Abstract
Hepatitis E virus (HEV) is the most common cause of viral hepatitis in the world. It is estimated that millions of people are infected every year, resulting in tens of thousands of deaths. However, these estimates do not include industrialized regions and are based on studies which employ assays now known to have inferior sensitivity. As such, this is likely to represent a massive underestimate of the true global burden of disease. In the developing world, HEV causes large outbreaks and presents a significant public-health problem. Until recently HEV was thought to be uncommon in industrialized countries, and of little relevance to clinicians in these settings. We now know that this is incorrect, and that HEV is actually very common in developed regions. HEV has proved difficult to study in vitro, with reliable models only recently becoming available. Our understanding of the lifecycle of HEV is therefore incomplete. Routes of transmission vary by genotype and location: endemic regions experience large waterborne epidemics, while sporadic cases in industrialized regions are zoonotic infections likely spread via the food chain. Both acute and chronic infection has been observed, and a wide range of extrahepatic manifestations have been reported. This includes neurological, haematological and renal conditions. As the complete clinical phenotype of HEV infection is yet to be characterized, a large proportion of cases go unrecognized or misdiagnosed. In many cases HEV infection does not feature in the differential diagnosis due to a lack of knowledge and awareness of the disease amongst clinicians. In combination, these factors have contributed to an underestimation of the threat posed by HEV. Improvements are required in terms of recognition and diagnosis of HEV infection if we are to understand the natural history of the disease, improve management and reduce the burden of disease around the world.
Keywords: emerging disease, extrahepatic manifestations, hepatitis E, neurological injury, zoonotic infection
Introduction
The existence of an epidemic, non-A, non-B hepatitis unrelated to blood transfusion was first recognized in India in the late 1970s.1 Subsequently, a Soviet scientist investigating outbreaks of unexplained non-A, non-B hepatitis amongst troops in Afghanistan ingested a pooled stool sample from affected soldiers. He developed an acute hepatitis, and a novel virus was identified in his faeces by electron microscopy.2 Almost a decade later, the viral genome of the newly named hepatitis E virus (HEV) was successfully sequenced.3,4
HEV is now recognized as the most common cause of acute viral hepatitis worldwide. There are an estimated 20 million infections per year, resulting in 3 million symptomatic cases and around 70,000 HEV-related deaths.5–7 As large as these figures are, this is likely to represent a gross underestimate of the true global burden of disease.8 In resource-poor countries HEV represents a significant health issue, causing both sporadic cases and large outbreaks affecting thousands of people.9 Several large-scale epidemics of acute viral hepatitis in southern Asia, which were previously considered to be epidemics of hepatitis A, have since been retrospectively identified as hepatitis E.1,10 Seroprevalence studies suggest that as much as one third of the world’s population will be infected at some point during their lifetime.11 In endemic regions the case-to-infection ratio is between 1:3 and 1:412,13; taken together, these figures indicate that in excess of half a billion people have had clinically apparent hepatitis E.11
Despite this, the clinical phenotype of HEV infection remains incompletely characterized, and the majority of infections are unrecognized or misdiagnosed. How can we have got it so wrong for so long? There are a number of reasons. In developed nations, the received wisdom for many years was that hepatitis E was clinically indistinguishable from hepatitis A and restricted to travellers returning from endemic areas.14 This view was reinforced by successive generations of serology assays which were not fit for purpose, leading to missed diagnoses and underestimates of levels of seroprevalence.15 As such, HEV was considered of little relevance to clinicians in industrialized countries. This is now known to be incorrect, and locally acquired cases of HEV infections have been reported in nearly all European countries,16–18 North America,19 Australasia20,21 and Japan.22,23 Until recently there were no efficient cell-culture models available,24,25 which limited progress in elucidating the lifecycle and characteristics of HEV. The range of clinical presentations, epidemiological patterns and routes of transmission vary widely by genotype, host characteristics, geographical location and over time. Taken together, these factors present significant challenges to understanding and appreciating the threat posed by HEV to human health.
Virology of HEV
HEV is a member of the Orthohepevirus genus, which itself is part of the Hepeviridae family. Orthohepevirus contains four species (A to D),26 with human disease being caused by strains of species A. There are eight genotypes of species A.27 Two of these are obligate human pathogens (HEV1, HEV2), and two are endemic in several animal species, causing zoonotic infections in humans (HEV3, HEV4). The remaining genotypes appear to be restricted to wild boar (HEV5, HEV6) and camels (HEV7, HEV8), although a case of human HEV7 infection has been reported.28
HEV is an icosahedral, positive-strand RNA virus with a 7.2kb genome which contains three open reading frames (ORFs).29 These ORFs are translated into proteins responsible for RNA replication (ORF1), the viral capsid (ORF2) and viral particle secretion (ORF3).29 While ORF2 is the most well-studied of the HEV proteins, given its role as an antigen, the less well characterized ORF3 protein may play a key role in how HEV interacts with the host’s immune response.29 HEV has always been considered to be a nonenveloped virus. This was based on the appearance of virions isolated from faeces, which are naked. The same is true of virions found in bile. However, virions found in blood are wrapped in host-cell membranes.30 The process by which HEV particles become enveloped is not fully understood, but ORF3 appears to play a key role.31 It has been suggested that the detergent action of bile may degrade the envelope of HEV virions, resulting in the nonenveloped particles observed in bile and faeces.32 The quasi-enveloped HEV virions do not have any antigenic proteins on their surfaces, and as such are resistant to the neutralizing effects of anti-ORF2 antibodies.31 Interestingly, HEV isolated from serum is less infectious than that derived from faeces,33,34 and quasi-enveloped and nonenveloped virions have separate mechanisms for entering host cells.35 By adopting these two distinct forms, HEV is able both to evade an existing host’s immune response and maximize infectivity of new hosts.
In addition to providing protection against the host immune response, the quasi-envelope may also grant HEV access to otherwise inaccessible areas. A wide range of extrahepatic manifestations of HEV infection have been described,36 and HEV is able to replicate in human neuronal and placental-derived cell lines.37,38 It has been suggested that the quasi-envelope may lend HEV exosome-like properties,39 which allow it to enter a range of cell types via an endocytic mechanism.32 These similarities to exosomes may also allow quasi-enveloped HEV to enter immunologically privileged sites, including the central nervous system40 and the testes.41
Clinical picture of HEV infection
Acute infection
There is substantial heterogeneity in the clinical presentation of HEV infection both between and within developing and developed countries. In resource-poor settings the majority of cases, both sporadic and epidemic, involve either HEV1 (Asia) or HEV2 (Africa and Central America9). Young adults are most commonly affected, especially the 15–35 age group, and men are more likely to be infected than women.42,43 Most patients experience an acute, self-limiting hepatitis. Chronic infection with HEV1 or HEV2 is yet to be reported. The overall mortality rate ranges from 0.2% to 4%,9 but this can be significantly higher in at-risk groups, such as those with pre-existing liver disease44 and very young children.45,46 Pregnant women in developing countries are particularly vulnerable, with mortality as high as 25%,47,48 with preterm delivery in 66% of cases.48 HEV-related deaths in pregnancy typically occur in the third trimester and are most often caused by fulminant hepatic failure or obstetric complications.48 The mechanisms which underlie this excess mortality and increase in prematurity and pregnancy loss are not currently understood.
