Abstract
Non-coding RNAs (ncRNAs) have been shown to regulate gene expression involved in tumor progression of multiple malignancies. Numerous studies have indicated that N-acetylglucosaminyltransferase V (MGAT5), is an important tumorigenesis and metastasis-associated enzyme in breast cancer (BC). But, the underlying molecular mechanisms by which ncRNAs modulate MGAT5 expression in BC remain undetermined. In this study, we demonstrated that miR-124 expression at a low level in BC tissue was associated with poor prognosis of BC patients. Meanwhile, miR-124 reduced BC cell proliferation and metastasis. MGAT5 was confirmed as a direct target of miR-124. MGAT5 restoration attenuated the inhibitory effects of miR-124 on BC proliferation and metastasis in vitro and vivo. Overall, we provide new insight into the mechanisms by which miR-124 inhibits BC progression, suggesting the potential of miR-124 and MGAT5 as biomarkers for early diagnosis of breast cancer to provide innovative ideas and methods for the diagnosis and treatment of BC.
Keywords: miR-124-3p, MGAT5, proliferation, metastasis, breast cancer
Introduction
Breast cancer is a malignant tumor that seriously harms women’s health worldwide. The latest published “2018 American Cancer Report” [1] shows that the most common malignant tumor in women is breast cancer, accounting for 30% of all female cancers, and the mortality rate is the second, accounting for 14%. Tumor recurrence and metastasis are the main causes of poor prognosis in breast cancer. Therefore, exploring biomarkers of breast cancer cell metastasis can help develop new diagnostic and therapeutic methods to improve disease-free survival and overall survival rate of breast cancer.
N-acetylglucosaminyltransferase V (MGAT5) is an important N-glycan processing enzyme distributed in the Golgi apparatus. It can catalyze the formation of β-1, 6 branches of N-glycans. It is also beneficial to expand the outer chain, change the sugar chain structure of cell surface glycoproteins such as cadherin, integrin and cell surface growth factor receptor, and affect the malignant transformation and tumor metastasis of cells [2,3].
MicroRNAs are a class of important endogenous non-coding RNA molecules consisting of approximately 21-25 nucleotides. MicroRNAs can control the expression of genes at the transcriptional, post-transcriptional and epigenetic levels, participate in a series of important processes such as cell proliferation, differentiation, apoptosis and invasive metastasis, and affect the growth and development of the body and various pathological processes [4]. Many studies have shown that the binding of miRNA to the non-coding region of mRNA (3’-UTR region), can be used as a tumor suppressor gene to down-regulate the activity of proto-oncogenes, and can also be used as an oncogene to down-regulate the activity of tumor suppressor genes [5]. Therefore, miRNA may be a new biological marker for disease diagnosis or a new drug target, and it is also likely to simulate this molecule for new drug development, which will provide a new means for the treatment of diseases.
In this study, we forecasted miRNA molecules which negatively associated with MGAT5, and confirmed their biological functions in breast cancer cells by experiments in vitro and vivo. We explored the use of miRNA molecules as biomarkers or drugs for early diagnosis of breast cancer to provide innovative ideas and methods for the diagnosis and treatment of breast cancer.
Materials and methods
Cell culture and human tissues
The human breast cancer cell lines (MCF-7 and MDAMB-231) and 293T, were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China), and cultured in Dulbecco’s Modified Eagle medium (DMEM) medium supplemented with 10% heat-inactivated fetal bovine serum (FBS, GIBCO, CA, USA), 100 U/ml of penicillin, and 100 μg/ml of streptomycin (HyClone). Cells in this medium were placed in a humidified atmosphere containing 5% CO2 at 37°C. All cells were used for study within 6 months.
Breast cancer and normal breast tissues are provided by the Changhai Hospital with Second Military Medical University (Shanghai, China) from patients during surgery. All protocols concerning the use of patient samples in this study were approved by the Medical Ethics Committee of the Changhai Hospital with Second Military Medical University (Shanghai, China). All samples collection is done following guidelines of Institutional Review Board-approved protocol after a written agreement approval by the patients. Liquid nitrogen is used to freeze samples soon after their collection from surgery and stored at -80°C later. The clinical features of the patients are listed in Table 1.
Table 1.
Clinicopathological data of BC patients
| Parameters | Cases n (%) | |
|---|---|---|
| Total | 20 (100.00%) | |
| Age | ≥40 | 16 (80%) |
| <40 | 4 (20%) | |
| Gender | Female | 20 (100%) |
| Male | 0 (0%) | |
| Estrogen receptor (ER) | Positive | 10 (50%) |
| Negative | 10 (50%) | |
| Progesterone receptor (PR) | Positive | 12 (60%) |
| Negative | 8 (40%) | |
| Fish (HER2) | Positive | 5 (25%) |
| Negative | 15 (75%) | |
| Ki-67 | ≥14 | 19 (95%) |
| <14 | 1 (5%) | |
| Luminal | A | 1 (5%) |
| B | 12 (60%) | |
| Three-negative Breast Cancer (TNBC) | 3 (15%) | |
| Pathological type | Infiltrating ductal carcinoma (IDC) | 19 (95%) |
| Ductal carcinoma in situ (DCIS) | 1 (5%) | |
| Clinical stage | I | 6 (30%) |
| II | 6 (30%) | |
| III | 6 (30%) | |
| IV | 2 (10%) | |
| T classification | T1 | 9 (45%) |
| T2 | 9 (45%) | |
| T3 | 2 (10%) | |
| N classification | N0 | 10 (50%) |
| N1 | 6 (30%) | |
| N2 | 4 (20%) | |
| N3 | 0 (0%) | |
| M (distant metastasis) | Positive | 2 (10%) |
| Negative | 18 (90%) |
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using TRIzol and reverse transcription was performed using M-MLV (Invitrogen) and cDNA amplification using the SYBR Premix Ex Taq (TaKaRa, China) The primers were listed in Table 2.
