Abstract
Four phospholipase C β (PLCB) isoforms, PLCB1, PLCB2, PLCB3 and PLCB4, have been previously investigated regarding their roles in the metabolism of inositol lipids and cancer. The present study aimed to explore the association between PLCB1-4 and hepatocellular carcinoma (HCC). Data from 212 patients with hepatitis B virus-associated HCC were used to analyze the diagnostic and prognostic significance of PLCB genes in. A nomogram predicted the survival probability. Gene set enrichment analysis explored gene ontology terms and the metabolic pathways associated with PLCB genes. Validation of the prognostic values of PLCB genes was performed using the Gene Expression Profiling Interactive Analysis website. PLCB1 and PLCB2 were revealed to have diagnostic value for HCC (0.869 and 0.836 area under the curve, respectively; both P≤0.05). The combination analysis of these genes had an advantage over each alone (0.905 PLCB1 and PLCB2, and 0.877 PLCB1 and PLCB3 area under the curve; P≤0.05). PLCB1 was associated with overall survival (OS) and recurrence-free survival (RFS; adjusted P=0.002 and P=0.001, respectively). A nomogram predicted survival probability of patients with HCC at 1, 3- and 5-years. Gene set enrichment analysis indicated that PLCB1 and PLCB2 are involved in the cell cycle, cell division and the PPAR signaling pathway, among other functions. Validation using GEPIA revealed that PLCB1 and PLCB2 were associated with OS and PLCB1 and PLCB4 were associated with RFS. PLCB1 and PLCB2 exhibited diagnostic value for HCC and their combination had an advantage over each individually. PLCB1 has OS and RFS prognostic value for patients with HCC.
Keywords: hepatocellular carcinoma, diagnosis, prognosis, mRNA expression, PLCB1, PLCB2
Introduction
Hepatocellular carcinoma (HCC) is one of the main causes of tumor-associated mortality, being the fifth most common malignancy worldwide (1). In 2018, the number of new cases and liver cancer-associated mortalities was 841,080 and 781,631, respectively, worldwide (2). Many risk factors, including dietary aflatoxin exposure (3), hepatitis B and C virus (HBV) infection (4) and cirrhosis, contribute to the initiation and progression of HCC. To date, many diagnostic and treatment procedures, including ultrasound, computed tomography, liver resection, liver transplantation, radiofrequency, thermal and non-thermal ablation, trans-arterial chemoembolization (5), immunotherapies and therapeutic cancer vaccines (4), have been used for patients with HCC. However, the prognosis of HCC is remains poor and the 5-year relative survival rate is ~12% due to tumor metastasis and recurrence (6,7). Due to the characteristics of systemic disease, the evolution and progression of HCC involves deregulation of genes, cells and tissues (1). Therefore, it is crucial to identify novel biomarkers that may be involved in the course of tumor metastasis and recurrence, for early diagnosis and recurrence prediction for HCC.
Phospholipase C (PLC) is encoded by four genes, PLCA, PLCB, PLCC and PLCD, and is involved in the pathogenesis of several bacterial infections, including Clostridium perfringens, Listeria monocytogene, and Pseudomonas aeruginosa (8,9). The activity of PLCA and PLCB in L. monocytogenes appears to overlap in the course of intracellular infection (10). In Listeria, three genes, PLCA, PLCB and PLCC, are clustered together on the same chromosome, whereas the PCLD gene is located in another region (11,12). Under the transcriptional control of PrfA regulator, PLCA, PLCB and HLY (encoding listeriolysin O precursor) have a role encoding the Listeria Pathogenicity Island 1, leading to the escape from endocytic and secondary vacuoles (13–15). PLCB isoforms in mice include PLCB1, PLCB2, PLCB3 and PLCB4, which are stimulated by G protein activation (Gαq/11 and/or Gβγ) (16,17). The roles of PLCB isoforms in immune defense and escape, and their functions in tumors are currently being investigated. PLCB1 has been reported to be associated with HCC prognosis in tumor proliferation (1) and an aberrant expression pattern has been reported in patients with schizophrenia (18). The PLCB2 and PLCB4 genes were found to be differentially expressed in human breast cancer MCF-7 cells, and to be associated with multidrug resistance using RNA-seq technology (19). PLCB3 has been reported to be regulated by multiple protein kinases and to control hormonal signaling (20).
HBV infection is regarded as a main risk factor for the development of HCC (4). HBV is classified into ten genotypes, from A to J, and >40 associated sub-genotypes (21). The 10 genotypes are based on an intergroup divergence of ≥8% in the complete nucleotide sequence; whereas the sub-genotypes are based on a divergence of 4–7.5% (22,23). Notably, genotypes A and B are associated with earlier hepatitis B e antigen seroconversion, less active liver disease, and a slower rate of progression to cirrhosis and HCC compared with genotypes C and D (24–27).
Some PLCB isoforms have been explored with regard their associations with tumor development; therefore, the present study aimed to explore the association between four PLCB genes and HCC.
Materials and methods
Patient data collection
The GSE14520 dataset was used for analysis (ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14520; accessed June 10th, 2018) (28,29). This dataset contains two platforms: GPL571 (GeneChip® Human Genome U133A 2.0 Array; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and GPL3921 (GeneChip® HT Human Genome U133 Array Plate Set; Thermo Fisher Scientific, Inc.). To avoid a batch effect, patients from GPL3921 were used. Patients with HBV infection were used, including a total of 212 patients. In addition, patient survival, including overall survival (OS) and recurrence-free survival (RFS), validated findings in the GSE14520 dataset using the Gene Expression Profiling Interactive Analysis (GEPIA; gepia.cancer-pku.cn/index.html; accessed June 10th, 2018) website with data from The Cancer Genome Atlas (TCGA) database (30).
Gene, protein and tissue expression, and the body map
Gene expression, the body map and transcripts per million of the PLCB genes were collected from the GEPIA website (gepia.cancer-pku.cn/index.html; accessed June 12th, 2018). Tissue and protein expression of the PLCB genes were collected from the GTEx portal (gtexportal.org/home/; accessed June 12th, 2018) (31) and The Human Protein Atlas (proteinatlas.org/; accessed June 12th, 2018) (32) websites, respectively.
Gene set enrichment analysis (GSEA)
GSEA (software.broadinstitute.org/gsea/index.jsp) was performed to explore potential mechanisms that PLCB genes are involved in, including biological processes and metabolic pathways. Datasets of c2.cp.kegg.v6.1.symbols.gmt, c5.bp.b6.1.symbols.gmt, c5.cc.v6.1.symbols.gmt, c5.mf.v6.1.symbols.gmt and c5.all.v6.1.symbols.gmt were used to analyze statistically significant Gene Ontology (GO) terms, including biological process (BP), cellular component (CC), and molecular function (MF), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (33,34).
Association and interaction analysis
The Pearson correlation matrix among PLCB genes was constructed using R version 3.5.0 (r-project.org/). Pearson correlation and associations between PLCB gene expression and tumor stage were validated using the GEPIA website. The co-expression interactive network of gene-gene interactions was constructed using the geneMANIA plugin of Cytoscape software version 3.6.0 (35,36). The protein-protein interaction (PPI) network was constructed using the STRING (string-db.org/cgi/input.pl, accessed June 20th, 2018) website (37). GO enrichment analysis was visualized using the BiNGO plugin of Cytoscape software version 3.6.0 (38).
Diagnostic and prognostic analysis and stratified, joint-effect analysis
Diagnostic receiver operating characteristic (ROC) curves were constructed using the expression of PLCB genes in tumor and non-tumor tissues. Gene expressions were categorized into two groups of low and high expression at a cut-off value of median expression levels. OS and RFS were calculated using the Kaplan-Meier and Cox proportional hazards regression models. Statistically significant clinical factors were adjusted for multivariate Cox models. Then, prognosis-associated genes were further stratified for analysis by clinical factors. In addition, prognosis-associated genes were combined for a joint-effect analysis with α-fetoprotein (AFP) based low and high expression.
Expression model and nomogram construction
To further explore prognosis-associated genes for HCC survival, expression models for OS and RFS prediction were constructed. Gene expression, patient survival status, expression heatmaps and prognostic ROC curves were constructed in the model (39–42). Nomograms for OS and RFS were also constructed using clinical factors and genes to predict patient survival probability at 1, 3 and 5 years.
Genome-wide analysis of prognosis-associated genes
Prognosis-associated genes were further explored in genome-wide analysis. A cut-off value of 0.4 was determined for further analysis. The cut-off 0.4 can filter a lot of genes with weak relationships with PLCB1 and leads to a better presentation of GO and pathway results compared with other cut-off values. Gene-gene interactions, and BP, CC and MF were constructed using Cytoscape software.
Statistical analysis
Unpaired t test was used to analyze expressions of PLCBs in tumor and non-tumor tissues. Box plots and survival plots were generated using GraphPad software version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Survival analyses were performed using SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA). Median survival time and log-rank P-value were calculated by the Kaplan-Meier method, and the 95% confidence interval (CI) and hazard ratio (HR) were calculated by univariate and multivariate Cox proportional hazards regression models, respectively. P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic and clinical characteristics
Data from 212 patients (GSE14520) with HBV-associated HCC were used in the study. AFP, BCLC stage, tumor size and cirrhosis were associated with OS (P=0.049, P<0.0001, P=0.002 and P=0.041, respectively). Gender, cirrhosis and BCLC stage were associated with RFS (P=0.002, P=0.036 and P<0.0001, respectively). Other factors were not associated with prognosis (P>0.05; Table SI).
Gene, protein, tissue expressions and transcription analysis
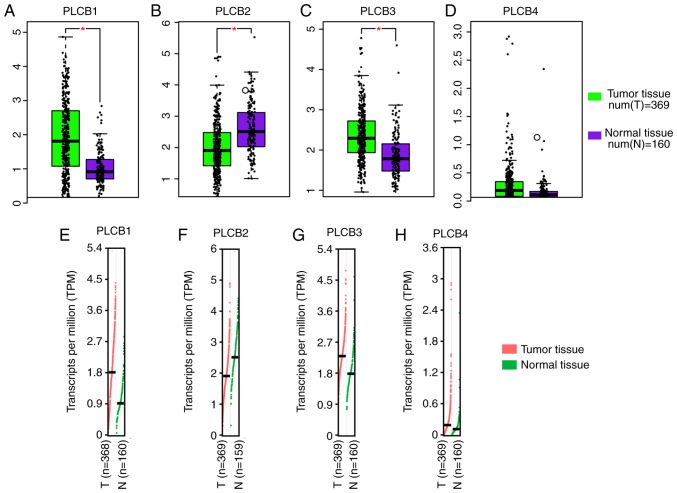
PLCB1 and PLCB3 were highly expressed in tumor tissues compared with normal tissue, whereas PLCB2 had the opposite result (all P≤0.05; Fig. 1A-C). However, there was no difference in PLCB4 expression between the tumor and normal tissue (Fig. 1D). Transcriptional analysis indicated that PLCB1, PLCB3 and PLCB4 consistently exhibited higher transcripts per millions in tumor tissues compared with normal tissues (Fig. 1E-H). Tissue and protein expression of the PLCB genes were collected from the GTEx portal.
Figure 1.
Relative mRNA expressions and transcriptional levels of PLCB1-4 in tumor and non-tumor tissues. Relative mRNA expressions of (A) PLCB1, (B) PLCB2, (C) PLCB3 and (D) PLCB4 in tumor and non-tumor tissues Transcriptional levels of (E) PLCB1, (F) PLCB2, (G) PLCB3 and (H) PLCB4 in tumor and non-tumor tissues. PLCB, phospholipase C β.