In developed countries, locally acquired infections are most commonly caused by HEV3, with HEV4 largely confined to Japan and China.8 Acute infection in these areas produces a wide variety of clinical presentations. Only a small minority of patients, probably less than 5%, present with a typical picture of acute viral hepatitis. Despite this, HEV is the major cause of acute viral hepatitis in several European countries; in France, Germany and the UK during 2014 and 2015, there were more reported cases of acute hepatitis E than hepatitis A or acute hepatitis B.49 Older men are predominantly affected, with a male to female ratio of around 3:1 and a median age around 63 years.17 It is thought that this is due to host factors rather than any difference in exposure, with pre-existing subclinical liver disease suggested as a possible risk factor. An English study found an overrepresentation of excess alcohol consumption and diabetes amongst individuals with acute hepatitis E, both of which are risk factors for hepatic fibrosis and steatosis.50 Generally, those that do present with clinically apparent hepatitis will experience an acute, self-limiting illness that lasts between 4 weeks and 6 weeks.16 Progression to acute liver failure is rare, but a small number of individual cases have been reported in Europe, and a single-centre German study of 80 patients with acute liver failure found that HEV was the most likely cause in around 10% of cases.51 Patients with pre-existing liver disease are at risk of acute-on-chronic liver failure; elderly patients are especially at risk. HEV is a less common cause of decompensation in developed countries52,53 than it is in resource-poor settings, perhaps reflecting differences in the pathogenicity of the dominant genotypes in different regions.54
Chronic infection
Chronic HEV infection can occur in immunosuppressed individuals, including transplant recipients,55–57 HIV-positive patients,58,59 and patients receiving chemotherapy for haematological malignancies.60 All cases reported to date have involved HEV3 or HEV4. The bulk of the literature on chronic HEV infection concerns solid-organ transplant recipients, but the clinical presentation is similar in other immunocompromised cohorts. The majority of cases are asymptomatic, with persistent mild to moderate derangement of liver function tests (LFTs).61 A proportion of patients have normal or near-normal liver enzyme levels, and some remain anti-HEV IgG and IgM negative despite the presence of persistent viral replication.61
Somewhere between half62 and two-thirds61 of solid-organ transplant recipients who are exposed to HEV will develop a chronic infection. In patients infected with HEV3, rapid progression of liver fibrosis to cirrhosis, decompensation and death has been described.55–57,63 In solid-organ transplant recipients, risk factors for developing a chronic infection include a greater degree of immunosuppression and tacrolimus treatment.61 In HIV-positive patients, chronic co-infection is uncommon, and seen only in individuals with a very low CD4+ count (< 200 cells/mm3).59 Factors predictive of chronic infection in other immunosuppressed groups are yet to be identified.
Extrahepatic manifestations
Extrahepatic manifestations of HEV infection have been reported in both acute and chronic cases. A wide range of disparate conditions have been linked to HEV, including glomerulonephritis, haematological disorders, pancreatitis and autoimmune conditions including Henoch–Schönlein purpura,64 thyroiditis65 and myasthenia gravis.66 However, by far the most commonly reported extrahepatic complications of HEV infection are neurological conditions.67
HEV and neurological injury
Around 150 cases of HEV-associated neurological injury have been described.67 These cases are mostly from Europe, and involve HEV3, although HEV1-associated neurological injury has been reported in Asia.68 The majority of cases involve immunocompetent patients, but there have been reports of neurological injury in chronically infected individuals. In all cases, the neurological signs and symptoms dominate the presentation. The patients’ LFTs are usually only mildly or moderately deranged and they are typically anicteric. A proportion of patients have entirely normal LFTs. The range of neurological conditions which have been linked to HEV includes mononeuritis multiplex, Bell’s palsy, vestibular neuritis, myositis and peripheral neuropathy. However, the best characterized HEV-associated neurological illnesses are Guillain–Barré syndrome (GBS), neuralgic amyotrophy (NA) and encephalitis/myelitis.67
GBS is an immune-mediated polyradiculopathy characterized by rapid onset, progressive muscle weakness which can cause respiratory compromise and autonomic dysfunction.69 Approximately two-thirds of patients have a preceding infective illness; most commonly this is Campylobacter jejuni gastroenteritis, accounting for approximately 30% of cases.69 However, in more than half of cases the preceding infection is not identified.70 The first description of a HEV-associated neurological disorder was a case of GBS in India, which was reported in 2000.71 However, 4 years prior to this a Dutch longitudinal study demonstrated that 30% of patients with GBS had moderately elevated LFTs at presentation, with no identifiable explanation for this derangement.72 In addition to a growing number of case reports,36 there have been three case-control studies examining the association between HEV infection and GBS. A Dutch study found that 5% of 201 patients with GBS had evidence of HEV infection at presentation, compared with 0.5% of controls (p = 0.02673). HEV3 RNA was isolated from the stool or serum of three patients. Another study, from Bangladesh, found that 11/100 patients with GBS were infected with HEV.68 The only case to be genotyped in this study involved HEV1.68 A third case-control study, conducted in Japan, found that 4.8% of 63 patients with GBS were anti-HEV IgM positive during the acute phase of their illness.74 None of the control subjects had evidence of acute HEV infection.74 More recently, a Belgian cohort study found that 8% of patients with GBS had evidence of HEV infection.75 The clinical picture of HEV-associated GBS is indistinguishable from cases not associated with HEV.
NA, also known as brachial neuritis or Parsonage–Turner syndrome, is an acute monophasic neurological injury affecting the brachial plexus.76 The aetiology of this condition is not fully understood, but in common with GBS it is thought to have a postinfectious, immune-mediated component.76 The typical presentation involves sudden onset, unilateral pain in the dominant upper limb or shoulder, followed by progressive weakness and sensory impairment in the affected limb.76 There have been a number of cohort and case studies of HEV-associated NA, virtually all from Europe and involving HEV3. In an Anglo–Dutch cohort study, 10.6% (5/47) of patients with NA were infected with HEV at the onset of their neurological symptoms.77 An international, multicentre study of 118 European patients with NA identified a characteristic phenotype of HEV-associated disease compared with other patients with NA.78 In HEV-associated cases symptoms tend to be more severe. Bilateral brachial plexus involvement is significantly more likely, and the damage tends to be more extensive.78 There is also an increased risk of neurological injury outside the brachial plexus, most notably involving the phrenic nerve.78 Another recent study, which prospectively tested consecutive patients with acute, nontraumatic neurological injury, found evidence of HEV infection in 2.4% of subjects (n = 464); three of these patients had NA, and all of them presented with the characteristic HEV-associated phenotype.79 The association between HEV and this phenotype is so strong that in one UK centre, the combination of a middle-aged man presenting with bilateral shoulder pain and deranged LFTs is regarded as acute HEV infection until proven otherwise.