Table 2.
Sequence of the primers in this study
| Pimer | Sequence |
|---|---|
| MGAT5 | Forward Primer AGGGCCATCCTGGGTTCATTA |
| Reverse Primer AAACTGCTGCGGGTTCAGATTC | |
| β-actin | Forward Primer CTGGTGCCTGGGGCG |
| Reverse Primer AGCCTCGCCTTTGCCGA | |
| miR-124 | Forward Primer CGACGTAAGGCACGCG |
| Reverse Primer CAGTGCAGGGTCCGAGGTAT | |
| RT-Primer GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGCATTCAC | |
| miR-101 | Forward Primer CGACGTACAGTACTGTG |
| Reverse Primer CAGTGCAGGGTCCGAGGTAT | |
| RT-Primer GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTTCAGTTAT | |
| U6 | Forward Primer CGAGCACAGAATCGCTTCA |
| Reverse Primer CTCGCTTCGGCAGCACATAT | |
| RT-Primer CGAGCACAGAATCGCTTCACGAATTTGCGTGTCAT | |
| miR-124 mimic | UAAGGCACGCGGUGAAUGCC |
| miR-124 inhibitor | GGCAUUCACCGCGUGCCUUA |
| miR-101 mimic | UACAGUACUGUGAUAACUGAA |
| miR-101 inhibitor | UUCAGUUAUCACAGUACUGUA |
| Inhibitor NC | CAGUACUUUUGUGUAGUACAA |
| Mimic NC | UUGUACUACACAAAAGUACUG |
| Dual luciferase vector plasmid | GP-miRGLO |
| MGAT5 3’UTR | WT AGTAATGCAG GATTCTGCAA ACAAGGTGTC GCCGTCCAAA TGTACTGTCC TGGCATAGAG |
| miR-101 | MUT AGTAATGCAG GATTCTGCAA ACAAGGTGTC GCCGTCCAAA TCATGACACC TGGCATAGAG |
| MGAT5 3’UTR | WT GGAGATATTA GTCAGTTTCT TTAGTGATAT TTGTTTCCTT GATGTGCCTT TTCGTTTTTC |
| miR-124 | MUT GGAGATATTA GTCAGTTTCT TTAGTGATAT TTGTTTCCTT GATCACGGAA TTCGTTTTTC |
| RNAi Lentiviral Vector | pGMLV-SC5 |
| Sh-NC | Primer-T gatcTGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTc |
| Sh-NC | Primer-B aattgAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACa |
| sh-MGAT5 | Primer-T GATCCGGCGGAAATTCGTACAGATTTCAAGAGAATCTGTACGAATTTCCGCCTTTTTTG |
| sh-MGAT5 | Primer-B AATTCAAAAAAGGCGGAAATTCGTACAGATTCTCTTGAAATCTGTACGAATTTCCGCCG |
| Lentiviral Vector | pGMLV-MI7 |
| miR-124 inhibitor | Primer-F GATCCGACGGCGCTAGGATCATCAACGGCATTCACCATCTGCGTGCCTTACAAGTATTCTGGTCACAGAATACAACGGCATTCACCATCTGCGTGCCTTACAAGATGATCCTAGCGCCGTCTTTTTTG |
| miR-124 inhibitor | Primer-R AATTCAAAAAAGACGGCGCTAGGATCATCTTGTAAGGCACGCAGATGGTGAATGCCGTTGTATTCTGTGACCAGAATACTTGTAAGGCACGCAGATGGTGAATGCCGTTGATGATCCTAGCGCCGTCG |
| Lentiviral Vector | PGMLV-6395 |
| MGAT5 | Primer-F CCGCTCGAGGCCACCATGGCTCTCTTCA |
| MGAT5 | Primer-R CCGGACGCGTCTATAGGCAGTCTTTGCAGAGAGC |
| miR-124 | Primer-F CCGGAATTCGGATTCAGGGCGAACCAACTG |
| miR-124 | Primer-R CCGCTCGAGGCACAGGCGGGAACTACTGC |
Western blotting analysis
The MCF-7 and MDA-MB-231 cells were washed in phosphate-buffered saline (PBS) and extracted using RIPA Lysis Buffer (Beyotime) and 1 mmol/L Phenylmethanesulfonyl fluoride (Beyotime). Cell extracts were boiled in SDS-PAGE Sample Loading Buffer (Beyotime) (70°C, 10 minutes) and equal amount of cell extracts were separated on 15% SDS-PAGE gels. Separated protein bands were transferred into polyvinylidene fluoride (PVDF) membranes. The Anti-MGAT5 antibody (ab87977, Mouse monoclonal antibody, Abcam, USA), and Anti-beta Actin (ab184576, Mouse monoclonal antibody, Abcam, USA) were diluted at a ratio of 1:1000 according to the instructions and incubated overnight at 4°C. Horseradish peroxidase-linked secondary antibodies were added at a dilution ratio of 1:10,000, and incubated at room temperature for 1 h. The membranes were washed with Tris Buffered Saline with Tween-20 (TBST) for three times and the immunoreactive bands were visualized using ECL-PLUS/Kit (GE Healthcare, Piscataway, NJ, USA) according to the kit’s instruction.