Gene expression levels in 212 patients with HBV-HCC (GSE14520) indicated that there were significant differences in PLCB1 and PLCB2 expression between tumor and non-tumor tissues, whereas there was not difference in PLCB3 and PLCB4 between the samples (Fig. 2A). In addition, when tumor samples were divided into high and low expression groups using the median as the cutoff there were significant differences in PLCB1, PLCB2 and PLCB4; whereas PLCB3 did not exhibit significance (Fig. 2B). The bodymap distribution of PLCB genes in different organs is shown in Fig. S1. Protein expression levels demonstrated that PLCB2 is the most highly expressed of the PLCB family (Fig. S2). The different tissue expression levels of PLCB family members demonstrated that all were expressed at low levels in the liver (Fig. S3).
Figure 2.
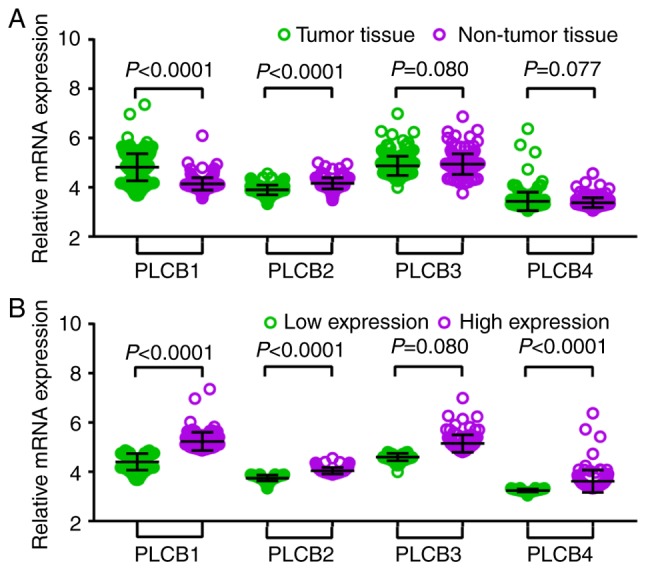
Relative mRNA expressions of PLCB1-4 in tumor and normal tissues and low, high expression groups. (A) Relative mRNA expressions of PLCB1-4 in tumor and normal tissues; (B) Relative mRNA expressions of PLCB1-4 in low and high expression groups. PLCB, phospholipase C β.
Diagnostic and prognostic analysis
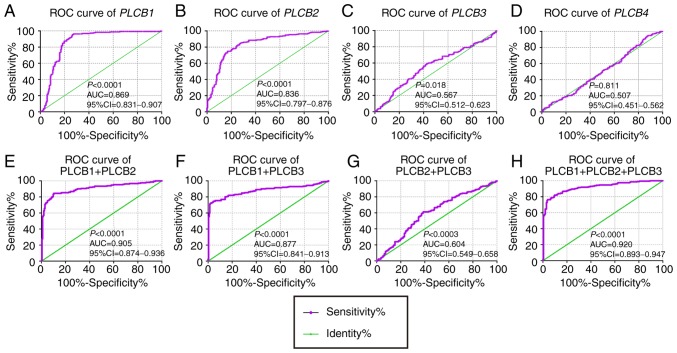
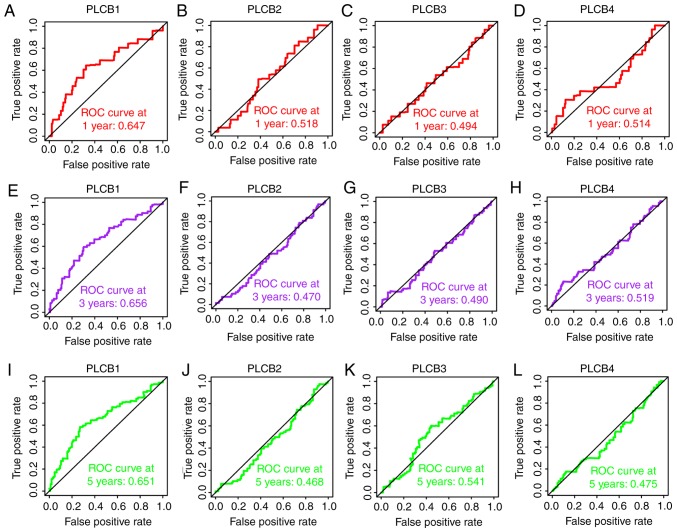
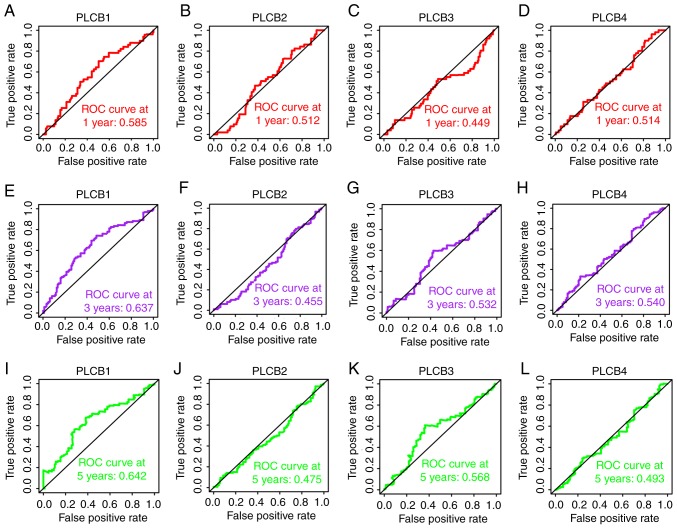
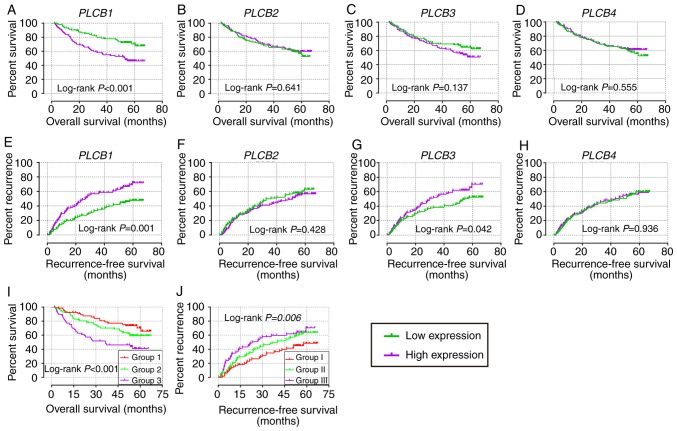
In the diagnostic analysis of PLCB genes, PLCB1 and PLCB2 exhibited diagnostic value for HCC, while PLCB3 showed potential diagnostic value [P<0.0001, P<0.0001 and P=0.018, respectively; area under the curve (AUC), 0.869, 0.836 and 0.567, respectively; Fig. 3A-C]. However, PLCB4 did not have any diagnostic value (P=0.811; Fig. 3D). In the combined diagnostic analysis for PLCB1, PLCB2 and PLCB3, the combinations of PLCB1 + PLCB2, PLCB1 + PLCB3, and PLCB1 + PLCB2 + PLCB3 exhibited diagnostic value for HCC with an advantage over PLCB1, PLCB2 or PLCB3 alone (AUC, 0.905, 0.877 and 0.920, respectively; all P<0.05; Fig. 3E, F and H). The combination of PLCB2 and PLCB3 exhibited potential diagnostic value for HCC (AUC, 0.604; P=0.0003; Fig. 3G). In the prognostic analysis (Figs. 4 and 5), only PLCB1 expression was associated with patient OS at 1-, 3- and 5-years (all AUC >0.6; Fig. 4A, E and I). In addition, PLCB1 expression was associated with patient RFS at 3- and 5-years (both AUC >0.6; Fig. 5E and I).
Figure 3.
Diagnostic ROC curves of PLCB1-4. A-D: Diagnostic ROC curves of (A) PLCB1, (B) PLCB2, (C) PLCB3 and (D) PLCB4; Diagnostic ROC curves of combination of (E) PLCB1 and PLCB2, (F) PLCB1 and PLCB3, (G) PLCB2 and PLCB3, and (H) PLCB1, PLCB2 and PLCB3. ROC, receiver operating characteristics; PLCB, phospholipase C β; AUC, area under the curve; CI, confidence interval.
Figure 4.
Overall survival ROC curves of PLCB1-4 at 1, 3 and 5 years. ROC curves of (A) PLCB1, (B) PLCB2, (C) PLCB3 and (D) PLCB4 at 1 year; ROC curves of (E) PLCB1, (F) PLCB2, (G) PLCB3 and (H) PLCB4 at 3 years; ROC curves of (I) PLCB1, (J) PLCB2, (K) PLCB3 and (L) PLCB4 at 5 years. PLCB, phospholipase C β; ROC, receiver operating characteristics.
Figure 5.
Recurrence-free survival ROC curves of PLCB1-4 at 1, 3 and 5 years. ROC curves of (A) PLCB1, (B) PLCB2, (C) PLCB3 and (D) PLCB4 at 1 year; ROC curves of (E) PLCB1, (F) PLCB2, (G) PLCB3 and (H) PLCB4 at 3 years; ROC curves of (I) PLCB1, (J) PLCB2, (K) PLCB3 and (L) PLCB4 at 5 years. PLCB, phospholipase C β; ROC, receiver operating characteristics.
In the univariate analysis (Tables I and II; Fig. 6), PLCB1 expression was associated with OS (crude P=0.002; Fig. 6A); PLCB1 and PLCB3 expression was associated with RFS (crude P=0.001 and P=0.042, respectively; Fig. 6E and G). In the multivariate analysis, PLCB1 expression was associated with OS and RFS (adjusted P=0.002 and 0.001, respectively; Tables I and II). Other genes were not associated with prognosis (adjusted P>0.05; Tables I and II).
Table I.
Prognostic analysis of PLCB genes for overall survival.
| Variable | Patients (n=212) | No. of events | MST (months) | HR (95% CI) | Crude P-value | HR (95% CI) | Adjusted P-valuea |
|---|---|---|---|---|---|---|---|
| PLCB1 | |||||||
| Low expression | 106 | 29 | NA | Ref. | Ref. | ||
| High expression | 106 | 53 | 53 | 2.246 (1.426–3.536) | <0.001 | 2.100 (1.310–3.367) | 0.002 |
| PLCB2 | |||||||
| Low expression | 106 | 41 | NA | Ref. | Ref. | ||
| High expression | 106 | 41 | NA | 0.902 (0.585–1.391) | 0.641 | 1.041 (0.660–1.641) | 0.863 |
| PLCB3 | |||||||
| Low expression | 106 | 36 | NA | Ref. | Ref. | ||
| High expression | 106 | 46 | NA | 1.394 (0.900–2.159) | 0.137 | 1.035 (0.659–1.625) | 0.882 |
| PLCB4 | |||||||
| Low expression | 106 | 44 | NA | Ref. | Ref. | ||
| High expression | 106 | 38 | NA | 0.877 (0.568–1.354) | 0.555 | 0.870 (0.555–1.363) | 0.534 |
P-values were adjusted for tumor size, cirrhosis, Barcelona Clinic Liver Cancer stage and α-fetoprotein; bold indicates significant P-values. Ref., reference value (1); NA, not available; MST, median survival time; HR, hazard ratio; 95% CI, 95% confidence interval; PLCB, phospholipase B.