A total of 12 cases of HEV-associated encephalitis/myelitis have been described in individual reports and small case series. Seven were from Europe,80–85 four were from Asia86–89 and one was from the USA.90 Five of these cases involved chronically infected solid-organ transplant patients.80,81 Five patients developed ataxic symptoms, and these patients tended to have poorer outcomes: two patients died, and those who survived had more significant long-term neurological sequelae.81,84,85,90 Six patients had HEV RNA in their serum and cerebrospinal fluid (CSF) at the onset of their illness.80–82,84,85,90 In one case, the HEV RNA found in the CSF differed significantly from that found in the serum.80 This quasispecies compartmentalization may suggest the emergence of directly neurotropic strains of HEV.80
HEV and renal injury
HEV-associated glomerular disease has been reported in both immunocompetent and immunosuppressed patients.91–94 All but one case91 have involved HEV3. A French study examined renal function and histology in a cohort of 51 solid-organ transplant recipients infected with HEV.95 Renal function was significantly impaired in both the acute and chronic phases of infection. Renal biopsies were performed on patients with concomitantly increased proteinuria, and these showed features of membranoproliferative glomerulonephritis, IgA nephropathy and nephroangiosclerosis. Most patients also had cryoglobulinaemia. These effects all appear to be related to HEV infection. Other causes of renal impairment were excluded and viral clearance resulted in improved estimated glomerular filtration rate, reduced proteinuria and resolution of cryoglobulinaemia.
The mechanisms which underlie HEV-associated renal injury remain unclear. Cryoglobulinaemia-associated glomerulonephritis is a well-recognized complication of hepatitis C virus (HCV) infection, and HEV may cause renal impairment in a similar way. As mentioned previously, cryoglobulinaemia appears to be common in HEV-associated renal disease. Notably, HEV RNA was isolated from the cryoprecipitate of one patient with HEV-associated glomerulonephritis.94
HEV and haematological disorders
Thrombocytopaenia has been reported in the context of both HEV1 and HEV3 infection. A UK study found that 12/106 patients infected with HEV presented with a low platelet count. Only three of these patients have platelet counts below 100 × 109/L, and there were no significant clinical consequences. Nine cases of more severe thrombocytopaenia have been reported,91,96–101 with a median platelet count of 10 × 109/L. All of these patients had elevated alanine transaminase levels (median: 1045 IU/L). Platelet-associated antibodies were detected in two patients,98 and it has been suggested that HEV may induce thrombocytopaenia via an immune-mediated mechanism61 similar to that seen with HCV infection.102
A number of other haematological conditions have been reported in association with HEV infection, mostly as single case reports. This includes autoimmune haemolytic anaemia103 aplastic anaemia104 and pure red-cell aplasia.105 One UK study reported monoclonal gammopathy of uncertain significance (MGUS) in over 25% of patients infected with HEV;106 prevalence in the general population is around 3%.107 The significance of this observation is yet to be determined, but MGUS is associated with an increased risk of progression to multiple myeloma.108
Diagnosis of HEV
The varied clinical phenotype of HEV infection makes diagnosis challenging, and this difficulty is compounded by number of other factors. Still regarded as an ‘emerging’ disease, knowledge of the condition is still limited amongst clinicians and as a result it can often be overlooked in the differential diagnosis of LFT derangement (Table 1). Even amongst specialists HEV infection can be easily confused for other hepatobiliary conditions. Studies have shown that a significant proportion of patients diagnosed with drug-induced liver injury (DILI) in fact have acute hepatitis E.109,110 There is substantial overlap in the main demographic for hepatitis E, polypharmacy and DILI so it is easy to make this misdiagnosis if HEV infection is not considered. Similarly, it can be challenging to distinguish acute hepatitis E from autoimmune hepatitis, which commonly presents in older people, and can also produce false positive HEV serology tests due to nonspecific, cross-reactive antibodies. When a diagnosis of HEV infection is considered, there may not be a reliable test available. In the USA, there is no diagnostic assay approved by the US Food and Drug Administration. Elsewhere, some of the assays which are available have questionable sensitivity and specificity, while others can be limited by the natural history of HEV infection.
Table 1.
Who should we test for HEV?.
| Immunological status | Criteria for testing |
|---|---|
| Immunocompetent patients | • ALT > 300 IU/L • Clinical suspicion of DILI • Decompensated chronic liver disease (regardless of LFT results) • Guillain–Barré syndrome (regardless of LFT results) • Neuralgic amyotrophy (regardless of LFT results) • Patients with unexplained acute neurology and a raised ALT |
| Immunocompromised patients | • As above • Persistently elevated ALT • Annual PCR screening |
Testing algorithm for HEV (adapted from Wallace, et al.115) ALT, alanine transaminase; DILI, drug-induced liver injury; HEV, hepatitis E virus; LFT, liver function test.
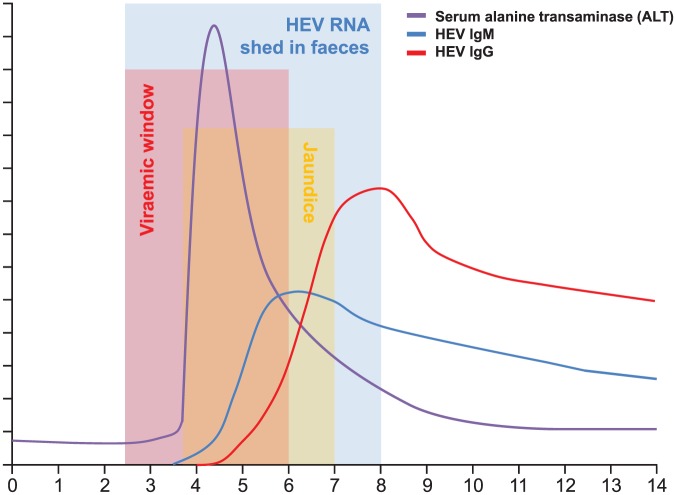
HEV has an incubation period of between 2 weeks and 8 weeks. During this time viraemia reaches its peak, before sharply declining. In acute infection HEV RNA becomes undetectable around 3 weeks after clinical symptoms appear, with the virus continuing to be shed in the stool for another 1–2 weeks.111,112 The initial immune response involves a rise in anti-HEV IgM, which remains detectable for 3–12 months.113 This is superseded by a more sustained IgG response, which peaks around 4 weeks later than IgM and remains detectable for more than 1 year113 (Figure 1).
Figure 1.
HEV viral detection in blood and stool: serological and biochemical response to acute HEV infection over time. ALT, alanine transaminase; HEV, hepatitis E virus.
The assays that are available to diagnose HEV infection do so either indirectly or directly. Serological assays indirectly detect infection via the host immune response, and can help characterize the phase of HEV infection. Wide variation in the sensitivity and specificity of commercially available serological assays exists,114 and the previous ‘gold standard’ test substantially underestimated seroprevalence in comparison to newer assays.15 Also, in chronically infected, immunocompromised patients anti-HEV antibodies are often undetectable. Molecular analysis can be used to directly detect HEV RNA in blood or stool. These techniques offer a more reliable diagnostic assay, but have obvious limitations in terms of cost. In addition, as outlined previously, in cases of acute infection the viraemic window can be very narrow. Taken together, these diagnostic hurdles not only impair the clinical care of individual patients, but also impact our understanding of the significance, prevalence, transmission and epidemiology of HEV.