miRNA mimic and inhibitor
MiR-124 mimic and inhibitor were purchased from GenePharma (Shanghai, PR, China) and negative control (mimic NC or inhibitor NC) used as a control. The sequences were shown in Table 2. MCF-7 and MDA-MB-231 cells were planted in 6-well plates 24 h prior to miR-124 mimic or inhibitor transfection with 50-60% confluence, and then were transfected with Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacture instructions.
Luciferase reporter assay
293T cells were seeded into 24-well plates and were transfected with 100 ng of miR-101 mimics, miR-124 mimics, or mimics NC and GP-miRGLO-MGAT5’-UTR-WT or GP-miRGLO-MGAT5’-UTR-MUT plasmid (The sequences were shown in Table 2) using Lipofectamine 3000 (Invitrogen). Luciferase reporter gene assay was conducted with the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Renilla luciferase gene was co-transfected as a control for normalization.
Lentiviral packaging and stable cell lines establishment
The lentiviral packaging kit was purchased from Open Biosystems (Genomeditech). Lentivirus carrying miR-124, MGAT5, miR-124 inhibitor, sh-MGAT5 or negative control (NC and sh-NC) (The sequences were shown in Table 2) was packaged in 293T cells and collected from the supernatant as instructed by the manufacturer’s manual. The lentiviruses were infected into MDA-MB-231 or MCF7 cells to establish stable cell lines, followed by puromycin selection.
Cell proliferation assay
For each well in 96-well plates, 8 × 103 MCF-7 cells and 3 × 103 MDA-MB-231 cells were seeded and transfected 24 hours later. After transfection, 10 μl WST-8 solution from the Cell Counting Kit-8 (CCK8, Beyotime, China) was added into each well. Absorbance of each well was measured after 2 hours incubation by reading plates at 450 nm at time 12, 24, 36, 48, 60 and 72 hours. The relative cell number was calculated as the ratio of absorbance at 24, 36, 48, 60 and 72 hours to 12 hours.
Colony formation assay
MCF-7 and MDA-MB-231 cells were trypsinized, and 1 × 103 cells were plated in 6-well plates and incubated at 37°C for 7 days. Colonies were dyed with dyeing solution containing 0.1% crystal violet and 20% methanol. Cell colonies were then counted and analyzed.
5-Ethynyl-20-deoxyuridine (EdU) incorporation assay
The EdU assay was carried out with a Cell-Light EdU DNA Cell Proliferation Kit (RiboBio, Shanghai, PR, China). 2 × 104 cells were seeded in 12-well plate. After incubation with 50 mM EdU for 2 hours, the cells were fixed in 4% paraformaldehyde and stained with Apollo Dye Solution. Hoechst-33,342 was used to stain the nucleic acid within the cells. Images were acquired with Zeiss axiophot photomicroscope (Carl Zeiss) and Image-Pro plus 6.0 software, and the percentage of EdU-positive cells was calculated.
Wound healing assay
MDA-MB-231 and MCF-7 cells were seeded into 6-well plates, and then allowed to grow until 100% confluent. Next, the cell layer was scratched through the central axis using a sterile plastic tip, and loose cells were washed away with phosphate-buffered saline. Wound healing was observed and photographed at 0 and 48 hours in three randomly selected microscopic fields for each condition and time-point. The degree of motility 48 hours after confluent cells had been scratched was expressed as the percentage of wound closure calculated as follows: (Distance of cell migration at 48 h/distance of scratch at 0 h) × 100%. The experiment was performed in triplicate and data are summarized as means ± SEM.
Animal studies
The animal studies were approved by the Committee on Ethics of Biomedicine, Second Military Medical University. Female athymic BALB/c nude mice (4 weeks old) were used for animal studies. Cells (3 × 106) were injected subcutaneously into the left and the right flanks of mice. Tumors were allowed to grow for 3-5 weeks. Tumors growth was recorded every 3 days with a caliper and tumor volume was calculated as a × b2 × 0.5 (a, longest diameter; b, shortest diameter). The investigators recording tumor growth and colonization were blinded to mouse allocation.
Statistical analysis
Statistical analyses were carried out by using SPSS 20.0 (IBM, SPSS, Chicago, IL, USA) and GraphPad Prism. Student’s t-test or Chi-square test was used to assess the statistical significance for comparisons of two groups. The Pearson’s correlation coefficient analysis was used to analyze the correlations. Overall survival (OS) was defined as the interval between the dates of surgery and death and OS and disease-free survival (DFS or recurrence) curves were analyzed with the Kaplane-Meier method and log-rank test. Univariate analysis and multivariate models were performed by using a Cox proportional hazards regression model. Receiver operating characteristic (ROC) curves were obtained using cutoff finder online software (http://molpath.charite.de/cutoff/load.jsp). P<0.05 was considered statistically significant.
