactive TG2 (TG2-C277S) expressing MCF7 cells that are stably transfected with vectors containing control miRNA and miR-205, and TG2-knockdown MDA-MB-231 cells (MDA-MB-231/shTG2) that are stably transfected with anti-microRNA control and anti-miR-205 constructs.
GTP binding activity of TG2 in breast cancer cell contributes to in vivo bone metastasis by inhibiting miR-205 expression
Next, we investigated if the enforced expression of transamidase-deficient TG2 that harbors GTP binding activity was sufficient to promote bone metastasis by using MCF7/TG2-C277S cells that co-expressing luciferase (Figure 4). We monitored the progression of bone metastasis after intracardiac injection of tumor cells by bioluminescence imaging (BLI) on a weekly basis. As expected, we demonstrated bone metastasis in the mice injected with TG2-overexpressing MCF7 cells, MCF7/TG2-C277S/Luc cells within 10 weeks, whereas mice injected with mock-transfected MCF7 cells did not show bone metastasis in 10 weeks (Figure 4A). These findings indicate that enforced expression of TG2 is sufficient to promote bone metastasis of MCF7 breast cancer cells.
Figure 4.
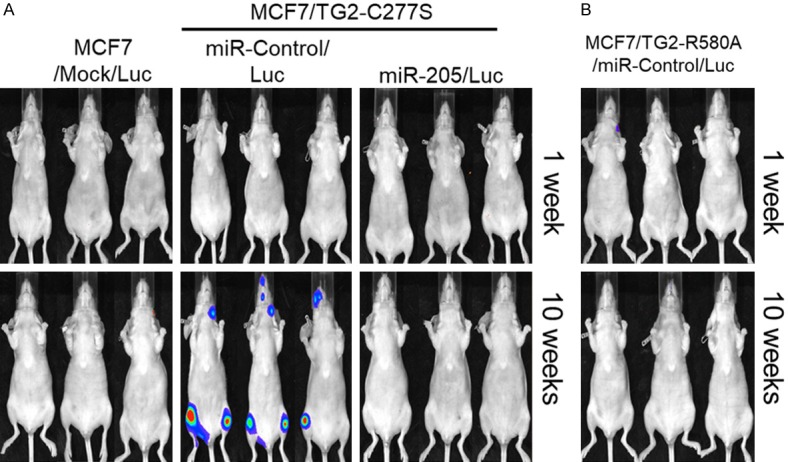
MCF7 breast cancer cells expressing TG2 with GTP binding activity promotes in vivo bone metastasis via miR-205 inhibition. Representative images show bioluminescence monitoring of mice at 1 week and 10 weeks after intracardiac injection of equal number (3 × 105 cells) of (A) luciferase-transfected MCF7/mock cells, transamidase-inactive TG2 (TG2-C277S) expressing MCF7 cells (MCF7/TG2-C277S) and miR-205 overexpressing MCF7/TG2-C277S cells and (B) GTP-binding inactive TG2 (TG2-R580A) expressing MCF7 cells (MCF7/TG2-R580A).
We further tested if miR-205 overexpression could functionally inhibit bone metastasis of TG2 expressing breast cancer cells. We stably transfected miR-205 expression vector into the MCF7/TG2-C277S/Luc cells. Then, we monitored bone metastasis weekly by bioluminescence imaging (BLI) mice injected intracardially with miR-205 expressing MCF7/TG2-C277S/Luc cells. Our data showed that miR-205 induction effectively inhibited bone metastasis of MCF7/TG2-C277S/Luc cells after 10 weeks relative to control-miR expressing MCF7/TG2-C277S/Luc cells (Figure 4A). Intracardiac injection of the MCF7/TG2-C277S cells that express the transamidation-inactive form resulted in bone metastases, especially jaw and knee, in the mice in 10 weeks. However, mice that received an intracardiac injection of MCF7/TG2-R580A cells, which express the GTP-binding-deficient form of TG2, did not show any bone metastases in 10 weeks (Figure 4B). These findings indicate that over-expression of miR-205 inhibits bone metastasis of breast cancer cells expressing the transamidation-inactive form of TG2 (TG2-C277S).