Routes of transmission and epidemiology of HEV
HEV1 and HEV2 are both restricted to humans and are primarily spread faecal–orally via contaminated water. In hyperendemic regions with poor sanitation there are regular, large-scale epidemics resulting in significant morbidity and mortality. This includes most of southern Asia,116–118 parts of Africa,119 rural areas of China, particularly the remote Xinjiang region,42,120 and several Latin American countries.121 However, outbreaks do not occur year on year in the same geographical region. Instead they appear periodically, despite the perennial presence of faecal contamination in the water supply. It has been suggested that this pattern is the result of a cohort effect.122 Anti-HEV IgG seropositivity increases by as much as five times following an epidemic. Over the following years there is a decline in IgG seroprevalence, until it reaches a critical level where it no longer offers herd immunity and another epidemic occurs.122
Direct person-to-person transmission via the faecal–oral route has been suggested as an additional factor contributing to both epidemic and sporadic cases of HEV. A large outbreak in Uganda in 2007–2008 has been proposed as an example of an epidemic spread via this route of transmission.123,124 The prolonged epidemic curve, high secondary attack rate within households and lack of an identified common source of infection were identified as evidence of person-to-person transmission.123
Parenteral transmission of HEV via blood products has been reported in several countries.125–131 In line with this, numerous studies have found healthy blood donors to be viraemic at the time of donation.126,132–139 The rates of viraemia amongst blood donors vary widely from country to country, ranging from 1:27 in India126 to 1:74,131 in Australia.138 A Canadian study did not find any evidence of viraemia in nearly 14,000 blood donors.140 The extent to which transfusion-related HEV infections contribute to the overall burden of disease is unclear.141 However, many of the patients who are most likely to receive blood products are also those at most risk of chronic infection or severe acute hepatitis E. This includes pregnant women, with liver disease, transplant recipients and other immunosuppressed patients. In view of this, several European countries, including UK, Ireland, the Netherlands and Switzerland, now routinely screen donated blood for HEV.142
The routes of transmission which are particular to industrialized regions add a further layer of complexity. Both HEV3 and HEV4 are zoonotic infections. The primary host species is thought to be pigs,143 but the virus has been found in a range of mammalian species including wild boar,144 deer145 and rabbits.146 Prevalence of antibodies to HEV exceeds 90% in UK pigs, and around 20% have evidence of active infection at the time of slaughter.147 The virus is apathogenic in pigs,148 making identification of infected animals difficult. It is thought that consumption of infected meat is the most important vector for HEV3 and HEV4, and the virus has been isolated from pork products at the point of sale.149 Other food stuffs such as shellfish150 and arable crops151–153 have also been implicated.
The evidence suggests that locally acquired HEV infection is actually very common in richer nations; seroprevalence data and studies of blood donors suggest that at least two million individuals are infected in Europe each year.8 The incidence of HEV infection has been observed to vary both between and within countries, and also changes over time. It is not currently understood why this is the case. In France the overall incidence is higher than in many other European countries, but is not uniformly distributed throughout the country. The incidence in France ranges from 0.4% to 4.6%, with the highest rates found in the southwest and southeast, to the extent that these regions are considered hyperendemic.154 There are other hotspots of HEV infection throughout the continent, including the Netherlands,155 western Germany,156 Czech Republic,49 western/central Poland157 and central Italy.158 In some European countries there has been a decline in HEV seroprevalence, suggesting a cohort effect where the incidence of infection was highest in the mid-20th century but has since been in decline.159,160 At the same time, the number of laboratory-confirmed cases has increased significantly across the continent.49 This to some degree reflects improvements in case ascertainment as awareness of HEV is raised, but that is not the whole story. There have also been substantial increases in incidence in several countries. For example, in Scotland the number of viraemic blood donors has risen from 1:14,500135 to 1:2481161 in the space of 5 years. Over the same period there has also been a shift in the source of human HEV infection in Scotland. In the past the strains of HEV identified in humans in Scotland shared sequence homology with the strain found in local pig herds. More recent infections, by contrast, have been found to involve HEV strains more closely related to those found in pigs from continental Europe. The obvious implication of this is that there has been some change leading to an increased amount of contaminated pork products entering the UK food chain from Europe.162
Treatment
The majority of cases of acute HEV infection do not require any specific treatment. However, a subset of patients may progress to hepatic failure or develop serious extrahepatic sequelae. This raises the question of whether disease progression can be prevented, or ameliorated, with antiviral therapy. In both hepatitis B and C early treatment with antivirals has been shown to improve the natural history of the condition and produce more favourable outcomes.163–165 A small number of patients with hepatitis E in both Europe and Asia have been treated with ribavirin.166–169 Following treatment liver enzyme levels quickly returned to normal and viral clearance was achieved. However, these results must be interpreted with caution due to the lack of controls and the substantial heterogeneity in the treatment regimens employed.
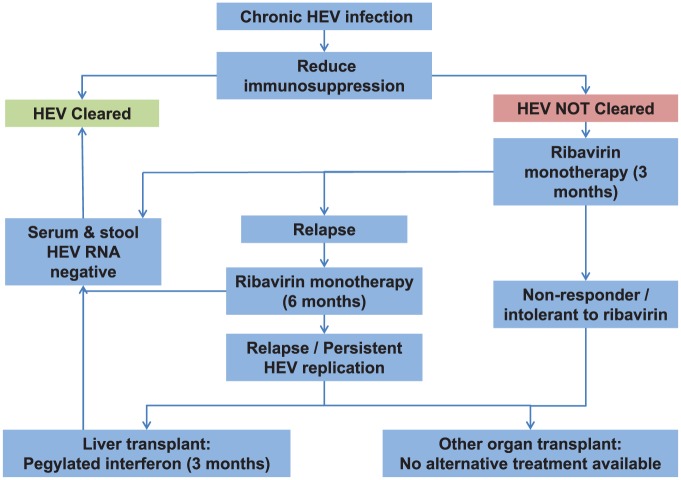
In the context of immunosuppression following solid-organ transplant, the first-line treatment of chronic HEV infection should be a reduction of immunosuppressive drugs, particularly those which target T cells.61,63 Ideally, this should be done as soon after diagnosis as is practicable; this will lead to viral clearance in around 33% of patients.63 For patients who still have active HEV replication after 3 months, ribavirin may be of benefit (Figure 2). A cohort of 59 French solid-organ transplant recipients with chronic hepatitis E was treated with ribavirin.170 The duration of treatment ranged between 1 month and 18 months; 66% of patients were treated for less than 3 months. HEV clearance was achieved in 95% of cases, and 78% had a sustained virologic response (SVR). Four patients who relapsed achieved SVR after a further period of treatment.169
Figure 2.
Treatment algorithm for chronic HEV infection in solid-organ transplant recipients (adapted from Dalton, et al.8 ) HEV, hepatitis E virus.