Results
MiR-124 downregulation is inversely associated with prognosis and MGAT5 expression in BC
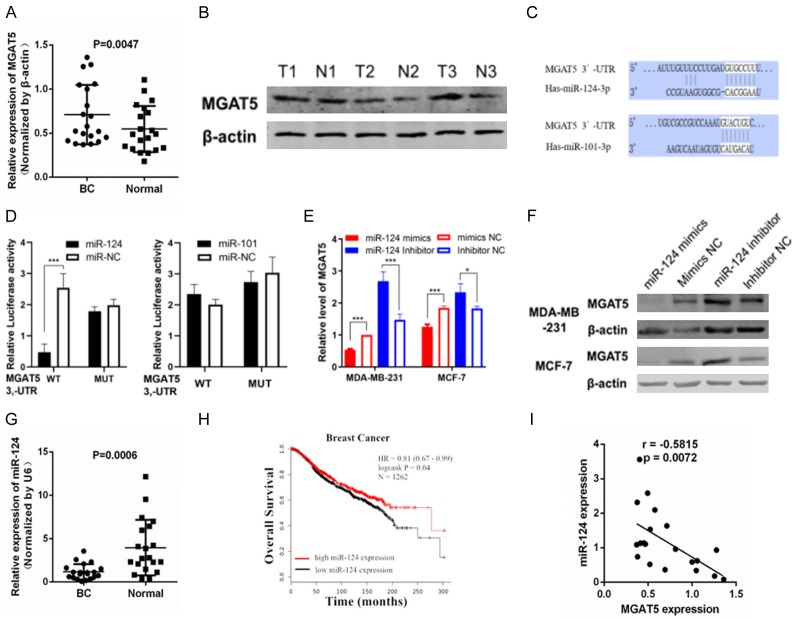
qPCR assay and western blot were performed to quantify MGAT5 levels in 20 pairs of frozen human BC specimens and the corresponding adjacent normal breast specimens. Figure 1A and 1B shows higher MGAT5 mRNA and protein expression in BC tissues than that in normal breast tissues.
Figure 1.
MiR-124 downregulation is inversely associated with prognosis and MGAT5 expression in BC. A: qRT-PCR analyses of MGAT5 mRNA levels in BC and normal tissues (n=20). B: Western blot analyses of MGAT5 protein expression in T and N tissues. β-actin was used as the internal control. C: miR-124, miR-101-3p and their deductive domains in the 3’-UTR of MGAT5. D: Luciferase reporter assay of the connection between the 3’UTR of MGAT5 and miR-124 in 293T cells; E: qRT-PCR analysis of MGAT5 mRNA expression level after infected miR-124 mimics or inhibitor in MDA-MB-231 and MCF-7 cells. F: Western blot analyses of MGAT5 protein expression after infected miR-124 mimics or inhibitor in MDA-MB-231 and MCF-7 cells. β-actin was used as the internal control. G: qRT-PCR analyses of miR-124 levels in BC and normal tissues (n=20). H: Kaplan-Meier survival curves of OS based on miR-124 expression in 1262 BC patients using the online bioinformatics tool Kaplan-Meier plotter (METABRIC); I: Spearman’s correlation assay showed that miR-124 was negatively correlated with MGAT5 mRNA expression in BC specimens. *P<0.05, **P<0.01.
We forecast the potential miRNAs that target MGAT5-3’-UTR, by using the Targetscan (http://www.targetscan.org), and identified has-miR-124-3p (miR-124) and has-miR-101-3p (miR-101) could bind to MGAT5 gene 3’-UTR with high stringency (Figure 1C). Dual-luciferase reporter assays revealed that miR-124 suppressed luciferase activity of the reporter plasmid with MGAT5-3’-UTR-WT but had insignificant effect on the activity of a reporter fused to MGAT5-3’-UTR-MUT in 293T cells. However, miR-101 had insignificant effect on the activity of a reporter fused to both MGAT5-3’-UTR-WT and MGAT5-3’-UTR-MUT (Figure 1D). Therefore, miR-124 was selected for further analysis. qPCR and Western blot analyses demonstrated that miR-124 suppressed MGAT5 mRNA and protein expression both in MDA-MB-231 and MCF-7 cells (Figure 1E and 1F). These results suggested that MGAT5 is a direct target of miR-124 in BC cells.
Figure 1G shows lower miR-124 expression in BC tissues than in normal breast tissues. The online bioinformatics tool Kaplan-Meier plotter was used to analyze the relationship between the expression of miR-124 and the overall survival rate (OS) in 1262 breast cancer patients (METABRIC). It was found that patients with high expression level of miR-124 had better OS benefit, compared with low miR-124 expression levels (Figure 1H). MGAT5 mRNA expression was inversely associated with miR-124 level in BC specimens (Figure 1I). These results indicated that miR-124 downregulation is negatively associated with prognosis and MGAT5 expression in BC.