Discussion
During the past decades, several studies have reported increased expression of TG2 in various types of cancer cells [9,43]. Moreover, several reports have demonstrated that high expression of TG2 in cancer cells is associated with metastasis and drug-resistance [10,12,44-48]. Our research focused on the role of TG2 in the EMT induction of breast cancer cells. Previous studies have reported that TG2 induces EMT in various tumors. The GTP-binding domain of TG2 is essential for epithelial-to-mesenchymal transition (EMT) in mammary epithelial cells [41] and ovarian cancer [16,49]. Besides, inhibition of TG2 by siRNA and small-molecule TG2 inhibitors renders drug-resistant cancer cells sensitive to chemotherapeutic drugs and inhibits EMT in both in vitro and in vivo models [17,50-52].
First of all, we confirmed that the TG2-expressing MDA-MB-231 cells showed higher expression of EMT markers than the TG2-deficient MCF7 cells that didn’t express EMT markers (Figure 1A, 1B). This data was consistent with previous data showing that TG2 induces EMT via ZEB1 and Vimentin [14] and NF-κB activation via I-κBα depletion, which are well known in literature [48].
The GTP-binding and transamidation activities of TG2 are known to be mechanistically exclusive to each other and are regulated by GTP and calcium, respectively. The transamidase activity of TG2 is promoted by calcium and blocked by GTP, whereas, the GTPase activity of TG2 is inhibited by calcium [33]. It is still not clear whether intracellular TG2 acts as a transamidase or as a GTPase under physiological conditions.
We used breast cancer cells expressing either the wild-type TG2 (TG2-WT), transamidation-inactive TG2 (TG2-C277S) or the GTP-binding-deficient TG2 (TG2-R580A mutant) to determine the contribution of the transamidation and GTP binding activities of TG2 to EMT induction in breast cancer cells (Figure 1C).
Our results confirmed previous reports that transamidation activity of TG2 did not correlate with EMT in various tumor cells [16,41,49]. We showed that transamidation-inactive TG2-C277S was as competent as TG2-WT in inducing EMT (Figures 1B, 1C and 3C). On the contrary, the GTP-binding-inactive R580A mutant inhibited EMT induction, despite high expression and possessing transamidation activity (Figure 3C). These results are consistent with previously reported findings [40,41,53-55]. However, previous reports had not investigated the mechanistic details of EMT induction by TG2.
Previous studies demonstrate that microRNAs such as miR-495, miR-622 and miR-1 regulate the metastasis of various tumors including gastric cancer [56], thyroid carcinoma [57] and squamous cell carcinoma [58]. Recently, preclinical and clinical studies are underway to determine the therapeutic relevance of several noncoding RNAs such as miRNAs [59]. We measured the expression of miR-205 and miR-200 in MCF7 (TG2-negative) and MDA-MB-231 (TG2-positive) breast cancer cells by qPCR because these two microRNAs are associated with regulation of ZEB1 expression [23,24,38]. Moreover, miR-205 modulates breast cancer metastasis [26]. Furthermore, miR-205, but not miR-200, is down-regulated in TG2-expressing MDA-MB-231 cells compared to TG2-negative MCF7 cells (Figure 2A). TG2 inhibition induces miR-205 expression in MDA-MB-231 cells, whereas, TG2-transfected MCF7 cells show decreased miR-205 expression than the mock-transfected MCF7 cells. We also demonstrate that the GTP-binding activity of TG2 plays a critical role in regulating miR-205 in the breast tumor cells (Figure 2B).
Next, we tested if miR-205 regulates EMT signaling induction by TG2. As expected, miR-205 blocked TG2-induced EMT signaling by reducing ZEB1 and Vimentin levels (Figure 3B) and cell invasiveness (Figure 3C). These results suggest that miR-205 acts as a potential tumor suppressor by inhibiting EMT in TG2 expressing breast tumor cells.
Finally, we confirmed that mice intracardially injected with MCF7 cells transfected with the transamidation-inactive TG2 (TG2-C277S) form tumors in the bones, especially jaw and knees. On the contrary, mice intracardially injected with MCF7 cells transfected with the GTP-binding-deficient TG2 (TG2-R580A) did not generate any bone tumors. Moreover, ectopic miR-205 expression inhibited bone metastasis of TG2-C277S transfected MCF7 cells (Figure 4). These results suggest that miR-205 potentially mediates inhibition of ZEB1 by binding to its putative target sequences in the 3’-UTR of ZEB1 [38]. Our study indicates that TG2 induces EMT via downregulation of miR-205. Further studies are necessary to investigate the mechanisms by which TG2 regulates miR-205 expression and also address if miR-205 restores drug sensitivity in TG2-induced drug-resistant tumor cells.