Ribavirin is effective against a wide range of RNA viruses. As such, several antiviral mechanisms have been proposed, one of which is lethal mutagenesis.171 RNA virus intra-host populations have high levels of variation due to rapid RNA replication and the lack of proof-reading capability in RNA-dependent RNA polymerase.172 This means that these viruses run close to the genomic error threshold, the point at which the burden of mutation is incompatible with transmission of the virus’ master sequence.173 Ribavirin is incorporated into newly synthesized viral RNA, introducing mutations.171 By increasing the error rate, ribavirin pushes the virus over this threshold and induces extinction.173 Ribavirin has been observed to exert this effect on HEV.174–177 Higher rates of mutation increase the likelihood of acquiring mutations associated with the development of hepatic failure, progression to chronic infection and reduced immunoreactivity.178 Mutations which confer resistance to ribavirin have also been reported.174–177 It has been suggested that selection of such variants which increase viral fitness may explain the treatment failure seen in some patients.172
Pegylated interferon-α (IFNα) has been used to good effect in a small number of chronically infected liver transplant recipients,179,180 and one haemodialysis patient.181 In general IFNα is not recommended in patients who have received transplants however, because the risk of acute rejection is increased. In other immunocompromised patients, this is not an issue. A handful of case reports and small case series have described effective treatment with IFNα, ribavirin and a combination of the two in patients with haematological malignancies182–184 being treated with chemotherapy and HIV-positive patients.82,185,186
Prevention
In endemic regions where most infections are waterborne the primary prevention strategy involves improving sanitation and drinking water facilities.187,188 It has been shown that failure to take action to sterilize drinking water during epidemics is associated with larger scale outbreaks.189 In areas where zoonotic transmission is predominant infection can be prevented by ensuring proper preparation of food products. Meat products, particularly pork and game, should be cooked thoroughly.190,191 At-risk individuals, such as pregnant women, those with pre-existing liver disease and immunosuppressed individuals should take particular care and avoid uncooked meat. Individuals who work with pigs, wild boar, game and their products should take steps to minimize direct contact and use protective equipment.192
A highly effective vaccine has been commercially available in China for almost 6 years.193 This vaccine has been designed to offer long-term protection against all HEV genotypes.194 However, it is not yet licensed for use in the rest of the world, pending phase IV trials to determine its safety in children, the elderly, immunosuppressed patients and individuals with pre-existing liver disease.195
Research questions
There are many remaining questions regarding HEV.
(1) What other animals are reservoirs for HEV infection, and what implications do they have for human health?
(2) Where are the ‘hot-spots’ of animal and human HEV located?
(3) How have these changed over time?
(4) Why does the force of infection vary between countries?
(5) Why does the force of infection vary within countries?
(6) How does HEV cause neurological injury?
(7) What other extrahepatic manifestations can HEV infection cause?
(8) Should all blood donors be screened for HEV?
(9) What is the role of HEV3 and 4 in adverse outcomes in pregnancy?
(10) How should we treat patients with chronic HEV infection who are unresponsive or intolerant to ribavirin?
(11) What is the burden of disease caused by HEV in developed countries?
One interesting possibility is the issue of whether HEV3 and HEV4 could have an adverse outcome in pregnancy. Vertical transmission from an infected mother to the foetus is an important route of HEV infection in Asia and Africa. Mother-to-child transmission rates in excess of 75% have been reported, leading to substantial foetal and perinatal morbidity and mortality.196,197 Thus far, this route of transmission has only been associated with HEV1 and HEV2; no cases have been reported with HEV3 or HEV4.198 However, a recent in vitro study demonstrated that HEV3 can replicate on placenta-derived human cell lines.37 In addition, rabbit HEV (rHEV), which is closely related to HEV3,199 has been linked to increased rates of stillbirth and miscarriage amongst experimentally infected animals.200 It has also been demonstrated that rHEV can be transmitted vertically.201 While no cases of vertically transmitted HEV3 have been reported, the possibility cannot be excluded. At present, the causes of miscarriage are often unknown, but infections are implicated in up to 15% of early miscarriages (< 12 weeks) and 66% of late miscarriages (12–24 weeks202). Subclinical and occult infections are similarly thought to play an important role in both spontaneous preterm labour and preterm premature rupture of the membranes.203 Importantly, up to 25% of stillbirths are unexplained by current investigations.204 As mentioned previously, HEV infection is often subclinical or presents atypically, and the seroprevalence in many developed nations is much higher than was previously thought.114 The role of HEV infection in adverse pregnancy outcomes warrants further study.
Conclusion
It is increasingly clear that we have been slow to recognize the threat posed by HEV. A range of factors have contributed to this. Unreliable assays have led to both misdiagnosis and underestimates of disease burden, particularly in developed nations. As a result there is a lack of awareness, knowledge and recognition of this disease amongst clinicians. We still do not fully understand the lifecycle of the virus, not least because of the longstanding difficulties experienced in developing a reliable in vitro model. The substantial heterogeneity in clinical presentation between geographical locations, genotypes and host demographic groups pose significant challenges both in terms of diagnosis and in understanding the natural history of the disease. Similarly, the complex web of transmission routes and variation in incidence and prevalence in different regions and over time makes understanding the epidemiology of HEV extremely difficult.
It is vital that we continue to improve recognition and diagnosis of HEV, by increasing both awareness and knowledge of the disease amongst clinicians. In order to achieve this we must continue to elucidate the clinical phenotype of HEV infection, particularly the myriad extrahepatic manifestations with which it has been linked. Similarly, we must improve our understanding of how HEV is transmitted, especially the zoonotic strains primarily seen in developed countries. Only then will we be able to fully appreciate the emerging challenge of HEV.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Glynn W. Webb, University of Manchester NHS Foundation Trust, 7 Radnor Rd London NW6 6TT Manchester, UK
Harry R. Dalton, Truro, Cornwall, UK.
References
- 1. Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med 1980; 68: 816–824. [DOI] [PubMed] [Google Scholar]
- 2. Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983; 20: 23–31. [DOI] [PubMed] [Google Scholar]
- 3. Reyes GR, Purdy MA, Kim JP, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990; 247: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 4. Tam AW, Smith MM, Guerra ME, et al. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 1991; 185: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rein DB, Stevens GA, Theaker J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012; 55: 988–997. [DOI] [PubMed] [Google Scholar]
- 6. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Hepatitis E: fact sheet. http://www.who.int/mediacentre/factsheets/fs280/en/ (2018, accessed 29 October 2018).