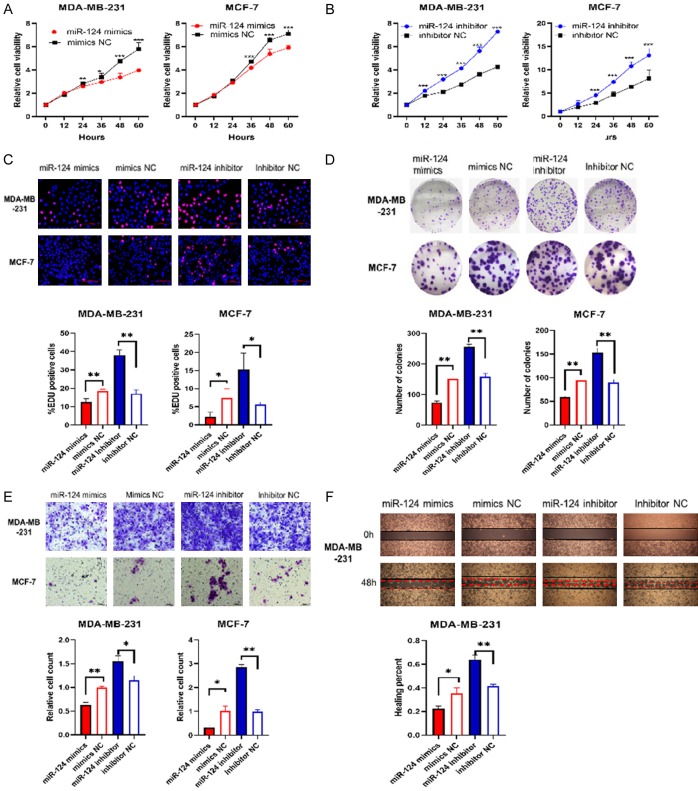
MiR-124 suppress the proliferation, migration of BC cells
MiR-124 mimics, mimics NC, miR-124 inhibitor, inhibitor NC were transfected into MDA-MB-231 and MCF-7 cells to explore biological functions of miR-124 in BC. CCK-8 assay showed that transfection of the miR-124 mimics significantly decreased the proliferation of the MDA-MB-231 and MCF-7 breast cancer cell lines compared with cells transfected with mimics NC (Figure 2A). In contrast, transfection of the miR-124 inhibitor significantly increased the proliferation of the breast cancer cell lines MDA-MB-231 and MCF-7 compared with cells transfected with the inhibitor NC (Figure 2B). EdU incorporation assay showed that miR-124 suppressed MDA-MB-231 and MCF-7 cell proliferation (Figure 2C). In addition, cells transfected with miR-124 mimics exhibited the decrease in colony formation (Figure 2D), and cells transfected with miR-124 inhibitor showed the increase, compared with cells transfected corresponding NC.
Figure 2.
MiR-124 suppress the proliferation, migration of BC cells. MDA-MB-231 and MCF-7 cells were transfected with miR-124 mimics, mimics NC, miR-124 inhibitor and inhibitor NC. A and B: Cell viability was measured using CCK-8 assays. C: EDU staining was performed and the percentage of EDU-positive cells were counted. Scale bar 100 µm. D: Representative photographs of the colony formation of MDA-MB-231 and MCF-7 cells, and related quantitative analysis. E: Cell migrations were determined using transwell. Scale bars, 50 µm. F: Cell migrations were determined using wound healing assay and the percentage of migrated was calculated. All data are shown as means ± SD of three separate experiments. *P<0.05, **P<0.01.
Subsequently, transwell assays and wound healing assay were performed to evaluate whether miR-124 inhibits BC cell migration. As shown in Figure 2E and 2F, miR-124 markedly reduced the migration of MDA-MB-231 and MCF-7 cells. These results demonstrated that miR-124 suppressed the proliferation and migration of BC cells in vitro.
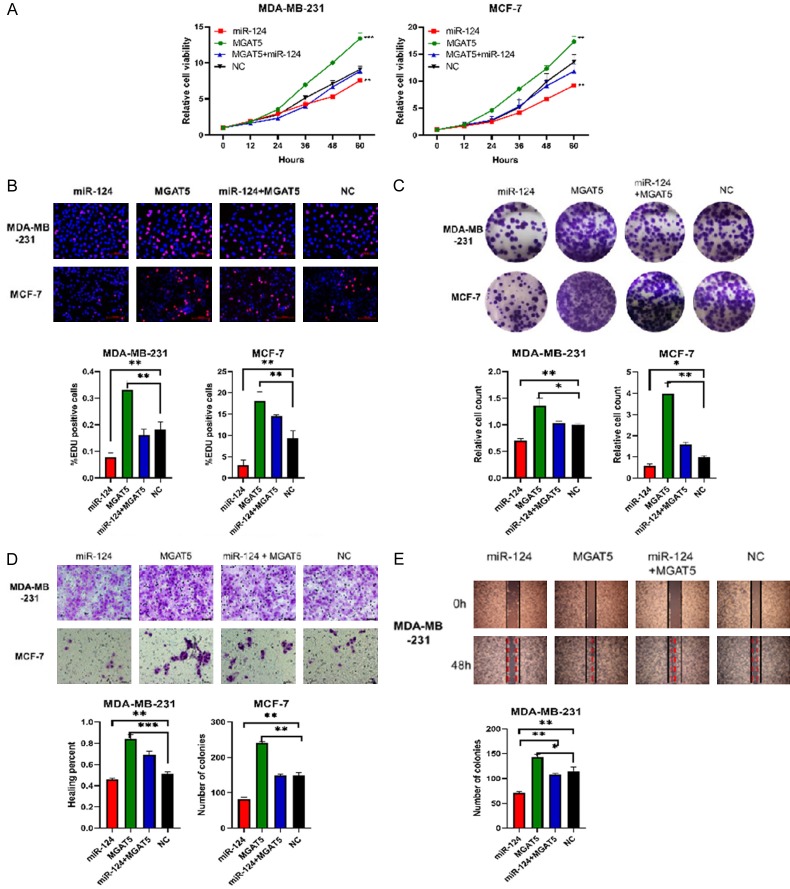
MGAT5 restoration counteracts the suppressive effects of miR-124 on BC cell proliferation and migration
We investigated whether MGAT5 was the functional target of miR-124 in BC.