Table II.
Prognostic analysis of PLCB genes for recurrence-free survival.
| Variable | Patients (n=212) | No. of events | MST (months) | HR (95% CI) | Crude P-value | HR (95% CI) | Adjusted P-valuea |
| PLCB1 | |||||||
| Low expression | 106 | 46 | NA | Ref. | Ref. | ||
| High expression | 106 | 70 | 26.9 | 1.914 (1.318–2.781) | 0.001 | 1.861 (1.273–2.271) | 0.001 |
| PLCB2 | |||||||
| Low expression | 106 | 59 | 36.0 | Ref. | Ref. | ||
| High expression | 106 | 57 | 51.1 | 0.863 (0.599–1.243) | 0.429 | 0.956 (0.654–1.398) | 0.817 |
| PLCB3 | |||||||
| Low expression | 106 | 52 | 54.8 | Ref. | Ref. | ||
| High expression | 106 | 64 | 29.9 | 1.466 (1.015–2.118) | 0.042 | 1.244 (0.853–1.814) | 0.257 |
| PLCB4 | |||||||
| Low expression | 106 | 59 | 46.3 | Ref. | Ref. | ||
| High expression | 106 | 57 | 43.2 | 1.015 (0.705–1.461) | 0.936 | 0.962 (0.664–1.395) | 0.840 |
P-values were adjusted for gender, cirrhosis and Barcelona Clinic Liver Cancer stage. Ref., reference value (1); MST, median survival time; HR, hazard ratio; 95% CI, 95% confidence interval; PLCB, phospholipase B. Bold indicates significant P-values.
Figure 6.
Overall survival and recurrence-free survival analysis plots of PLCB1-4. Overall survival analysis plot of (A) PLCB1, (B) PLCB2, (C) PLCB3 and (D) PLCB4; recurrence-free survival analysis plot (E) PLCB1, (F) PLCB2, (G) PLCB3 and (H) PLCB4; joint-effects analysis of (I) α-fetoprotein and (J) PLCB1 for overall survival and recurrence-free survival. Group 1, AFP low expression and PLCB1 low expression; Group 2, AFP low expression and PLCB1 high expression, and AFP high expression and PLCB1 low expression; Group 3, AFP high expression and PLCB1 high expression; Group I, AFP low expression and PLCB1 low expression; Group II, AFP low expression and PLCB1 high expression, and AFP high expression and PLCB1 low expression; Group III, AFP high expression and PLCB1 high expression. PLCB, phospholipase C β.
Stratified and joint-effect survival analysis
Stratification analysis was performed for PLCB1 on OS and RFS. Male gender, age <60 years, chronic carrying of HBV, cirrhosis, single nodular, AFP levels <300 ng/ml, and A stage in the BCLC staging system were associated with OS and RFS (all adjusted P≤0.05; Table III). Tumor size <5 cm was associated with OS and any group of tumor size was associated with RFS (all adjusted P≤0.05; Table III).
Table III.
Stratified analysis of PLCB1 for overall survival and recurrence-free survival.
| Overall survival | Recurrence-free survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Low | High | Adjusted HR (95% CI) | Adjusted P-value | Low | High | Adjusted HR (95% CI) | Adjusted P-value |
| Sex | ||||||||
| Male | 86 | 89 | 1.967 (1.174–3.24) | 0.010 | 86 | 89 | 1.877 (1.249–2.820) | 0.002 |
| Female | 20 | 17 | 2.619 (0.711–9.652) | 0.148 | 20 | 17 | 0.754 (0.168–3.382) | 0.713 |
| Age (years) | ||||||||
| ≤60 | 91 | 92 | 2.252 (1.370–3.702) | 0.001 | 91 | 92 | 1.736 (1.129–2.670) | 0.012 |
| >60 | 15 | 14 | 0.850 (0.140–5.148) | 0.860 | 15 | 14 | 2.043 (0.701–5.953) | 0.191 |
| HBV | ||||||||
| AVR-CC | 20 | 36 | 1.987 (0.695–5.687) | 0.200 | 20 | 36 | 1.486 (0.663–3.332) | 0.336 |
| CC | 86 | 70 | 1.957 (1.114–3.438) | 0.020 | 86 | 70 | 1.864 (1.166–2.979) | 0.009 |
| Tumor size (cm) | ||||||||
| ≤5 | 75 | 62 | 2.100 (1.149–3.838) | 0.016 | 75 | 62 | 1.627 (1.012–2.616) | 0.045 |
| >5 | 30 | 44 | 1.790 (0.821–3.901) | 0.143 | 30 | 44 | 2.204 (1.049–4.629) | 0.037 |
| Cirrhosis | ||||||||
| Yes | 93 | 102 | 1.922 (1.196–3.091) | 0.007 | 93 | 102 | 1.678 (1.124–2.503) | 0.011 |
| No | 13 | 4 | 476.586 (5.21E-12-4.36E16) | 0.707 | 13 | 4 | 3.758 (0.379–37.311) | 0.258 |
| Multinodular | ||||||||
| Yes | 21 | 24 | 1.399 (0.544–3.598) | 0.487 | 21 | 24 | 1.186 (0.474–2.965) | 0.716 |
| No | 85 | 82 | 2.662 (1.522–4.656) | 0.001 | 85 | 82 | 2.163 (1.395–3.355) | 0.001 |
| AFP (ng/ml) | ||||||||
| ≤300 | 68 | 47 | 2.098 (1.097–4.015) | 0.025 | 68 | 47 | 2.180 (1.307–3.635) | 0.003 |
| >300 | 35 | 59 | 1.886 (0.934–3.806) | 0.077 | 35 | 59 | 1.294 (0.710–2.359) | 0.399 |
| BCLC stage | ||||||||
| 0 | 8 | 12 | 0.535 (0.033–8.559) | 0.658 | 8 | 12 | 0.597 (0.097–3.665) | 0.577 |
| A | 79 | 64 | 2.214 (1.210–4.051) | 0.010 | 79 | 64 | 1.928 (1.200–3.097) | 0.007 |
| B | 10 | 12 | 0.746 (0.225–2.478) | 0.633 | 10 | 12 | 0.903 (0.310–2.627) | 0.851 |
| C | 9 | 18 | 2.746 (0.836–9.021) | 0.096 | 9 | 18 | 2.370 (0.774–7.252) | 0.131 |
Ref., reference value (1); PLCB, phospholipase B; HR, hazard ratio; 95% CI, 95% confidence interval; HBV, hepatitis B virus; AVR-CC, acute viral replication-chronic carrier; CC, chronic carrier; AFP, AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer. Bold indicates significant P-values.
In the joint-effect analysis (OS/RFS: group 1/I, AFP low + PLCB1 low; group 2/II, AFP low + PLCB1 high, and AFP high + PLCB1 low; groups 3/III, AFP high + PLCB1 high), when combining PLCB1 and AFP, prognostic significance was observed among the three groups for OS (adjusted P=0.008; Table IV); group 3 exhibited the worst prognosis [adjusted P=0.002, adjusted HR (95% CI)=4.382 (1.703–11.276); Table IV]. Prognostic significance was not observed among the three groups in RFS (adjusted P=0.075; Table IV). However, group III exhibited the worst prognosis [adjusted P=0.045, adjusted HR (95% CI)=1.670 (1.012–2.755); Table IV].
Table IV.
Joint-effect analysis of PLCB1 and AFP for overall survival and recurrence-free survival.
| A, Overall survival | ||||||
|---|---|---|---|---|---|---|
| Group | AFP expression | PLCB1 expression | Events/total | MST (months) | Adjusted HR (95% CI) | Adjusted P-value |
| 1 | Low | Low | 18/68 | NA | Ref. | 0.008 |
| 2 | Low | High | 32/82 | NA | 2.162 (1.143–4.089) | 0.018 |
| High | Low | |||||
| 3 | High | High | 32/59 | 36.4 | 4.382 (1.703–11.276) | 0.002 |
| B, Recurrence-free survival | ||||||
| Group | AFP expression | PLCB1 expression | Events/total | MST (months) | Adjusted HR (95% CI) | Adjusted P-value |
| I | Low | Low | 29/68 | NA | Ref. | 0.075 |
| II | Low | High | 50/82 | 40.1 | 1.613 (1.019–2.555) | 0.041 |
| High | Low | |||||
| III | High | High | 37/59 | 23.0 | 1.670 (1.012–2.755) | 0.045 |
Group 1, AFP low expression and PLCB1 low expression; Group 2, AFP low expression and PLCB1 high expression, and AFP high expression and PLCB1 low expression; Group 3, AFP high expression and PLCB1 high expression; Group I, AFP low expression and PLCB1 low expression; Group II, AFP low expression and PLCB1 high expression, and AFP high expression and PLCB1 low expression; Group III, AFP high expression and PLCB1 high expression. Ref., reference value (1); PLCB, phospholipase B; AFP, α-fetoprotein; MST, median survival time; HR, hazard ratio; 95% CI, 95% confidence interval. Bold indicates significant P-values.