- 8. Dalton HR, Kamar N, Baylis SA, et al. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018; 68: 1256–1271. [DOI] [PubMed] [Google Scholar]
- 9. Kamar N, Bendall R, Legrand-Abravenal F, et al. Hepatitis E. Lancet 2012; 379: 2477–2488. [DOI] [PubMed] [Google Scholar]
- 10. Naidu SS, Viswanathan R. Infectious hepatitis in pregnancy during Delhi epidemic. Indian J Med Res 1957; 45: 71–76. [PubMed] [Google Scholar]
- 11. Purcell RH, Emerson SU. Prevention. In: Thomas HC, Lemon S, Zuckerman AJ. (eds) Viral hepatitis. 3rd ed. Malden, MA: Blackwell Publishing, 2005, pp.635–645. [Google Scholar]
- 12. Clayson ET, Vaughn DW, Innis BL, et al. Association of hepatitis E virus with an outbreak of hepatitis at a military training camp in Nepal. J Med Virol 1998; 54: 178–182. [DOI] [PubMed] [Google Scholar]
- 13. Clayson ET, Shrestha MP, Vaughn DW, et al. Rates of hepatitis E virus infection and disease among adolescents and adults in Kathmandu, Nepal. J Infect Dis 1997; 176: 763–766. [DOI] [PubMed] [Google Scholar]
- 14. Aggarwal R, Naik SR. Epidemiology of hepatitis E: past, present and future. Trop Gastroenterol 1997; 18: 49–56. [PubMed] [Google Scholar]
- 15. Kmush BL, Labrique AB, Dalton HR, et al. Two generations of “gold standards”: the impact of a decade in hepatitis E virus testing innovation on population seroprevalence. Am J Trop Med Hyg 2015; 93: 714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansuy JM, Peron JM, Abravanel F, et al. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol 2004; 74: 419–424. [DOI] [PubMed] [Google Scholar]
- 17. Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol 2008; 20: 784–790. [DOI] [PubMed] [Google Scholar]
- 18. Wichmann O, Schimanski S, Koch J, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis 2008; 198: 1732–1741. [DOI] [PubMed] [Google Scholar]
- 19. Tsang TH, Denison EK, Williams HV, et al. Acute hepatitis E infection acquired in California. Clin Infect Dis 2000; 30: 618–619. [DOI] [PubMed] [Google Scholar]
- 20. Dalton HR, Fellows HJ, Gane EJ, et al. Hepatitis E in New Zealand. J Gastroenterol Hepatol 2007; 22: 1236–1240. [DOI] [PubMed] [Google Scholar]
- 21. Yapa CM, Furlong C, Rosewell A, et al. First reported outbreak of locally acquired hepatitis E virus infection in Australia. Med J Aust 2016; 204: 274. [DOI] [PubMed] [Google Scholar]
- 22. Mizuo H, Yazaki Y, Sugawara K, et al. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol 2005; 76: 341–349. [DOI] [PubMed] [Google Scholar]
- 23. Mitsui T, Tsukamoto Y, Hirose A, et al. Distinct changing profiles of hepatitis A and E virus infection among patients with acute hepatitis, patients on maintenance hemodialysis and healthy individuals in Japan. J Med Virol 2006; 78: 1015–1024. [DOI] [PubMed] [Google Scholar]
- 24. Okamoto H. Hepatitis E virus cell culture models. Virus Res 2011; 161: 65–77. [DOI] [PubMed] [Google Scholar]
- 25. Shukla P, Nguyen HT, Faulk K, et al. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depends on an inserted human gene segment acquired by recombination. J Virol 2012; 86: 5697–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith DB, Simmonds P, Jameel S, et al. ; International Committee on Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J Gen Virol 2014; 95: 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Purdy MA, Harrison TJ, Jameel S, et al. ICTV virus taxonomy profile: hepeviridae. J Gen Virol 2017; 98: 2645–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee GH, Tan BH, Teo EC, et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016; 150: 355–357, e353. [DOI] [PubMed] [Google Scholar]
- 29. Debing Y, Moradpour D, Neyts J, et al. Update on hepatitis E virology: implications for clinical practice. J Hepatol 2016; 65: 200–212. [DOI] [PubMed] [Google Scholar]
- 30. Takahashi M, Yamada K, Hoshino Y, et al. Monoclonal antibodies raised against the ORF3 protein of hepatitis E virus can capture HEV particles in culture supernatant and serum but not those in feces. Arch Virol 2008; 153: 1703–1713. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi M, Tanaka T, Takahashi H, et al. Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol 2010; 48: 1112–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yin X, Li X, Feng Z. Role of envelopment in the HEV life cycle. Viruses 2016; 8: E229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van de Garde MD, Pas SD, Van der Net G, et al. Hepatitis E virus (HEV) genotype 3 infection of human liver chimeric mice as a model for chronic HEV Infection. J Virol 2016; 90: 4394–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sayed IM, Verhoye L, Cocquerel L, et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut 2016; 66: 920–929. [DOI] [PubMed] [Google Scholar]
- 35. Yin X, Ambardekar C, Lu Y, et al. Distinct entry mechanisms for non-enveloped and quasi-enveloped hepatitis E virus. J Virol 2016; 90: 4232–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamar N, Marion O, Abravanel F, et al. Extrahepatic manifestations of hepatitis E virus. Liver Int 2016; 36: 467–472. [DOI] [PubMed] [Google Scholar]
- 37. Knegendorf L, Drave SA, Dao Thi VL, et al. Hepatitis E virus replication and interferon responses in human placental cells. Hepatol Commun 2018; 2: 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drave SA, Debing Y, Walter S, et al. Extra-hepatic replication and infection of hepatitis E virus in neuronal-derived cells. J Viral Hepat 2016; 23: 512–521. [DOI] [PubMed] [Google Scholar]
- 39. Nagashima S, Jirintai S, Takahashi M, et al. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J Gen Virol 2014; 95: 2166–2175. [DOI] [PubMed] [Google Scholar]
- 40. Zhou X, Huang F, Xu L, et al. Hepatitis E virus infects neurons and brains. J Infect Dis 2017; 215: 1197–1206. [DOI] [PubMed] [Google Scholar]
- 41. Huang F, Long F, Wenhai Y, et al. High prevalence of hepatitis E virus in semen of infertile male and causes testis damage. Gut 2018; 67: 1199–1201. [DOI] [PubMed] [Google Scholar]
- 42. Zhuang H, Cao XY, Liu CB, et al. Epidemiology of hepatitis E in China. Gastroenterol 1991; 26: 135–138. [DOI] [PubMed] [Google Scholar]
- 43. Aggarwal R, Kumar R, Pal R, et al. Role of travel as a risk factor for hepatitis E virus infection in a disease-endemic area. Indian J Gastroenterol 2002; 21: 14–18. [PubMed] [Google Scholar]
- 44. Kumar AS, Kumar SP, Singh R, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol 2007; 46: 387–394. [DOI] [PubMed] [Google Scholar]
- 45. Teshale EH, Howard CM, Grytdal SP, et al. Hepatitis E epidemic, Uganda. Emerg Infect Dis 2010; 16: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharapov MB, Favorov MO, Yashina TL, et al. Acute viral hepatitis morbidity and mortality associated with hepatitis E virus infection: Uzbekistan surveillance data. BMC Infect Dis 2009; 9: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumar A, Beniwal M, Kar P, et al. Hepatitis E in pregnancy. Int J Gynaecol Obstet 2004; 85: 240–244. [DOI] [PubMed] [Google Scholar]