First, we tested the proliferation and migration of four stably cell lines which transfected with miR-124, MGAT5, NC, and miR-124+MGAT5 lentiviral vectors respectively. Figure 3A shows that miR-124+MGAT5 group markedly restored the suppression of cell viability due by miR-124 group both in MDA-MB-231 and MCF-7 cells. EDU incorporation assay revealed that miR-124+MGAT5 group reduced miR-124-suppressed cell proliferation (Figure 3B). MGAT5 also upregulation counteracted the decline in colony formation caused by miR-124 (Figure 3B). Transwell and wound healing assays showed that miR-124 markedly reduced the migration of cells, and MGAT5 group was just the opposite, but miR-124+MGAT5 group rescued the suppression of mir-124 (Figure 3D, 3E).
Figure 3.
MGAT5 restoration counteracts the suppressive effects of miR-124 on BC cell proliferation and migration. MDA-MB-231 and MCF-7 cells were transfected with miR-124, MGAT5, NC, and miR-124+MGAT5 lentiviral vectors respectively to culture stably cell lines which followed by puromycin selection. A: Cell viability was measured using CCK-8 assays. B: EDU staining was performed and the percentage of EDU-positive cells were counted. Scale bar 100 µm. C: Representative photographs of the colony formation of MDA-MB-231 and MCF-7 cells, and related quantitative analysis. D: Cell migrations were determined using transwell. Scale bars, 50 µm. E: Cell migrations were determined using wound healing assay and the percentage of migrated was calculated. All data are shown as means ± SD of three separate experiments. *P<0.05, **P<0.01.
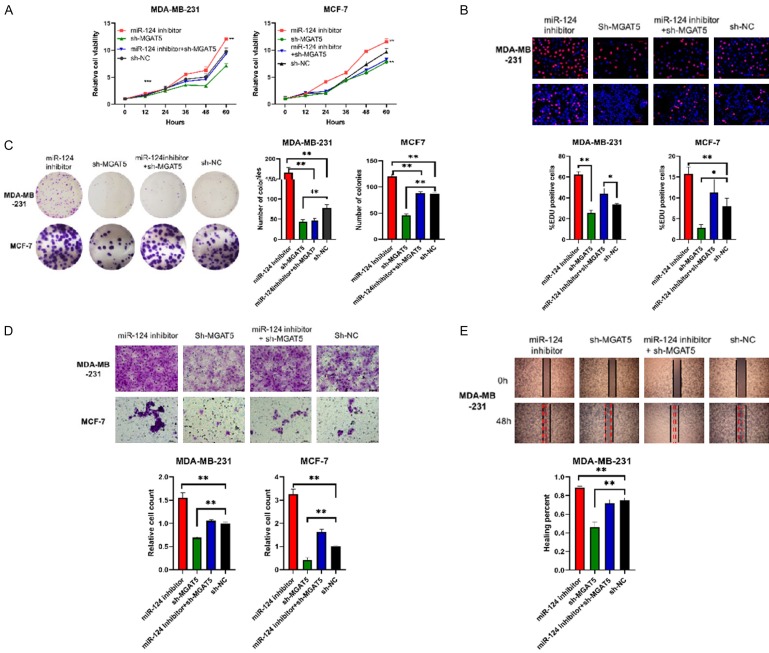
Furthermore, we determined the proliferation and migration of other four stably cell lines which transfected with miR-124 inhibitor, sh-MGAT5, sh-NC, and miR-124 inhibitor+sh-MGAT5 lentiviral vectors respectively. Cell proliferation assay showed that miR-124 inhibitor increased cell viability, but miR-124 inhibitor+sh-MGAT5 significantly reduced cell viability in contrast (Figure 4A). EDU incorporation assay displayed that miR-124 inhibitor+sh-MGAT5 group obviously decreased the proliferation of cells compared with miR-124 inhibitor group (Figure 4B). Colony formation showed the numbers of colonies in miR-124 inhibitor+sh-MGAT5 group distinct decreased than miR-124 inhibitor group (Figure 4C). Figure 4D and 4E reveal that the migration of cells in miR-124 inhibitor+sh-MGAT5 group was markedly down regulated compared with miR-124 inhibitor group. In summary, miR-124 might suppress the proliferation and migration ability of BC cells by targeting MGAT5.
Figure 4.