GSEA
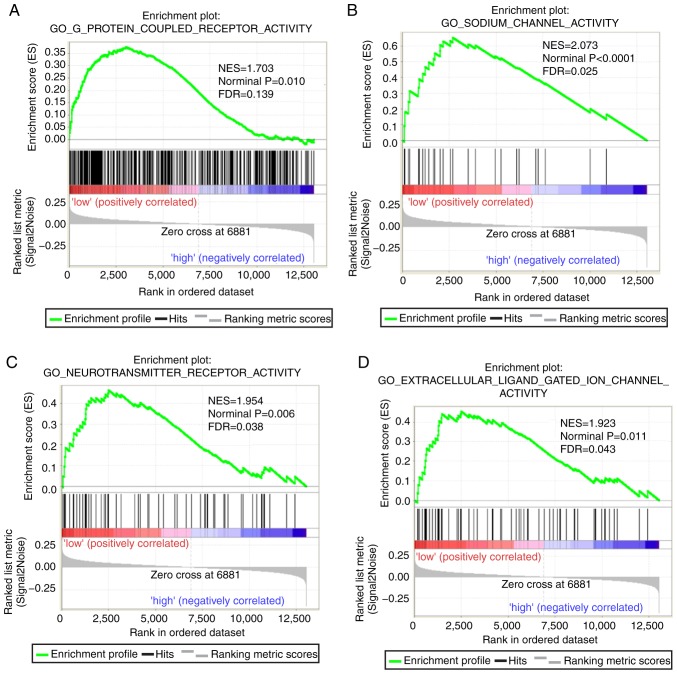
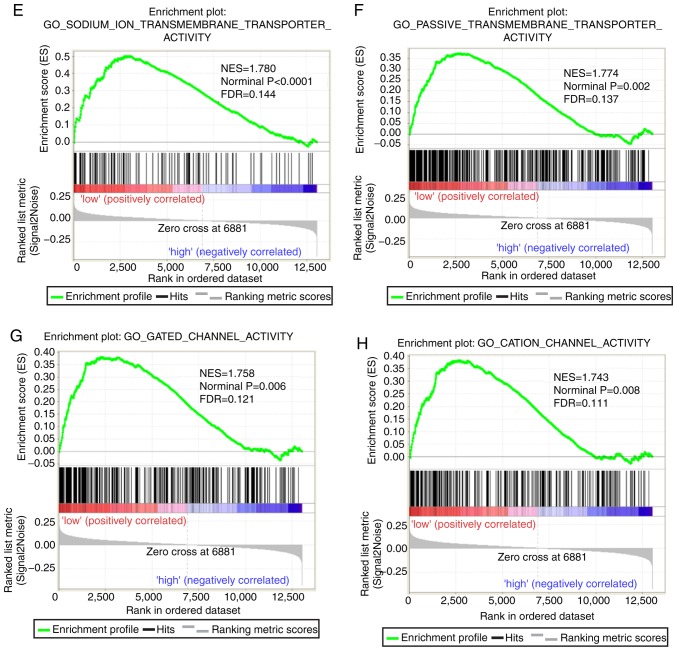
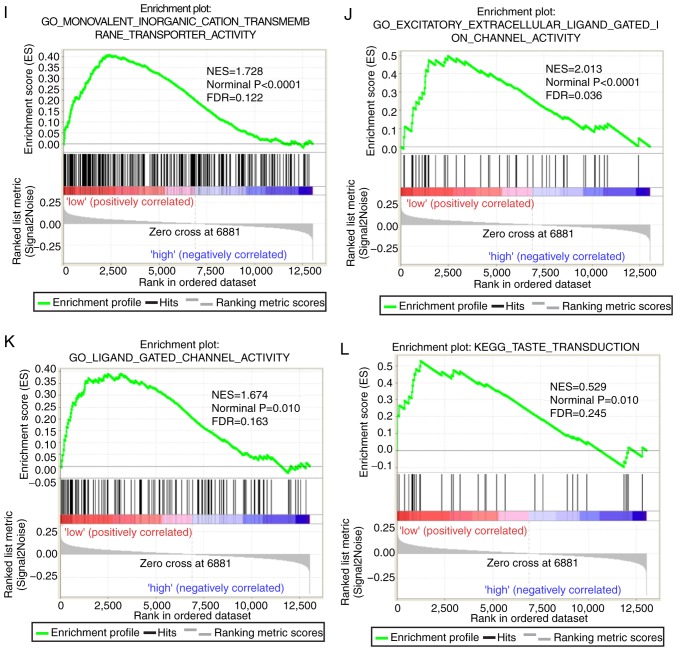
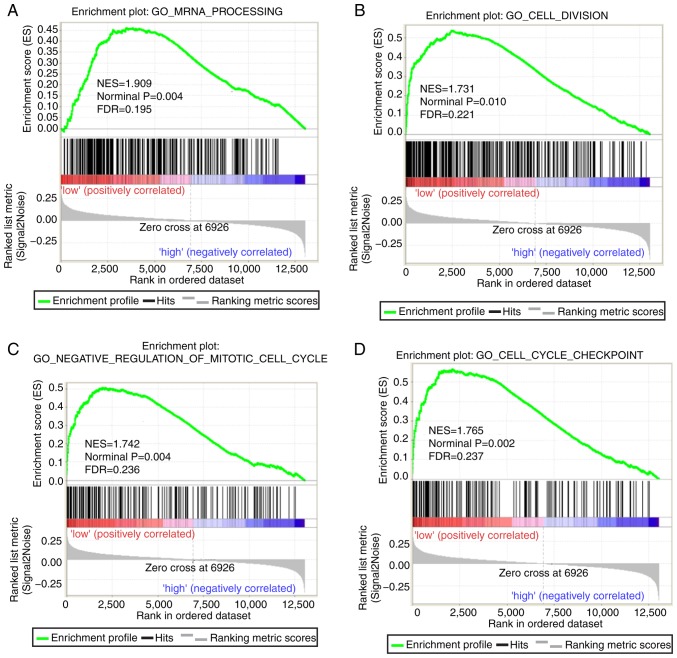
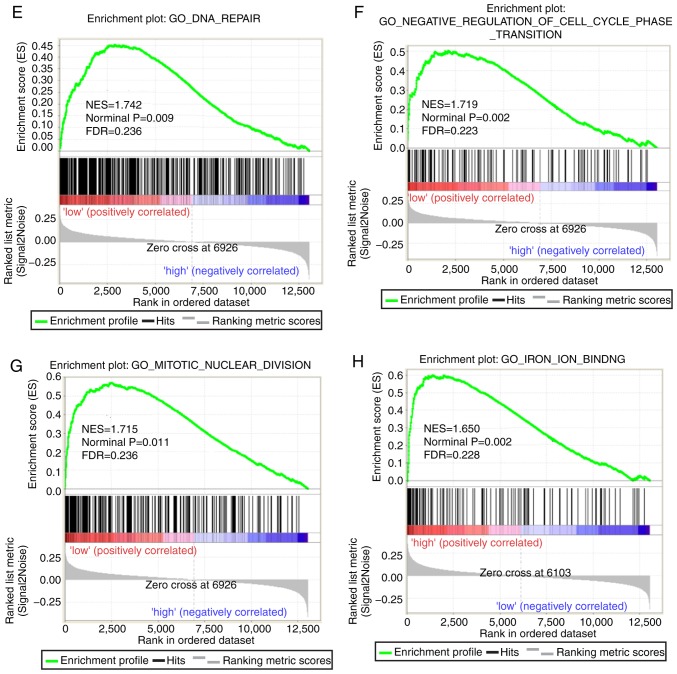
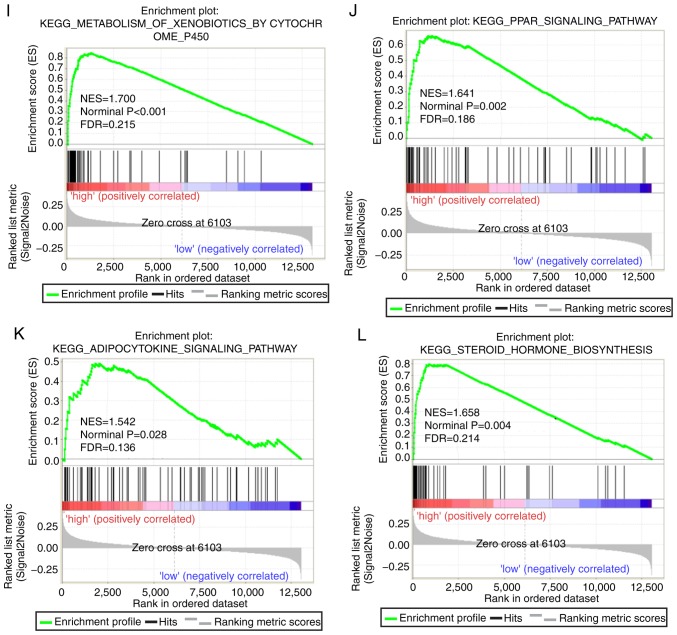
Both diagnostic- and prognostic-associated genes were explored to investigate the mechanisms that PLCBs are involved in. Enriched GO terms and KEGG pathways annotated with PLCB1 included ‘G protein coupled receptor activity’, ‘sodium channel activity’, ‘extracellular ligand gated ion channel activity’ and ‘taste transduction pathway’, among others (Fig. 7). Enriched GO terms and KEGG pathways annotated with PLCB2 included ‘mRNA processing’, ‘cell division’, ‘cell cycle checkpoints’, ‘DNA repair’, ‘PPAR signaling pathway’, ‘metabolism of xenobiotics by cytochrome P450’ and ‘adipocytokine signaling pathway’ among others (Fig. 8).
Figure 7.
Gene set enrichment analysis results of phospholipase C β1 gene. Results of gene ontologies: (A) G-protein coupled receptor activity; (B) sodium channel activity; (C) neutotransmitter receptor activity; (D) extracellular ligand gated ion channel activity. GO, gene ontology; NES, normalized enrichment score; FDR, false discovery rate; KEGG, Kyoto Encyclopedia of Genes and Genomes. Gene set enrichment analysis results of phospholipase C β1 gene. Results of gene ontologies: (E) sodium ion transmembrane transporter; (F) passive transmembrane transporter; (G) gated channel activity; (H) cation channel activity; (I) monovalent inorganic cation transmembrane transporter activity; (J) excitatory extracellular ligand gated ion channel activity; (K) ligand gated channel activity. (L) Taste transduction KEGG pathway. GO, gene ontology; NES, normalized enrichment score; FDR, false discovery rate; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Figure 8.
Gene set enrichment analysis results of phospholipase C β2 gene. Results of gene ontologies: (A) mRNA processing; (B) cell division; (C) negative regulation of mitotic cell cycle; (D) cell cycle checkpoint. GO, gene ontology; NES, normalized enrichment score; FDR, false discovery rate; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPAR peroxisome proliferator-activated receptor. Gene set enrichment analysis results of phospholipase C β2 gene. Results of gene ontologies: (E) DNA repair; (F) negative regulation of cell cycle phase transition (G) mitotic nuclear division; (H) iron ion binding. Results of KEGG pathways: (I) metabolism of xenobiotics by cytochrome P450; (J) PPAR signaling pathway; (K) adipocytokine signaling pathway; (L) steroid hormone biosynthesis. GO, gene ontology; NES, normalized enrichment score; FDR, false discovery rate; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPAR peroxisome proliferator-activated receptor.
Expression model and nomogram construction
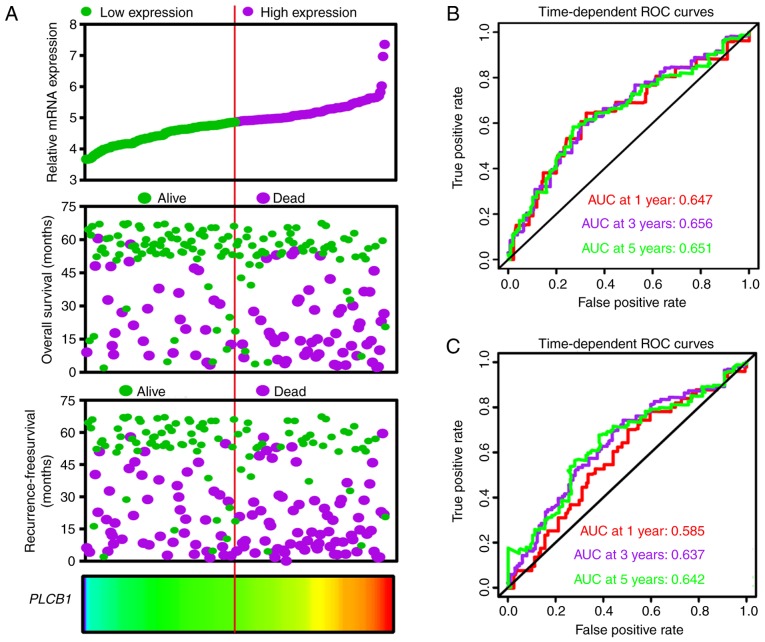
An expression model was constructed for OS and RFS prognosis prediction (Fig. 9). PLCB1 expression, OS and RFS survival status, and PLCB1 expression heatmaps are shown in Fig. 9A, and prognostic ROC curves demonstrated that PLCB1 expression has prognostic value for OS and RFS (Fig. 9B and C).
Figure 9.
Expression model constructed using PLCB1 gene. (A) Expression model including expression, overall survival status, recurrence-free survival status and heatmap. (B) Time dependent ROC curves of overall survival at 1, 3- and 5- years. (C) Time dependent ROC curves of recurrence-free survival at 1, 3- and 5-years. PLCB1, phospholipase C β1; ROC, receiver operating characteristics; AUC, area under the curve.