- 48. Tsega E, Krawczynski K, Hansson BG, et al. Hepatitis E virus infection in pregnancy in Ethiopia. Ethiop Med J 1993; 31: 173–181. [PubMed] [Google Scholar]
- 49. Adlhoch C, Avellon A, Baylis SA, et al. Hepatitis E virus: assessment of the epidemiological situation in humans in Europe, 2014/15. J Clin Virol 2016; 82: 9–16. [DOI] [PubMed] [Google Scholar]
- 50. Dalton HR, Bendall RP, Rashid M, et al. Host risk factors and autochthonous hepatitis E infection. Eur J Gastroenterol Hepatol 2011; 23: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 51. Manka P, Bechmann LP, Coombes JD, et al. Hepatitis E virus infection as a possible cause of acute liver failure in Europe. Clin Gastroenterol Hepatol 2015; 13: 1836–1842. [DOI] [PubMed] [Google Scholar]
- 52. Blasco-Perrin H, Madden RG, Stanley A, et al. Hepatitis E virus in patients with decompensated chronic liver disease: a prospective UK/French study. Aliment Pharmacol Ther 2015; 42: 574–581. [DOI] [PubMed] [Google Scholar]
- 53. Haim-Boukobza S, Coilly A, Sebagh M, et al. Hepatitis E infection in patients with severe acute alcoholic hepatitis. Liver Int 2015; 35: 870–875. [DOI] [PubMed] [Google Scholar]
- 54. Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis 2010; 51: 328–334. [DOI] [PubMed] [Google Scholar]
- 55. Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008; 358: 811–817. [DOI] [PubMed] [Google Scholar]
- 56. Kamar N, Mansuy JM, Cointault O, et al. Hepatitis E virus-related cirrhosis in kidney- and kidney pancreas-transplant recipients. Am J Transplant 2008; 8: 1744–1748. [DOI] [PubMed] [Google Scholar]
- 57. Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med 2008; 358: 859–886. [DOI] [PubMed] [Google Scholar]
- 58. Colson P, Kaba M, Moreau J, et al. Hepatitis E in an HIV-infected patient. J Clin Virol 2009; 45: 269–271. [DOI] [PubMed] [Google Scholar]
- 59. Dalton HR, Bendall RP, Keane FE, et al. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med 2009; 361: 1025–1027. [DOI] [PubMed] [Google Scholar]
- 60. Geng Y, Zhang H, Huang W, et al. Persistent hepatitis E virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat Mon 2014; 14: e15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011; 140: 1481–1489. [DOI] [PubMed] [Google Scholar]
- 62. Pischke S, Stiefel P, Franz B, et al. Chronic hepatitis E in heart transplant recipients. Am J Transplant 2012; 12: 3128–3133. [DOI] [PubMed] [Google Scholar]
- 63. Kamar N, Abravanel F, Selves J, et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation 2010; 89: 353–360. [DOI] [PubMed] [Google Scholar]
- 64. Thapa R, Biswas B, Mallick D. Henoch-Schonlein purpura triggered by acute hepatitis E virus infection. J Emerg Med 2010; 39: 218–219. [DOI] [PubMed] [Google Scholar]
- 65. Dumoulin FL, Liese H. Acute hepatitis E virus infection and autoimmune thyroiditis: yet another trigger? BMJ Case Rep 2012; pii: bcr1220115441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Belbezier A, Deroux A, Sarrot-Reynauld F, et al. Myasthenia gravis associated with acute hepatitis E infection in immunocompetent woman. Emerg Infect Dis 2014; 20: 908–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dalton HR, Kamar N, van Eijk JJ, et al. Hepatitis E virus and neurological injury. Nat Rev Neurol 2016; 12: 77–85. [DOI] [PubMed] [Google Scholar]
- 68. Geurtsvankessel CH, Islam Z, Mohammad QD, et al. Hepatitis E and Guillain–Barré syndrome. Clin Infect Dis 2013; 57: 1369–1370. [DOI] [PubMed] [Google Scholar]
- 69. Van den Berg B, Walgaard C, Drenthen J, et al. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 2014; 10: 469–482. [DOI] [PubMed] [Google Scholar]
- 70. Jacobs BC, Rothbarth PH, Van der Meché FG, et al. The spectrum of antecedent infections in Guillain–Barré syndrome: a case-control study. Neurology 1998; 51: 1110–1115. [DOI] [PubMed] [Google Scholar]
- 71. Sood A, Midha V, Sood N. Guillain-Barre syndrome with acute hepatitis E. Am J Gastroenterol 2000; 95: 3667–3668. [DOI] [PubMed] [Google Scholar]
- 72. Oomes PG, Van der Meche FG, Kleyweg RP. Liver function disturbances in Guillain-Barré syndrome: a prospective longitudinal study in 100 patients. Dutch Guillain-Barré study group. Neurology 1996; 46: 96–100. [DOI] [PubMed] [Google Scholar]
- 73. Van den Berg B, van der Eijk AA, Pas SD, et al. Guillain-Barre syndrome associated with preceding hepatitis E virus infection. Neurology 2014; 82: 491–497. [DOI] [PubMed] [Google Scholar]
- 74. Fukae J, Tsugawa J, Ouma S, et al. Guillain-Barre and Miller Fisher syndromes in patients with anti-hepatitis E virus antibody: a hospital-based survey in Japan. Neurol Sci 2016; 37: 1849–1851. [DOI] [PubMed] [Google Scholar]
- 75. Stevens O, Claeys KG, Poesen K, et al. Diagnostic challenges and clinical characteristics of hepatitis E virus-associated Guillain-Barre syndrome. JAMA Neurol 2017; 74: 26–33. [DOI] [PubMed] [Google Scholar]
- 76. Feinberg JH, Radecki J. Parsonage-Turner syndrome. HSS J 2010; 6: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Eijk JJ, Madden RG, van der Eijk AA, et al. Neuralgic amyotrophy and hepatitis E virus infection. Neurology 2014; 82: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van Eijk JJJ, Dalton HR, Ripellino P, et al. Clinical phenotype and outcome of hepatitis E virus-associated neuralgic amyotrophy. Neurology 2017; 89: 909–917. [DOI] [PubMed] [Google Scholar]
- 79. Dalton HR, Van Eijk JJJ, Cintas P, et al. Hepatitis E virus infection and acute non-traumatic neurological injury: a prospective multicentre study. J Hepatol 2017; 67: 925–932. [DOI] [PubMed] [Google Scholar]
- 80. Kamar N, Izopet J, Cintas P, et al. Hepatitis E virus-induced neurological symptoms in a kidney-transplant patient with chronic hepatitis. Am J Transplant 2010; 10: 1321–1324. [DOI] [PubMed] [Google Scholar]
- 81. Kamar N, Bendall RP, Peron JM, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis 2011; 17: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dalton H, Keane F, Bendall R, et al. Treatment of chronic hepatitis E in a HIV positive patient. Ann Intern Med 2011; 155: 479–480. [DOI] [PubMed] [Google Scholar]
- 83. Despierres LA, Kaphan E, Attarian S, et al. Neurologic disorders and hepatitis E, France, 2010. Emerg Infect Dis 2011; 17: 1510–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. de Vries MA, Samijn JP, De Man R, et al. Hepatitis E associated encephalopathy in a renal transplant recipient. BMJ Case Rep 2014; 2014: bcr2014204244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Deroux A, Brion JP, Hyerle L, et al. Association between hepatitis E and neurological disorders: two case studies and literature review. J Clin Virol 2014; 60: 60–62. [DOI] [PubMed] [Google Scholar]
- 86. Kejariwal D, Roy S, Sarkar N. Seizure associated with acute hepatitis E. Neurology 2001; 57: 1935. [DOI] [PubMed] [Google Scholar]