MGAT5 restoration counteracts the suppressive effects of miR-124 on BC cell proliferation and migration. MDA-MB-231 and MCF-7 cells were transfected with miR-124 inhibitor, sh-MGAT5, sh-NC, and miR-124 inhibitor+sh-MGAT5 lentiviral vectors respectively to culture stably cell lines which followed by puromycin selection. A: Cell viability was measured using CCK-8 assays. B: EDU staining was performed and the percentage of EDU-positive cells were counted. Scale bar 100 µm. C: Representative photographs of the colony formation of MDA-MB-231 and MCF-7 cells, and related quantitative analysis. D: Cell migrations were determined using transwell. Scale bars, 50 µm. E: Cell migrations were determined using wound healing assay and the percentage of migrated was calculated. All data are shown as means ± SD of three separate experiments. *P<0.05, **P<0.01.
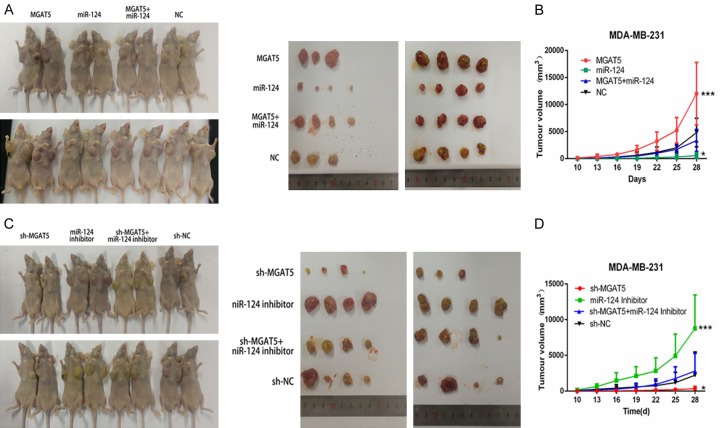
MiR-124 suppressed proliferation of BC cells by targeting MGAT5 in vivo
To further validate the significance of miR-124 and MGAT5 on tumor growth in vivo, we injected the upper eight groups MDA-MB-231 cells subcutaneously into the left and the right flanks of Female athymic BALB/c nude mice (4 weeks old). The tumor volumes were measured every three days, and the mice were sacrifced at 4 weeks after tumor cell implantation (Figure 5A and 5C). The size and volume of the tumors derived from MDA-MB-231 cells stably expressing miR-124 were lower than those of the tumors in the control group. The size and volume of the tumors derived from cells stably expressing miR-124+MGAT5 were bigger than those of the tumors in the miR-124 group, but lower than MGAT5 group (Figure 5B). The size and volume of the tumors derived from miR-124 inhibitor group were bigger than those of the tumors in the control group, otherwise, the size and volume of the tumors from miR-124 inhibitor+sh-MGAT5 group were lower than miR-124 inhibitor group and bigger than sh-MGAT5 group (Figure 5D). These results suggest that the suppressive effects of miR-124 on BC tumor growth by targeting MGAT5 in vivo.
Figure 5.
MiR-124 suppressed proliferation of BC cells by targeting MGAT5 in vivo. Female athymic BALB/c nude mice were injected with eight groups MDA-MB-231 cells subcutaneously. Tumors were allowed to grow for 4 weeks. Tumors growth was recorded every 3 days with a caliper and tumor volume was calculated as a × b2 × 0.5 (A, longest diameter; B, shortest diameter). Tumor growth curve was drawn (C, D). *P<0.05, **P<0.01.
Discussion
Breast cancer cause secondary number of deaths globally and is the most common type of cancer among women. The development of new treatments is halted mainly because of drug resistance and less knowledge about tumor cell signaling pathways [6]. The results of our previous experiments showed that the expression of MGAT5 was significantly higher in breast cancer tissues than in benign lesions (P<0.01). The high expression of MGAT5 in various pathological types of breast cancer was highly correlated with poor prognosis [7]. We try to explore the molecular mechanisms by which MGAT5 promotes breast cancer proliferation and metastasis, and to find new diagnostic and therapeutic targets.
MicroRNAs (miRNAs) are a class of endogenous small RNAs of about 20-24 nucleotides in length. Several miRNAs can regulate the same gene, and fine regulation of the expression of a gene can by a combination of several miRNAs. Studies have shown that miRNAs act as tumor suppressors or oncogenes in cancer. In this study, we forecasted miRNA molecules which negatively associated with MGAT5, and confirmed miR-124 can directly targets MGAT5 in BC cells. Meanwhile, miR-124 significantly under-expressed and inversely associated with prognosis of BC patients. Second, miR-124 markedly reduced BC cell proliferation and metastasis, which were reversed by MGAT5 restoration. Lastly, the suppression effects of miR-124 on malignant phenotypes of BC were rescued by MGAT5 in vivo. Overall, these results suggested the potential prognostic role of miR-124 in BC and indicated that miR-124 was a tumor suppressor of BC by targeting MGAT5.