Furthermore, nomograms were constructed for clinical factors and PLCB1. High expression always led to low points. The same points indicated a higher probability of survival at 1 year, yet a lower probability of survival at 5 years for both OS and RFS. Survival probability at 3 years was seated in the middle (Fig. 10).
Figure 10.
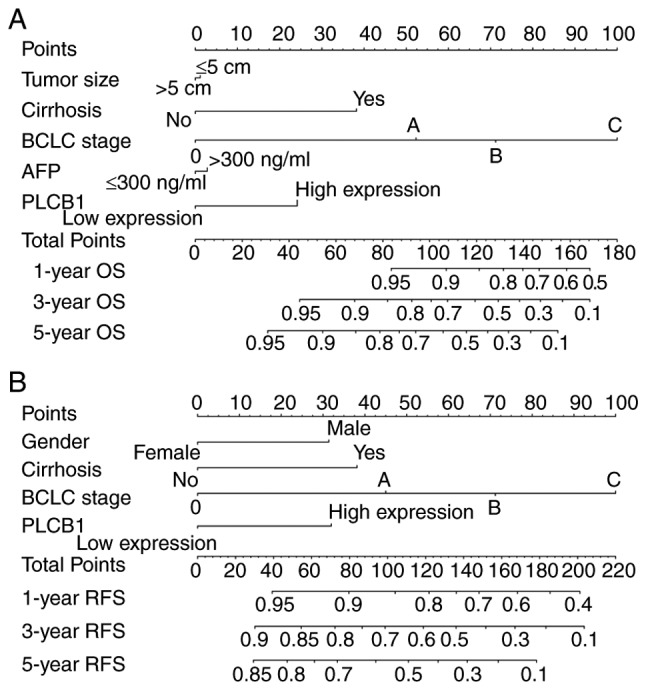
Nomograms constructed using overall survival and recurrence-free survival-related clinical factors and genes. (A) Nomogram of OS-associated genes and clinical factors. (B) Nomogram of RFS-associated genes and clinical factors. BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; PLCB1, phospholipase C β1; OS, overall survival; RFS, recurrence-free survival.
Interaction and co-expression networks and enrichment analysis
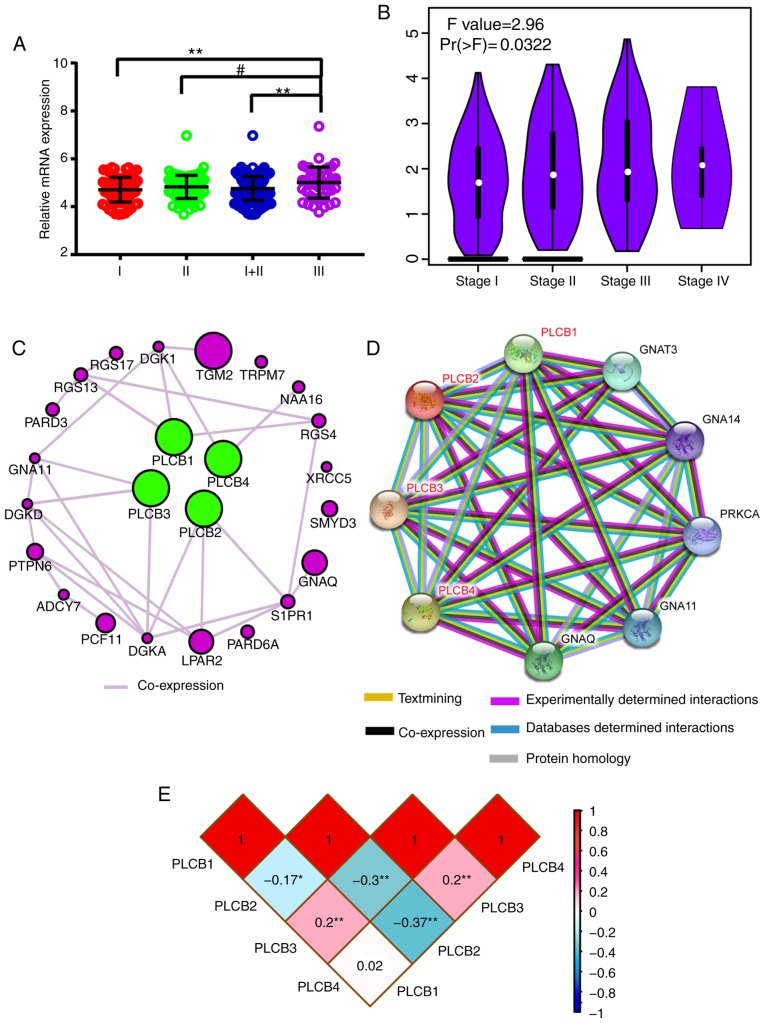
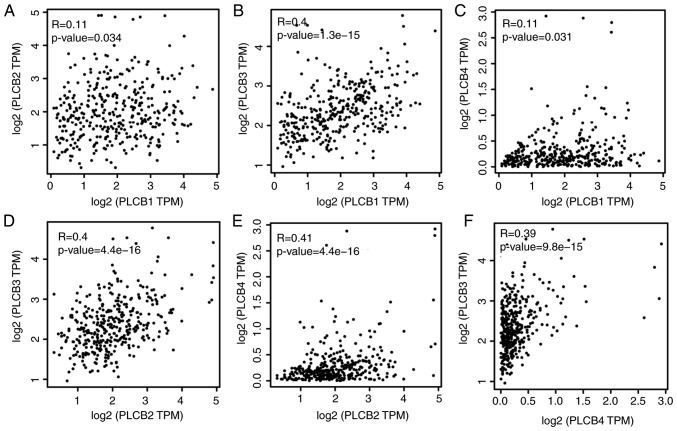
Associations between gene expression and TNM stage (I, II, III) were visualized; PLCB1 gene expression of 212 HBV-HCC was significantly different in early (I, II) stages compared with advanced (III) stage in (P≤0.01; Fig. 11A). Associations between gene expressions and TNM stage (I, II and III) in GEPIA indicated that PLCB1 expression was different in different tumor stages (Fig. 11B). Gene-gene co-expression interactions and PPI networks demonstrated interactions between PLCB members (Fig. 11C and D). The Pearson correlation matrix showed an association between PLCB members (Fig. 11E).
Figure 11.
Scatter plots, matrix and interaction networks analysis. (A) Scatter plot and (B) violin plot of PLCB1 expressions. (C) Co-expression network of PLCB1-4 genes. (D) Protein-protein interaction network of PLCB1-4. (E) Pearson correlation matrix of PLCB1-4. PLCB, phospholipase C β.
Furthermore, enriched GO terms are presented in Fig. S4A-C. KEGG pathways that PLCB members are involved in are presented in Fig. S5. All members were involved in diacylglycerol and IP3 metabolism and finally induced sustained angiogenesis, thus evading apoptosis and proliferation effects.
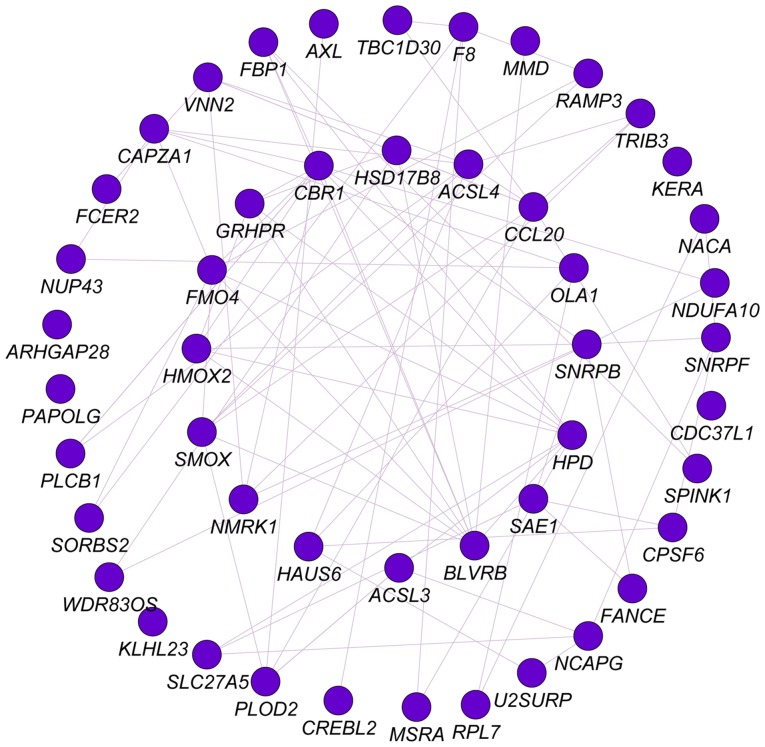
Analysis of PLCB1 and associated genes genome-wide
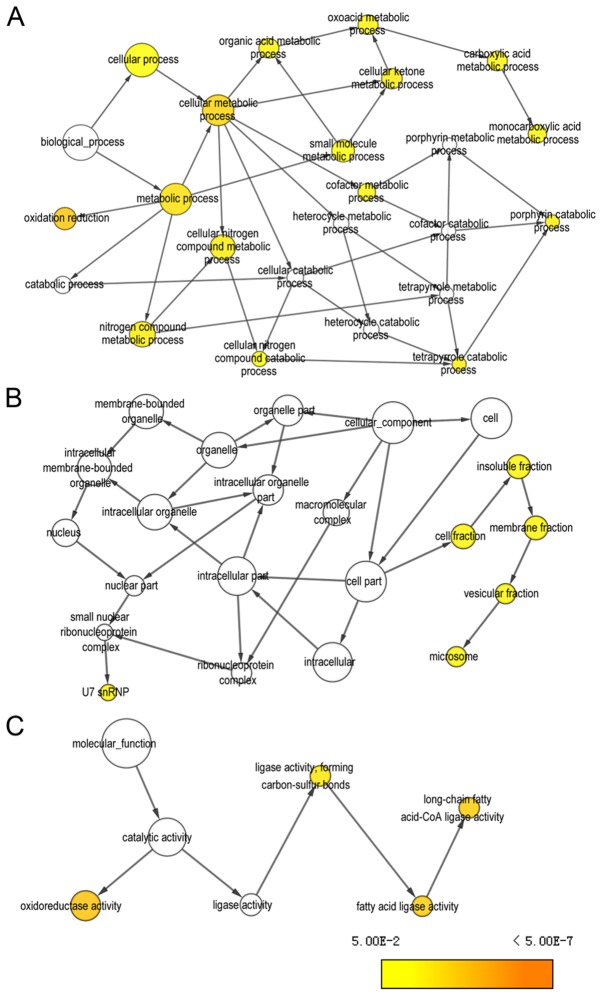
Pearson correlation analysis was performed for PLCB1 genome-wide. A total of 53 genes were identified at r≥0.4. Gene-gene interaction analysis was constructed and presented in Fig. 12. Networks of BP, CC and MF terms were constructed (Fig. 13). Enriched GO terms and KEGG pathways annotated by PLCB1 and correlated genes are presented in Table V.
Figure 12.
Co-expression network of PLCB1 gene with correlation-associated genes in genome-wide analysis. PLCB1, phospholipase C β1.
Figure 13.
Visualized gene ontologies of phospholipase C β1 and correlation-associated genes in genome-wide analysis. (A) Biological process, (B) cellular component and (C) molecular function.
Table V.