In summary, we demonstrate that TG2 (especially the GTP binding activity of TG2) induces EMT by increasing ZEB1 expression via miR-205 inhibition. Therefore, our study suggests that TG2 inhibition or ectopic miR-205 expression is a potential rational approach for the treatment of TG2-induced metastatic breast cancer patients.
Acknowledgements
We thank Dr. Soo-Youl Kim for kindly providing the TG2 expressing vector. This work was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2017R1D1A1B03033550).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Pinilla SM, Sarrio D, Honrado E, Hardisson D, Calero F, Benitez J, Palacios J. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12:1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 3.Wright HJ, Hou J, Xu B, Cortez M, Potma EO, Tromberg BJ, Razorenova OV. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc Natl Acad Sci U S A. 2017;114:E6556–E6565. doi: 10.1073/pnas.1703791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Liang Y, Sang Y, Song X, Zhang H, Liu Y, Jiang L, Yang Q. MiR-770 suppresses the chemo-resistance and metastasis of triple negative breast cancer via direct targeting of STMN1. Cell Death Dis. 2018;9:14. doi: 10.1038/s41419-017-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorand L, Weissmann LB, Epel DL, Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976;73:4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fesus L, Szucs EF, Barrett KE, Metcalfe DD, Folk JE. Activation of transglutaminase and production of protein-bound gamma-glutamylhistamine in stimulated mouse mast cells. J Biol Chem. 1985;260:13771–13778. [PubMed] [Google Scholar]
- 7.Palosuo K, Varjonen E, Nurkkala J, Kalkkinen N, Harvima R, Reunala T, Alenius H. Transglutaminase-mediated cross-linking of a peptic fraction of omega-5 gliadin enhances IgE reactivity in wheat-dependent, exercise-induced anaphylaxis. J Allergy Clin Immunol. 2003;111:1386–1392. doi: 10.1067/mai.2003.1498. [DOI] [PubMed] [Google Scholar]
- 8.Lai TS, Greenberg CS. Histaminylation of fibrinogen by tissue transglutaminase-2 (TGM-2): potential role in modulating inflammation. Amino Acids. 2013;45:857–864. doi: 10.1007/s00726-013-1532-y. [DOI] [PubMed] [Google Scholar]
- 9.Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol. 2010;80:1921–1929. doi: 10.1016/j.bcp.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Park KS, Kim HK, Lee JH, Choi YB, Park SY, Yang SH, Kim SY, Hong KM. Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:493–502. doi: 10.1007/s00432-009-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67:7194–7202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–10533. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 13.Yuan L, Behdad A, Siegel M, Khosla C, Higashikubo R, Rich KM. Tissue transgluaminase 2 expression in meningiomas. J Neurooncol. 2008;90:125–132. doi: 10.1007/s11060-008-9642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Xu J, Brady S, Gao H, Yu D, Reuben J, Mehta K. Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PLoS One. 2010;5:e13390. doi: 10.1371/journal.pone.0013390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann AP, Verma A, Sethi G, Manavathi B, Wang H, Fok JY, Kunnumakkara AB, Kumar R, Aggarwal BB, Mehta K. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–8795. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 16.Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-beta, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31:2521–2534. doi: 10.1038/onc.2011.429. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JY, Mangala LS, Fok JY, Lin YG, Merritt WM, Spannuth WA, Nick AM, Fiterman DJ, Vivas-Mejia PE, Deavers MT, Coleman RL, Lopez-Berestein G, Mehta K, Sood AK. Clinical and biological significance of tissue transglutaminase in ovarian carcinoma. Cancer Res. 2008;68:5849–5858. doi: 10.1158/0008-5472.CAN-07-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh K, Ko E, Kim HS, Park AK, Moon HG, Noh DY, Lee DS. Transglutaminase 2 facilitates the distant hematogenous metastasis of breast cancer by modulating interleukin-6 in cancer cells. Breast Cancer Res. 2011;13:R96. doi: 10.1186/bcr3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Griffin M. The role of TG2 in regulating S100A4-mediated mammary tumour cell migration. PLoS One. 2013;8:e57017. doi: 10.1371/journal.pone.0057017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Mehta K. Tissue transglutaminase, inflammation, and cancer: how intimate is the relationship? Amino Acids. 2013;44:81–88. doi: 10.1007/s00726-011-1139-0. [DOI] [PubMed] [Google Scholar]