MGAT5 promotes the proliferation, migration and invasion of breast cancer. Researches have shown that the expression of protein Caspase-3, Caspase-9, Bcl-2, MMP-2 and MMP-9 was significantly decreased in SMMC-7721/R cells after knocked out of MGAT5, while the expression of Bax-protein was upregulated [8-12]. MGAT5 which catalyzes the increase of β1, 6-branched sugar chains by FZD-7 (transmembrane receptor crimped protein), leads to abnormal Wnt signaling pathway, tumor stem cell (CSC) interval and tumor progression [13]. Experiments have shown that MGAT5 stimulates transcription of HER-2/neu, and the HER-2/neu response element is located at the 5’ end of the transcription start site and is 400 bp, including three ETS transcription factor binding sequences. The co-transfected/recessive Raf and ETS expression plasmids were co-transfected, demonstrating that the transcriptional activity of the novel MGAT5 promoter is mediated by the Ras-Raf-Ets signal transduction pathway [14,15]. Our study revealed that miR-124 directly binds the MGAT5 3’-UTR and inhibit MGAT5 expression, and that MGAT5 overexpression sufficiently attenuates the inhibitory effects of miR-124 on breast cancer cell proliferation and migration. Therefore, the modulation of MGAT5 by miR-124 may explain why the downregulation of miR-124 can promote the development of breast cancer. However, we have only verified from the perspective of epigenetics that miR-124 suppress the proliferation and metastasis of breast cancer cells by targeting MGAT5, and the deeper mechanism of action requires further exploration. Additional work is needed to characterize the feasibility of targeting miR-124 and MGAT5 in cancer therapy and develop simplified and cost-effective methods.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Saito T, Miyoshi E, Sasai K, Nakano N, Eguchi H, Honke K, Taniguchi N. A secreted type of beta 1,6-N-acetylglucosaminyltransferase V (GnT-V) induces tumor angiogenesis without mediation of glycosylation: a novel function of GnT-V distinct from the original glycosyltransferase activity. J Biol Chem. 2002;277:17002–8. doi: 10.1074/jbc.M200521200. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi N, Ihara S, Saito T, Miyoshi E, Ikeda Y, Honke K. Implication of GnT-V in cancer metastasis: a glycomic approach for identification of a target protein and its unique function as an angiogenic cofactor. Glycoconj J. 2001;18:859–65. doi: 10.1023/a:1022292223878. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Cortez MA, Anfossi S, Ramapriyan R, Menon H, Atalar SC, Aliru M, Welsh J, Calin GA. Role of miRNAs in immune responses and immunotherapy in cancer. Genes Chromosomes Cancer. 2019;58:244–253. doi: 10.1002/gcc.22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17:152–163. doi: 10.2174/1871520616666160502122724. [DOI] [PubMed] [Google Scholar]
- 7.Hu W, Shi JY, Sheng Y, et al. Epirubicin or pirarubicin plus paclitaxel for neoadjuvant chemotherapy of locally advanced breast cancer: a randomized controlled trial. Journal of the Second Military Medical University. 2010;31:70–3. [Google Scholar]
- 8.Guo H, Nagy T, Pierce M. Post-translational glycoprotein modifications regulate colon cancer stem cells and colon adenoma progression in Apc(min/+) mice through altered Wnt receptor signaling. J Biol Chem. 2014;289:31534–49. doi: 10.1074/jbc.M114.602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Yang X, Zhang H, Liu X, Pan S, Li C. The role of extracellular matrix metalloproteinase inducer glycosylation in regulating matrix metalloproteinases in periodontitis. J Periodontal Res. 2018;53:391–402. doi: 10.1111/jre.12524. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Liu J, Cai Y, Chen J, Xie W, Kong X, Huang W, Guo H, Zhao X, Lu Y, Niu L, Li X, Zhang H, Lei C, Lei Z, Yin J, Hu H, Yu F, Nie Y, Xia L, Wu K. Loss of Barx1 promotes hepatocellular carcinoma metastasis through up-regulating MGAT5 and MMP9 expression and indicates poor prognosis. Oncotarget. 2017;8:71867–80. doi: 10.18632/oncotarget.18288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Q, Chen Y, Yin N, Shan N, Luo X, Tong C, Zhang H, Baker PN, Liu X, Qi H. N-acetylglucosaminyltransferase V inhibits the invasion of trophoblast cells by attenuating MMP2/9 activity in early human pregnancy. Placenta. 2015;36:1291–9. doi: 10.1016/j.placenta.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Guo HB, Johnson H, Randolph M, Nagy T, Blalock R, Pierce M. Specific posttranslational modification regulates early events in mammary carcinoma formation. Proc Natl Acad Sci U S A. 2010;107:21116–21. doi: 10.1073/pnas.1013405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurimoto A, Kitazume S, Kizuka Y, Nakajima K, Oka R, Fujinawa R, Korekane H, Yamaguchi Y, Wada Y, Taniguchi N. The absence of core fucose up-regulates GnT-III and Wnt target genes: a possible mechanism for an adaptive response in terms of glycan function. J Biol Chem. 2014;289:11704–14. doi: 10.1074/jbc.M113.502542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, Nairn A, dela Rosa M, Nagy T, Zhao S, Moremen K, Pierce M. Transcriptional regulation of the protocadherin beta cluster during Her-2 protein-induced mammary tumorigenesis results from altered N-glycan branching. J Biol Chem. 2012;287:24941–54. doi: 10.1074/jbc.M112.369355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Zhang W, Fregien N, Pierce M. The her-2/neu oncogene stimulates the transcription of N-acetylglucosaminyltransferase V and expression of its cell surface oligosaccharide products. Oncogene. 1998;17:2087–93. doi: 10.1038/sj.onc.1202124. [DOI] [PubMed] [Google Scholar]