Enrichment results of gene ontologies and KEGG pathways of phospholipase B1 with genome-wide associated genes.
| Category | Term | Count | P-value | False discovery rate | Genes |
|---|---|---|---|---|---|
| Biological process | Oxidation-reduction process | 11 | 1.15E-06 | 0.001521 | FMO4, CBR1, MSRA, PLOD2, BLVRB, F8, SMOX, GRHPR, NDUFA10, HPD, HSD17B8 |
| Molecular function | Long-chain fatty acid-CoA ligase activity | 3 | 0.000399 | 0.455909 | ACSL4, ACSL3, SLC27A5 |
| Cellular component | Extracellular exosome | 16 | 0.000748 | 0.826682 | NACA, FCER2, CAPZA1, FBP1, AXL, SPINK1, GRHPR, CBR1, MSRA, RPL7, PLOD2, BLVRB, SNRPB, ACSL4, PLCB1, HPD |
| Biological process | Long-chain fatty acid metabolic process | 3 | 0.001186 | 1.559498 | ACSL4, ACSL3, SLC27A5 |
| Cellular component | Cytosol | 17 | 0.001409 | 1.552995 | CAPZA1, FBP1, ARHGAP28, TRIB3, SAE1, GRHPR, CBR1, MSRA, RPL7, NCAPG, BLVRB, SNRPB, SMOX, PLCB1, SNRPF, NUP43, HPD |
| Cellular component | Endoplasmic reticulum membrane | 7 | 0.012018 | 12.55815 | FMO4, HMOX2, PLOD2, ACSL4, ACSL3, SLC27A5, HPD |
| Cellular component | Actin cytoskeleton | 4 | 0.012886 | 13.40731 | MSRA, SORBS2, NCAPG, CAPZA1 |
| Cellular component | U7 snRNP | 2 | 0.015645 | 16.05626 | SNRPB, SNRPF |
| Biological process | Heme catabolic process | 2 | 0.01697 | 20.28229 | HMOX2, BLVRB |
| Molecular function | Decanoate-CoA ligase activity | 2 | 0.018337 | 19.08795 | ACSL4, ACSL3 |
| Molecular function | Very long-chain fatty acid-CoA ligase activity | 2 | 0.02287 | 23.26214 | ACSL4, SLC27A5 |
| Cellular component | U4 snRNP | 2 | 0.024478 | 24.04788 | SNRPB, SNRPF |
| Cellular component | Methylosome | 2 | 0.026674 | 25.92421 | SNRPB, SNRPF |
| Biological process | Cellular protein modification process | 3 | 0.027107 | 30.50786 | MSRA, PLOD2, SAE1 |
| Cellular component | Small nucleolar ribonucleoprotein complex | 2 | 0.028865 | 27.75428 | SNRPB, SNRPF |
| Biological process | Histone mRNA metabolic process | 2 | 0.028919 | 32.20282 | SNRPB, SNRPF |
| Biological process | Positive regulation of nitric-oxide synthase biosynthetic process | 2 | 0.031291 | 34.36424 | CCL20, FCER2 |
| Molecular function | RNA binding | 5 | 0.036659 | 34.78158 | RPL7, SNRPB, CPSF6, PAPOLG, SNRPF |
| Cellular component | Intracellular ribonucleoprotein complex | 3 | 0.037507 | 34.57769 | RPL7, SNRPB, CPSF6 |
| Cellular component | Small nuclear ribonucleoprotein complex | 2 | 0.037582 | 34.63438 | SNRPB, SNRPF |
| Cellular component | SMN-Sm protein complex | 2 | 0.037582 | 34.63438 | SNRPB, SNRPF |
| Cellular component | U1 snRNP | 2 | 0.041912 | 37.82519 | SNRPB, SNRPF |
| Biological process | Nuclear import | 2 | 0.043071 | 44.18227 | SNRPB, SNRPF |
| Cellular component | U12-type spliceosomal complex | 2 | 0.056916 | 47.81769 | SNRPB, SNRPF |
| Biological process | Metabolic process | 3 | 0.063294 | 57.93576 | GRHPR, ACSL4, ACSL3 |
| Biological process | Drug metabolic process | 2 | 0.063922 | 58.30767 | FMO4, CBR1 |
| Biological process | Spliceosomal snRNP assembly | 2 | 0.066211 | 59.63802 | SNRPB, SNRPF |
| Biological process | mRNA polyadenylation | 2 | 0.066211 | 59.63802 | CPSF6, PAPOLG |
| Biological process | Regulation of glucose transport | 2 | 0.077575 | 65.68049 | TRIB3, NUP43 |
| Biological process | Regulation of G-protein coupled receptor protein signaling pathway | 2 | 0.091035 | 71.75149 | RAMP3, PLCB1 |
| Biological process | Long-chain fatty-acyl-CoA biosynthetic process | 2 | 0.097693 | 74.37219 | ACSL4, ACSL3 |
| KEGG pathway | PPAR signaling pathway | 3 | 0.031778 | 29.78018 | ACSL4, ACSL3, SLC27A5 |
| KEGG pathway | Fatty acid biosynthesis | 2 | 0.053251 | 45.0671 | ACSL4, ACSL3 |
| KEGG pathway | Metabolic pathways | 10 | 0.058505 | 48.31368 | CBR1, FBP1, GRHPR, ACSL4, PLCB1, NDUFA10, ACSL3, SLC27A5, HPD, HSD17B8 |
KEGG, Kyoto Encyclopedia of Genes and Genomes.
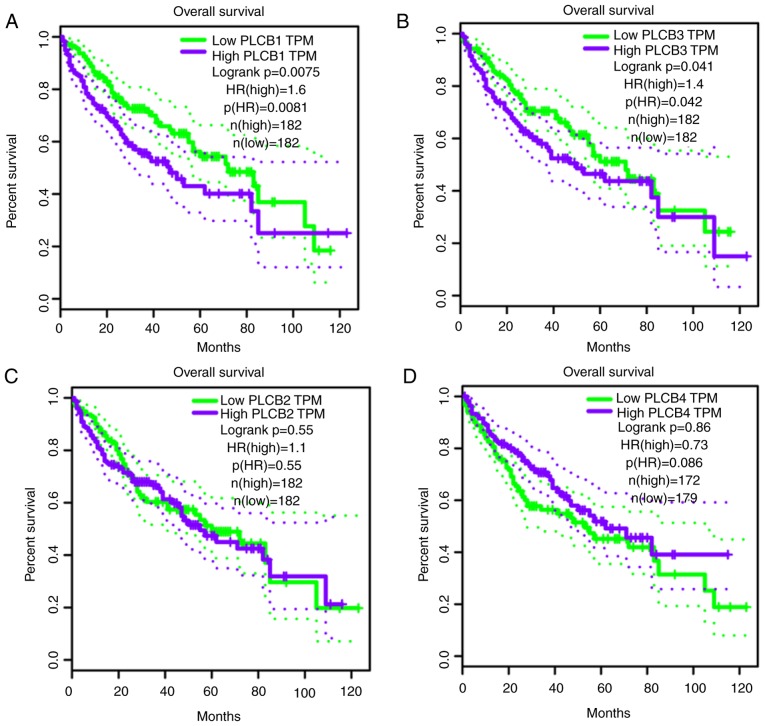
Validation of prognostic values of PLCB genes
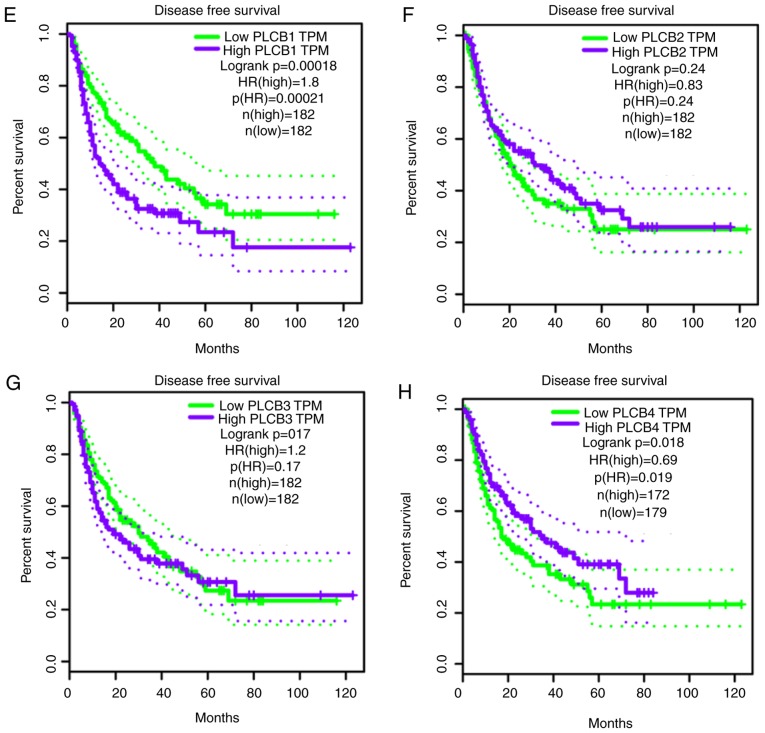
PLCB genes were further validated in GEPIA for OS and RFS (Fig. 14). PLCB1 and PLCB2 were associated with OS (P=0.0075 and P=0.041, respectively; Fig. 14A and B). In addition, PLCB1 and PLCB4 were associated with RFS (P<0.0001 and P<0.018, respectively; Fig. 14E and H). Other genes were not associated with OS or RFS (all P>0.05; Fig. 14). Pearson correlation in GEPIA (Fig. 15) indicated that PLCB1 was positively correlated with PLC3 and PLCB4, while PLCB3 was positively correlated with PLCB4, which is consistent with Fig. 11E.
Figure 14.
Overall survival and disease recurrence-free survival analysis plots of PLCB1-4. Overall survival analysis plots of (A) PLCB1, (B) PLCB2, (C) PLCB3 and (D) PLCB4. Disease recurrence-free survival analysis plot (E) PLCB1, (F) PLCB2, (G) PLCB3 and (H) PLCB4. PLCB, phospholipase C β; TPM, transcripts per million; HR, hazard ratio.
Figure 15.
Pearson correlation plots of PLCB1-4 genes. (A) PLCB1 vs. PLCB2; (B) PLCB3 vs. PLCB1; (C) PLCB4 vs. PLCB1; (D) PLCB3 vs. PLCB2; (E) PLCB4 vs. PLCB2; (E) PLCB3 vs. PLCB4. PLCB, phospholipase C β; TPM, transcripts per million.
Discussion
In the current study, it was identified that PLCB1 and PLCB2 genes are differently expressed in tumor and normal tissues. PLCB1 and PLCB2 have diagnostic value for HCC, while PLCB3 has potential diagnostic value for HCC. Combinations of these genes have an advantage over PLCB1, PLCB2 or PLCB3 alone with regard to HCC diagnosis. In addition, PLCB1 has prognostic value of OS and RFS for HCC. Combining PLCB1 and AFP had an advantage over PLCB1 alone for OS and RFS. GSEA indicated that PLCB1 and PLCB2 were involved in ‘G protein coupled receptor activity’, ‘sodium channel activity’, ‘cell division’, ‘cell cycle checkpoint’, ‘DNA repair’, ‘PPAR signaling pathway’, ‘metabolism of xenobiotics by cytochrome P450’ and ‘adipocytokine signaling pathway’, among others. Nomograms and gene expression models were constructed for HCC prognosis prediction. The validation of the prognostic values of PLCB genes revealed that PLCB1 and PLCB2 were associated with OS, and PLCB1 and PLCB4 were associated with RFS.