- 21.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Liu J, Wang G. The role of microRNAs in human breast cancer progression. Tumour Biol. 2014;35:6235–6244. doi: 10.1007/s13277-014-2202-8. [DOI] [PubMed] [Google Scholar]
- 23.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Zhu S, Mo YY. Suppression of cell growth and invasion by miR-205 in breast cancer. Cell Res. 2009;19:439–448. doi: 10.1038/cr.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Liao H, Deng Z, Yang P, Du N, Zhanng Y, Ren H. miRNA-205 affects infiltration and metastasis of breast cancer. Biochem Biophys Res Commun. 2013;441:139–143. doi: 10.1016/j.bbrc.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Lebanony D, Benjamin H, Gilad S, Ezagouri M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H, Nonaka D, Li J, Spector Y, Rosenfeld N, Chajut A, Cohen D, Aharonov R, Mansukhani M. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J. Clin. Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- 28.Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem. 2008;54:1696–1704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 29.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Chung TK, Cheung TH, Huen NY, Wong KW, Lo KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, Yu MY, To KF, Mok SC, Wang VW, Li C, Cheung AY, Doran G, Birrer MJ, Smith DI, Wong YF. Dysregulated microRNAs and their predicted targets associated with endometrioid endometrial adenocarcinoma in Hong Kong women. Int J Cancer. 2009;124:1358–1365. doi: 10.1002/ijc.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulf T, Sibbritt T, Wiklund ED, Bert S, Strbenac D, Statham AL, Robinson MD, Clark SJ. Discovery pipeline for epigenetically deregulated miRNAs in cancer: integration of primary miRNA transcription. BMC Genomics. 2011;12:54. doi: 10.1186/1471-2164-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dar AA, Majid S, de Semir D, Nosrati M, Bezrookove V, Kashani-Sabet M. miRNA-205 suppresses melanoma cell proliferation and induces senescence via regulation of E2F1 protein. J Biol Chem. 2011;286:16606–16614. doi: 10.1074/jbc.M111.227611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philippidou D, Schmitt M, Moser D, Margue C, Nazarov PV, Muller A, Vallar L, Nashan D, Behrmann I, Kreis S. Signatures of microRNAs and selected microRNA target genes in human melanoma. Cancer Res. 2010;70:4163–4173. doi: 10.1158/0008-5472.CAN-09-4512. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Brenn T, Brown ER, Doherty V, Melton DW. Differential expression of microRNAs during melanoma progression: miR-200c, miR-205 and miR-211 are downregulated in melanoma and act as tumour suppressors. Br J Cancer. 2012;106:553–561. doi: 10.1038/bjc.2011.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iorio MV, Casalini P, Piovan C, Di Leva G, Merlo A, Triulzi T, Menard S, Croce CM, Tagliabue E. microRNA-205 regulates HER3 in human breast cancer. Cancer Res. 2009;69:2195–2200. doi: 10.1158/0008-5472.CAN-08-2920. [DOI] [PubMed] [Google Scholar]
- 36.Greene SB, Herschkowitz JI, Rosen JM. The ups and downs of miR-205: identifying the roles of miR-205 in mammary gland development and breast cancer. RNA Biol. 2010;7:300–304. doi: 10.4161/rna.7.3.11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi R, Horiuchi S, Sakurazawa Y, Hasegawa T, Sato K, Sakamaki T. ErbB2 down-regulates microRNA-205 in breast cancer. Biochem Biophys Res Commun. 2011;411:804–808. doi: 10.1016/j.bbrc.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Mo YY. Targeting miR-205 in breast cancer. Expert Opin Ther Targets. 2009;13:1439–1448. doi: 10.1517/14728220903338777. [DOI] [PubMed] [Google Scholar]
- 40.Fisher ML, Adhikary G, Xu W, Kerr C, Keillor JW, Eckert RL. Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition. Oncotarget. 2015;6:20525–20539. doi: 10.18632/oncotarget.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A, Xu J, Sung B, Kumar S, Yu D, Aggarwal BB, Mehta K. Evidence that GTP-binding domain but not catalytic domain of transglutaminase 2 is essential for epithelial-to-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2012;14:R4. doi: 10.1186/bcr3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, Ross BD. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia. 2000;2:491–495. doi: 10.1038/sj.neo.7900121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chhabra A, Verma A, Mehta K. Tissue transglutaminase promotes or suppresses tumors depending on cell context. Anticancer Res. 2009;29:1909–1919. [PubMed] [Google Scholar]
- 44.Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004;10:8068–8076. doi: 10.1158/1078-0432.CCR-04-1107. [DOI] [PubMed] [Google Scholar]
- 45.Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007;26:2459–2470. doi: 10.1038/sj.onc.1210035. [DOI] [PubMed] [Google Scholar]
- 46.Fok JY, Ekmekcioglu S, Mehta K. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther. 2006;5:1493–1503. doi: 10.1158/1535-7163.MCT-06-0083. [DOI] [PubMed] [Google Scholar]
- 47.Choi CM, Jang SJ, Park SY, Choi YB, Jeong JH, Kim DS, Kim HK, Park KS, Nam BH, Kim HR Korean Thoracic Oncology Research Group (KTORG) Kim SY, Hong KM. Transglutaminase 2 as an independent prognostic marker for survival of patients with non-adenocarcinoma subtype of non-small cell lung cancer. Mol Cancer. 2011;10:119. doi: 10.1186/1476-4598-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park KS, Han BG, Lee KH, Kim DS, Kim JM, Jeon H, Kim HS, Suh SW, Lee EH, Kim SY, Lee BI. Depletion of nucleophosmin via transglutaminase 2 cross-linking increases drug resistance in cancer cells. Cancer Lett. 2009;274:201–207. doi: 10.1016/j.canlet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Shao M, Cao L, Shen C, Satpathy M, Chelladurai B, Bigsby RM, Nakshatri H, Matei D. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009;69:9192–9201. doi: 10.1158/0008-5472.CAN-09-1257. [DOI] [PubMed] [Google Scholar]
- 50.Verma A, Guha S, Diagaradjane P, Kunnumakkara AB, Sanguino AM, Lopez-Berestein G, Sood AK, Aggarwal BB, Krishnan S, Gelovani JG, Mehta K. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008;14:2476–2483. doi: 10.1158/1078-0432.CCR-07-4529. [DOI] [PubMed] [Google Scholar]
- 51.Yuan L, Choi K, Khosla C, Zheng X, Higashikubo R, Chicoine MR, Rich KM. Tissue transglutaminase 2 inhibition promotes cell death and chemosensitivity in glioblastomas. Mol Cancer Ther. 2005;4:1293–1302. doi: 10.1158/1535-7163.MCT-04-0328. [DOI] [PubMed] [Google Scholar]
- 52.Kim DS, Park SS, Nam BH, Kim IH, Kim SY. Reversal of drug resistance in breast cancer cells by transglutaminase 2 inhibition and nuclear factor-kappaB inactivation. Cancer Res. 2006;66:10936–10943. doi: 10.1158/0008-5472.CAN-06-1521. [DOI] [PubMed] [Google Scholar]
- 53.Colak G, Keillor JW, Johnson GV. Cytosolic guanine nucledotide binding deficient form of transglutaminase 2 (R580a) potentiates cell death in oxygen glucose deprivation. PLoS One. 2011;6:e16665. doi: 10.1371/journal.pone.0016665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begg GE, Carrington L, Stokes PH, Matthews JM, Wouters MA, Husain A, Lorand L, Iismaa SE, Graham RM. Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc Natl Acad Sci U S A. 2006;103:19683–19688. doi: 10.1073/pnas.0609283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Cerione RA, Clardy J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc Natl Acad Sci U S A. 2002;99:2743–2747. doi: 10.1073/pnas.042454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C, Jian M, Qi H, Mao WZ. MicroRNA-495 inhibits proliferation, metastasis and promotes apoptosis by targeting Twist1 in gastric cancer cells. Oncol Res. 2019;27:389–397. doi: 10.3727/096504018X15223159811838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Wang R, Ma Q, Ji L, Yao Y, Ma M, Wen Q. miR-622 suppresses tumor formation by directly targeting VEGFA in papillary thyroid carcinoma. Onco Targets Ther. 2018;11:1501–1509. doi: 10.2147/OTT.S156810. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Liu W, Li M, Chen X, Zhu S, Shi H, Zhang D, Cheng C, Li B. MicroRNA-1 suppresses proliferation, migration and invasion by targeting Notch2 in esophageal squamous cell carcinoma. Sci Rep. 2018;8:5183. doi: 10.1038/s41598-018-23421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christopher AF, Kaur RP, Kaur G, Kaur A, Gupta V, Bansal P. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect Clin Res. 2016;7:68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]