PLC proteins are key enzymes that metabolize inositol lipids and have a pivotal role in multiple transmembrane signaling transduction pathways that modulate a series of cellular processes, including cell proliferation and mobility (16). In mammalian cells, there are four PLCB isoforms: PLCB1, PLCB2, PLCB3 and PLCB4. PLCB2 and PLCB3 are activated by Gβγ dimers, which are released upon the activation of Gα protein coupled receptor families (43). PLCB2 can also be activated by Rho family members of monomeric G proteins, with the strongest activation by Rac1; these participate in the cytoskeletal rearrangements that accompany cell mobility (44).
The PLCB1 enzyme is encoded by the PLCB1 gene, which is located at chromosome of 20p12 (1). It was originally identified as a G protein coupled receptor-associated PLCB isoform that is able produce inositol 1,4,5-trisphosphate and diacylglycerol from phosphatidylinositol 4,5-bisphophate (45). The deregulation of signaling transduction pathways always leads to advantages for tumor patients (1). PLCB1 is activated by Gα and induces a variety of events, which may increase the total intracellular calcium levels (46); one possible result of this process is aberrant proliferation in the cell (1). PLCB1 has been reported to have a role in promoting cell cycle progression by targeting cyclin-cyclin kinase complexes (47).
In addition, PLCB1 has been documented to have a pivotal role in myoblast differentiation, regulating the delayed differentiation of skeletal muscle in myotonic dystrophy myoblasts (48). PLCB1 may also reduce cell damage under oxidative conditions and prevent α-synuclein aggregation (49). The amplification of PLCB1 increased K562 cell viability and enables cells to evade apoptosis (50,51); the overexpression of PLCB1 keeps Swiss 3T3 cells in the S phase of the cell cycle (52). Li et al (1) reported that upregulated PLCB1 expression is associated with tumor cell proliferation and infers a poor prognosis for HCC. The present study revealed that high expression has is undesirable for HCC prognosis (OS and RFS), which is consistent with the results of Li et al (1). Furthermore, PLCB1 had diagnostic value for HCC.
PLCB2 mediates mitogenic, proliferative and migratory events by interacting with heterotrimeric and monomeric G proteins, and can interact with γ-synuclein to regulate G protein activation (43). Bertagnolo et al (53) reported that PLCB2 induces cell cycle transition from G0/G1 to the S/G2/M phases, which is critical for tumor progression, without affecting cell cycle-associated enzymes. They also indicate that PLCB2, by modifying the phospholipase pool, may be responsible for the inositol lipid-associated modifications of the cytoskeleton architecture that occur in the course of division, motility and invasion of tumor cells (53). The current findings with regard to the role of PLCB2 in the cell cycle and cell division are consistent with the results of Bertagnolo et al (53).
PLCB2 has been reported to promote mitosis and the migration of human breast cancer-derived cells (54), is highly expressed in breast cancer and associated with poor prognosis (55); however, little is known about HCC PLCB2 expression, and the role in HCC diagnosis and prognosis. In the current study, PLCB2 expression as not associated with HCC prognosis, but may be a diagnostic signature for HCC.
PLCB3 is located on chromosome 11q13 in the vicinity of the multiple endocrine neoplasia type 1 gene; its loss leads to the development of neuroendocrine tumors (56). The transfection of PLCB3 to a human endocrine pancreatic tumor cell line can induce the activation of the human mismatch repair protein 3 gene (56). PLCB3 interacts with Na(+)/H(+) exchange regulatory cofactor NHERF-1, providing a structural basis for CXCR2 signaling in pancreatic cancer (57). Hoeppner et al (58) identified a novel role for PLCB3, functioning as a negative regulator of vascular endothelial growth factor-mediated vascular permeability by regulating intracellular Ca2+ release. PLCB3 may have a tumor suppressor role via SHP-1-mediated dephosphorylation of Stat5 (59). Ju et al (60) reported that PLCB and Gqα may have important roles in scar remodeling, cardiac hypertrophy and fibrosis following myocardial infarction rat hearts. In the present study, PLCB3 expression exhibited potential diagnostic value for HCC and without association with HCC prognosis. PLCB3 may have a weak role in HCC if at all, which requires further investigation.
Compared with other PLCB genes, PLCB4 is less well characterized, and associations between PLCB4 and cancer are unclear. The expression of PLCB4 and PLCB3 was previously explored in Purkinje cell subsets of the mouse cerebellum (61). PLCB4 and PLCB3 are differentially expressed in microarray databases of non-small cell lung cancer, but neither are associated with the prognosis and development of lung cancer (62). Orchel et al (63) reported that PLCB4 is differentially expressed in 50 endometrium samples from women with endometrial cancer, but is not associated with the treatment of endometrial cancer. Furthermore, the present study did not find any association between PLCB4 and HCC. Therefore, further studies are required to explore the relationship between PLCB4 expression and malignancy.
The findings of the present study indicate that PLCB1 expression was associated with OS, whereas PLCB1 and PLCB3 expression was associated with RFS in univariate analysis. In multivariate analysis, PLCB1 expression was associated with OS and RFS. Multivariate cox analysis contains several significant clinicopathological characteristics, which produces new adjusted results and conclusions. In addition, PLCB1 expression was associated with OS, whereas PLCB1 and PLCB3 expression was associated with RFS in univariate analysis. However, PLCB1 expression was associated with OS and RFS in multivariate analysis. Different results may be due to varied clinicopathological characteristics in the GSE14520 and TCGA dataset. Of course, HBV is a pivotal factor associated with HCC.
There are some limitations to the present study that should be recognized. Firstly, larger sample cohorts are required to validate these findings. Additionally, the results are based on a HBV-associated HCC population; therefore, further explorations are needed in a study including HBV-infected and non-infected patients. Finally, functional trials are required to further explore the roles of PLCB genes in HCC initiation, development, metastasis, proliferation and angiogenesis. BCLC stage is an important factor associated with HCC and treatments concerning BCLC stage should mentioned in the material section.
The present study demonstrated that the PLCB1 and PLCB2 genes are differentially expressed between tumor and normal tissues and have diagnostic values for HCC. PLCB3 has a potential diagnostic value for HCC. The combinations of these genes have an advantage over PLCB1, PLCB2 or PLCB3 used alone for HCC diagnosis. In addition, PLCB1 has OS and RFS prognostic value for HCC. Combining PLCB1 and AFP was advantageous over PLCB1 alone for predicting OS and RFS. Nomogram and gene expression models were used to construct and predict HCC prognosis. GO terms and metabolic pathways associated with PLCB1 and PLCB2 are include ‘G protein coupled receptor activity’, ‘cell division’, ‘cell cycle checkpoint’, ‘DNA repair’, ‘PPAR signaling pathway’ and ‘metabolism of xenobiotics by cytochrome P450’. Validation of the prognostic value of the PLCB genes revealed that PLCB1, PLCB2 and PLCB4 are associated with HCC prognosis.
Supplementary Material
Acknowledgements
The authors would like to acknowledge researchers for their contribution to open access data available via GTEx portal, GEPIA, Kaplan-Meier Plotter, STRING and The Human Protein Atlas websites. In addition, the authors would like to acknowledge invaluable help from peer reviewers.
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- PLCB
phospholipase C β
- HBV
hepatitis B virus
- GEO
gene expression omnibus
- GEPIA
gene expression profiling interactive analysis
- GSEA
gene set enrichment analysis
- BP
biological process
- CC
cellular component
- MF
molecular function
- GO
gene ontology
- OS
overall survival
- RFS
recurrence-free survival
- MST
median survival time
- CI
confidence interval
- HR
hazard ratio
- ROC
receiver operating characteristic
- PPI
protein-protein interaction
Funding
This work was supported in part by the National Nature Science Foundation of China (grant no. 81560535, 81072321, 30760243, 30460143, 30560133 and 81802874), Natural Science Foundation of Guangxi Province of China (grant no. 2017JJB140189y), Key Laboratory of High-Incidence-Tumor Prevention and Treatment (Guangxi Medical University), Ministry of Education (GKE2018-01), 2009 Program for New Century Excellent Talents in University (NCET), Guangxi Nature Sciences Foundation (grant no. GuiKeGong 1104003A-7), and Guangxi Health Ministry Medicine Grant (Key-Scientific Research-Grant; grant no. Z201018). The present study is also partly supported by Scientific Research Fund of the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region (grant no. Z2016318), The Basic Ability Improvement Project for Middle-aged and Young Teachers in Colleges and Universities in Guangxi (grant no. 2018KY0110). As well as, the present study is also partly supported by Research Institute of Innovative Think-tank in Guangxi Medical University (The gene-environment interaction in hepatocarcinogenesis in Guangxi HCCs and its translational applications in the HCC prevention). We also acknowledge the supported by the National Key Clinical Specialty Programs (General Surgery & Oncology) and the Key Laboratory of Early Prevention & Treatment for Regional High-Incidence-Tumor (Guangxi Medical University), Ministry of Education, China.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XW and TP designed this study and the manuscript. KH, XZ, ZL, XL, CY, TY, CH, GZ, WQ and TP conducted the study and analyzed the data. XW wrote the manuscript and TP guided the writing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Li J, Zhao X, Wang D, He W, Zhang S, Cao W, Huang Y, Wang L, Zhou S, Luo K. Up-regulated expression of phospholipase C, β1 is associated with tumor cell proliferation and poor prognosis in hepatocellular carcinoma. Onco Targets Ther. 2016;9:1697–1706. doi: 10.2147/OTT.S97189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al. Management of hepatocellular carcinoma in Asia: Consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1119–1127. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 4.Tagliamonte M, Petrizzo A, Napolitano M, Luciano A, Arra C, Maiolino P, Izzo F, Tornesello ML, Aurisicchio L, Ciliberto G, et al. Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model. Cancer Immunol Immunother. 2015;64:1305–1314. doi: 10.1007/s00262-015-1698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 6.Kanwal F, El-Serag HB, Ross D. Surveillance for hepatocellular carcinoma: Can we focus on the mission? Clin Gastroenterol Hepatol. 2015;13:805–807. doi: 10.1016/j.cgh.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Wang XY, Zhou J, Fan J. Heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol. 2015;12:10. doi: 10.1038/nrclinonc.2014.122-c1. [DOI] [PubMed] [Google Scholar]
- 8.Songer JG. Bacterial phospholipases and their role in virulence. Trends Microbiol. 1997;5:156–161. doi: 10.1016/S0966-842X(97)01005-6. [DOI] [PubMed] [Google Scholar]
- 9.Talarico S, Durmaz R, Yang Z. Insertion- and deletion-associated genetic diversity of Mycobacterium tuberculosis phospholipase C-encoding genes among 106 clinical isolates from Turkey. J Clin Microbiol. 2005;43:533–538. doi: 10.1128/JCM.43.2.533-538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, et al. Deciphering the biology of Mycobacterium tuberculosis from thecomplete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann RD, Alland D, Eisen JA, Carpenter L, White O, Peterson J, DeBoy R, Dodson R, Gwinn M, Haft D, et al. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol. 2002;184:5479–5490. doi: 10.1128/JB.184.19.5479-5490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-I. [DOI] [PubMed] [Google Scholar]
- 14.Vazquezboland JA, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci USA. 2011;108:19484–19491. doi: 10.1073/pnas.1112371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakahara M, Shimozawa M, Nakamura Y, Irino Y, Morita M, Kudo Y, Fukami K. A novel phospholipase C, PLC(eta)2, is a neuron-specific isozyme. J Biol Chem. 2005;280:29128–29134. doi: 10.1074/jbc.M503817200. [DOI] [PubMed] [Google Scholar]
- 18.Lo Vasco VR, Cardinale G, Polonia P. Deletion of PLCB1 gene in schizophrenia-affected patients. J Cell Mol Med. 2012;16:844–851. doi: 10.1111/j.1582-4934.2011.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M, Li H, Li Y, Ruan Y, Quan C. Identification of genes and pathways associated with MDR in MCF-7/MDR breast cancer cells by RNA-seq analysis. Mol Med Rep. 2018;17:6211–6226. doi: 10.3892/mmr.2018.8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong M, Murtazina DA, Phillips J, Ku CY, Sanborn BM. Multiple signals regulate phospholipase CBeta3 in human myometrial cells. Biol Reprod. 2008;78:1007–1017. doi: 10.1095/biolreprod.107.064485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu IC, Liu WC, Chang TT. Applications of next-generation sequencing analysis for the detection of hepatocellular carcinoma-associated hepatitis B virus mutations. J Biomed Sci. 2018;25:51. doi: 10.1186/s12929-018-0442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramvis A, Arakawa K, Yu MC, Nogueira R, Stram DO, Kew MC. Relationship of serological subtype, basic core promoter and precore mutations to genotypes/subgenotypes of hepatitis B virus. J Med Virol. 2008;80:27–46. doi: 10.1002/jmv.21049. [DOI] [PubMed] [Google Scholar]
- 23.Chu CJ, Keeffe EB, Han SH, Perrillo RP, Min AD, Soldevila-Pico C, Carey W, Brown RS, Jr, Luketic VA, Terrault N, Lok AS. Hepatitis B virus genotypes in the United States: Results of a nationwide study. Gastroenterology. 2003;125:444–451. doi: 10.1016/S0016-5085(03)00895-3. [DOI] [PubMed] [Google Scholar]
- 24.Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: Recent advances. J Gastroenterol Hepatol. 2011;26(Suppl 1):S123–S130. doi: 10.1111/j.1440-1746.2010.06541.x. [DOI] [PubMed] [Google Scholar]
- 25.Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494–1498. doi: 10.1136/gut.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology. 2002;122:1756–1762. doi: 10.1053/gast.2002.33588. [DOI] [PubMed] [Google Scholar]
- 27.Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19–26. doi: 10.1053/jhep.2003.50036. [DOI] [PubMed] [Google Scholar]
- 28.Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, Wang XW. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202–10212. doi: 10.1158/0008-5472.CAN-10-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roessler S, Long EL, Budhu A, Chen Y, Zhao X, Ji J, Walker R, Jia HL, Ye QH, Qin LX, et al. Integrative genomic identification of genes on 8p associated with hepatocellular carcinoma progression and patient survival. Gastroenterology. 2012;142:957–966.e912. doi: 10.1053/j.gastro.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, Compton CC, DeLuca DS, Peter-Demchok J, Gelfand ET, et al. A novel approach to high-quality postmortem tissue procurement: The GTEx project. Biopreserv Biobank. 2015;13:311–319. doi: 10.1089/bio.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 35.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montojo J, Zuberi K, Rodriguez H, Kazi F, Wright G, Donaldson SL, Morris Q, Bader GD. GeneMANIA Cytoscape plugin: Fast gene function predictions on the desktop. Bioinformatics. 2010;26:2927–2928. doi: 10.1093/bioinformatics/btq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maere S, Heymans K, Kuiper M. BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 39.Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, Levy R. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 40.Alizadeh AA, Gentles AJ, Alencar AJ, Liu CL, Kohrt HE, Houot R, Goldstein MJ, Zhao S, Natkunam Y, Advani RH, et al. Prediction of survival in diffuse large B-cell lymphoma based on the expression of 2 genes reflecting tumor and microenvironment. Blood. 2011;118:1350–1358. doi: 10.1182/blood-2011-03-345272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao X, Han C, Wang X, Huang K, Yu T, Yang C, Huang R, Liu Z, Han Q, Peng T. Prognostic value of minichromosome maintenance mRNA expression in early-stage pancreatic ductal adenocarcinoma patients after pancreaticoduodenectomy. Cancer Manag Res. 2018;10:3255–3271. doi: 10.2147/CMAR.S168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao X, Liu X, Yang C, Wang X, Yu T, Han C, Huang K, Zhu G, Su H, Qin W, et al. Distinct diagnostic and prognostic values of minichromosome maintenance gene expression in patients with hepatocellular carcinoma. J Cancer. 2018;9:2357–2373. doi: 10.7150/jca.25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golebiewska U, Guo Y, Khalikaprasad N, Zurawsky C, Yerramilli VS, Scarlata S. γ-Synuclein interacts with phospholipase Cβ2 to modulate G protein activation. PLoS One. 2012;7:e41067. doi: 10.1371/journal.pone.0041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu Rev Pharmacol Toxicol. 2006;46:355–379. doi: 10.1146/annurev.pharmtox.46.120604.141223. [DOI] [PubMed] [Google Scholar]
- 45.Martelli AM, Fiume R, Faenza I, Tabellini G, Evangelista C, Bortul R, Follo MY, Falà F, Cocco L. Nuclear phosphoinositide specific phospholipase C (PI-PLC)-beta 1: A central intermediary in nuclear lipid-dependent signal transduction. Histol Histopathol. 2005;20:1251–1260. doi: 10.14670/HH-20.1251. [DOI] [PubMed] [Google Scholar]
- 46.Ngoh A, Mctague A, Wentzensen IM, Meyer E, Applegate C, Kossoff EH, Batista DA, Wang T, Kurian MA. Severe infantile epileptic encephalopathy due to mutations in PLCB1: Expansion of the genotypic and phenotypic disease spectrum. Dev Med Child Neurol. 2014;56:1124–1128. doi: 10.1111/dmcn.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faenza I, Matteucci A, Manzoli L, Billi AM, Aluigi M, Peruzzi D, Vitale M, Castorina S, Suh PG, Cocco L. A role for nuclear phospholipase Cbeta 1 in cell cycle control. J Biol Chem. 2000;275:30520–30524. doi: 10.1074/jbc.M004630200. [DOI] [PubMed] [Google Scholar]
- 48.Cocco L, Finelli C, Mongiorgi S, Clissa C, Russo D, Bosi C, Quaranta M, Malagola M, Parisi S, Stanzani M, et al. An increased expression of PI-PLCβ1 is associated with myeloid differentiation and a longer response to azacitidine in myelodysplastic syndromes. J Leukoc Biol. 2015;98:769–780. doi: 10.1189/jlb.2MA1114-541R. [DOI] [PubMed] [Google Scholar]
- 49.Guo Y, Scarlata S. A loss in cellular protein partners promotes α-synuclein aggregation in cells resulting from oxidative stress. Biochemistry. 2013;52:3913–3920. doi: 10.1021/bi4002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poli A, Faenza I, Chiarini F, Matteucci A, Mccubrey JA, Cocco L. K562 cell proliferation is modulated by PLCβ1 through a PKCα-mediated pathway. Cell Cycle. 2013;12:1713–1721. doi: 10.4161/cc.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bavelloni A, Poli A, Fiume R, Blalock W, Matteucci A, Ramazzotti G, McCubrey JA, Cocco L, Faenza I. PLC-beta 1 regulates the expression of miR-210 during mithramycin-mediated erythroid differentiation in K562 cells. Oncotarget. 2014;5:4222–4231. doi: 10.18632/oncotarget.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho KK, Mann DJ. Nuclear signalling through phospholipase C and phosphatidylinositol 4,5-bisphosphate. Signal Transduction. 2006;6:92–100. doi: 10.1002/sita.200500078. [DOI] [Google Scholar]
- 53.Bertagnolo V, Benedusi M, Brugnoli F, Lanuti P, Marchisio M, Querzoli P, Capitani S. Phospholipase C-β2 promotes mitosis and migration of human breast cancer-derived cells. Carcinogenesis. 2007;28:1638–1645. doi: 10.1093/carcin/bgm078. [DOI] [PubMed] [Google Scholar]
- 54.Bertagnolo V, Benedusi M, Brugnoli F, Lanuti P, Marchisio M, Querzoli P, Capitani S. Phospholipase C-beta 2 promotes mitosis and migration of human breast cancer-derived cells. Carcinogenesis. 2007;28:1638–1645. doi: 10.1093/carcin/bgm078. [DOI] [PubMed] [Google Scholar]
- 55.Bertagnolo V, Benedusi M, Querzoli P, Pedriali M, Magri E, Brugnoli F, Capitani S. PLC-beta2 is highly expressed in breast cancer and is associated with a poor outcome: A study on tissue microarrays. Int J Oncol. 2006;28:863–872. [PubMed] [Google Scholar]
- 56.Stålberg P, Lopez-Egido JR, Wang S, Gobl A, Oberg K, Skogseid B. Differentially expressed cDNAs in PLCbeta3-induced tumor suppression in a human endocrine pancreatic tumor cell line: Activation of the human mismatch repair protein 3 gene. Biochem Biophys Res Commun. 2001;281:227–231. doi: 10.1006/bbrc.2001.4329. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Y, Wang S, Holcomb J, Trescott L, Guan X, Hou Y, Brunzelle J, Sirinupong N, Li C, Yang Z. Crystallographic analysis of NHERF1-PLCβ3 interaction provides structural basis for CXCR2 signaling in pancreatic cancer. Biochem Biophys Res Commun. 2014;446:638–643. doi: 10.1016/j.bbrc.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 58.Hoeppner LH, Phoenix KN, Clark KJ, Bhattacharya R, Gong X, Sciuto TE, Vohra P, Suresh S, Bhattacharya S, Dvorak AM, et al. Revealing the role of phospholipase Cβ3 in the regulation of VEGF-induced vascular permeability. Blood. 2012;120:2167–2173. doi: 10.1182/blood-2012-03-417824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao W, Hong H, Kawakami Y, Kato Y, Wu D, Yasudo H, Kimura A, Kubagawa H, Bertoli LF, Davis RS, et al. Tumor suppression by phospholipase C-beta3 via SHP-1-mediated dephosphorylation of Stat5. Cancer Cell. 2009;16:161–171. doi: 10.1016/j.ccr.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ju H, Zhao S, Tappia PS, Panagia V, Dixon IM. Expression of Gq alpha and PLC-beta in scar and border tissue in heart failure due to myocardial infarction. Circulation. 1998;97:892–899. doi: 10.1161/01.CIR.97.9.892. [DOI] [PubMed] [Google Scholar]
- 61.Sarna JR, Marzban H, Watanabe M, Hawkes R. Complementary stripes of phospholipase Cbeta3 and Cbeta4 expression by Purkinje cell subsets in the mouse cerebellum. J Comp Neurol. 2006;496:303–313. doi: 10.1002/cne.20912. [DOI] [PubMed] [Google Scholar]
- 62.Tan X, Chen M. MYLK and MYL9 expression in non-small cell lung cancer identified by bioinformatics analysis of public expression data. Tumour Biol. 2014;35:12189–12200. doi: 10.1007/s13277-014-2527-3. [DOI] [PubMed] [Google Scholar]
- 63.Orchel J, Witek L, Kimsa M, Strzalka-Mrozik B, Kimsa M, Olejek A, Mazurek U. Expression patterns of kinin-dependent genes in endometrial cancer. Int J Gynecol Cancer. 2012;22:937–944. doi: 10.1097/IGC.0b013e318259d8da. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.