- 87. Mandal K, Chopra N. Acute transverse myelitis following hepatitis E virus infection. Indian Pediatr 2006; 43: 365–366. [PubMed] [Google Scholar]
- 88. Joshi GG, Sircar S, Jain AK, et al. Acute viral hepatitis E and Japanese encephalitis: an unusual co-occurrence. Indian J Gastroenterol 2007; 26: 102–103. [PubMed] [Google Scholar]
- 89. Chen XD, Zhou YT, Zhou JJ, et al. Guillain–Barré syndrome and encephalitis/encephalopathy of a rare case of Northern China acute severe hepatitis E infection. Neurol Sci 2014; 35: 1461–1463. [DOI] [PubMed] [Google Scholar]
- 90. Maddukuri VC, Russo MW, Ahrens WA, et al. Chronic hepatitis E with neurologic manifestations and rapid progression of liver fibrosis in a liver transplant recipient. Dig Dis Sci 2013; 58: 2413–2416. [DOI] [PubMed] [Google Scholar]
- 91. Ali G, Kumar M, Bali S, et al. Hepatitis E associated immune thrombocytopenia and membranous glomerulonephritis. Indian J Nephrol 2001; 11: 70–72. [Google Scholar]
- 92. Taton B, Moreau K, Lepreux S, et al. Hepatitis E virus infection as a new probable cause of de novo membranous nephropathy after kidney transplantation. Transpl Infect Dis 2013; 15: E211–E215. [DOI] [PubMed] [Google Scholar]
- 93. Del Bello A, Guilbeau-Frugier C, Josse AG, et al. Successful treatment of hepatitis E virus-associated cryoglobulinemic membranoproliferative glomerulonephritis with ribavirin. Transpl Infect Dis 2015; 17: 279–283. [DOI] [PubMed] [Google Scholar]
- 94. Guinault D, Ribes D, Delas A, et al. Hepatitis E virus-induced cryglobulinemic glomerulonephritis in a non-immunocompromised person. Am J Kidney Dis 2016; 67: 660–663. [DOI] [PubMed] [Google Scholar]
- 95. Kamar N, Weclawiak H, Guilbeau-Frugier C, et al. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation 2012; 93: 617–623. [DOI] [PubMed] [Google Scholar]
- 96. Bulang T, Porst H. [Hepatitis E after travel to India–2 case reports] Hepatitis E nach Indienaufenthalt–zwei Fallberichte. Z Gastroenterol 2000; 38: 249–253. [DOI] [PubMed] [Google Scholar]
- 97. Fourquet E, Mansuy JM, Bureau C, et al. Severe thrombocytopenia associated with acute autochthonous hepatitis E. J Clin Virol 2010; 48: 73–74. [DOI] [PubMed] [Google Scholar]
- 98. Singh NK, Gangappa M. Acute immune thrombocytopenia associated with hepatitis E in an adult. Am J Hematol 2007; 82: 942–943. [DOI] [PubMed] [Google Scholar]
- 99. Thapa R, Mallick D, Ghosh A. Childhood hepatitis E infection complicated by acute immune thrombocytopenia. J Pediatr Hematol Oncol 2009; 31: 151. [DOI] [PubMed] [Google Scholar]
- 100. Masood I, Rafiq A, Majid Z. Hepatitis E presenting with thrombocytopaenia. Trop Doct 2014; 44: 219–220. [DOI] [PubMed] [Google Scholar]
- 101. Colson P, Payraudeau E, Leonnet C, et al. Severe thrombocytopenia associated with acute hepatitis E virus infection. J Clin Microbiol 2008; 46: 2450–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. de Almeida AJ, Campos-de-Magalhaes M, Antonietti CL, et al. Autoimmune thrombocytopenia related to chronic hepatitis C virus infection. Hematology 2009; 14: 49–58. [DOI] [PubMed] [Google Scholar]
- 103. Mishra P, Mahapatra M, Kumar R, et al. Autoimmune haemolytic anaemia and erythroid hypoplasia associated with hepatitis E. Indian J Gastroenterol 2007; 26: 195–196. [PubMed] [Google Scholar]
- 104. Shah SA, Lal A, Idrees M, et al. Hepatitis E virus-associated aplastic anaemia: the first case of its kind. J Clin Virol 2012; 54: 96–97. [DOI] [PubMed] [Google Scholar]
- 105. Li C, Wang HF. Hepatitis E virus-related acute liver failure associated with pure red cell aplasia. Hepatobiliary Pancreat Dis Int 2011; 10: 557–558. [DOI] [PubMed] [Google Scholar]
- 106. Woolson KL, Forbes A, Vine L, et al. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther 2014; 40: 1282–1291. [DOI] [PubMed] [Google Scholar]
- 107. Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc 2010; 85: 945–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kyle RA, Larson DR, Therneau TM, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med 2018; 378: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Davern TJ, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology 2011; 141: 1665–1672, e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dalton HR, Fellows HJ, Stableforth W, et al. The role of HEV testing in drug-induced liver injury. Aliment Pharmacol Therap 2007; 26: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 111. Chauhan A, Jameel S, Dilawari JB, et al. Hepatitis E virus transmission to a volunteer. Lancet 1993; 341: 149–150. [DOI] [PubMed] [Google Scholar]
- 112. Clayson ET, Myint KS, Snitbhan R, et al. Viremia, fecal shedding, and IgM and IgG responses in patients with hepatitis E. J Infect Dis 1995; 172: 927–933. [DOI] [PubMed] [Google Scholar]
- 113. Huang S, Zhang X, Jiang H, et al. Profile of acute infectious markers in sporadic hepatitis E. PLoS One 2010; 5: e13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hartl J, Otto B, Madden RG, et al. Hepatitis E seroprevalence in Europe: a meta-analysis. Viruses 2016; 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wallace SJ, Webb GW, Madden RG, et al. Investigation of liver dysfunction: who should we test for hepatitis E? Eur J Gastroenterol Hepatol 2017; 29: 215–220. [DOI] [PubMed] [Google Scholar]
- 116. Naik SR, Aggarwal R, Salunke PN, et al. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ 1992; 70: 597–604. [PMC free article] [PubMed] [Google Scholar]
- 117. Srestha A, Lama TK, Karki S, et al. Hepatitis E epidemic, Biratnagar, Nepal, 2014. Emerg Infect Dis 2015; 21: 711–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gurley SE, Hossain MJ, Paul RC, et al. Outbreak of hepatitis E in urban Bangladesh resulting in maternal and perinatal mortality. Clin Infect Dis 2014; 59: 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kim JH, Nelson KE, Panzner U, et al. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect Dis 2014; 14: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Huang RT, Li DR, Wei J, et al. Isolation and identification of hepatitis E virus in Xinjiang, China J Gen Virol 1992; 73: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 121. Fierro NA, Realpe M, Meraz-Medina T, et al. Hepatitis E virus: an ancient hidden enemy in Latin America. World J Gastroenterol 2016; 22: 2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Khuroo MS, Khuroo MS, Khuroo NS, et al. Transmission of HEV in developing countries. Viruses 2016; 8: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Teshale EH, Grytdal SP, Howard C, et al. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin Infect Dis 2010; 50: 1006–1010. [DOI] [PubMed] [Google Scholar]
- 124. Teshale EH, Howard CM, Grytdal SP, et al. Hepatitis E epidemic, Uganda. Emerg Infect Dis 2010; 16(1): 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dalton HR, Saunders M, Woolson KL. Hepatitis E virus in developed countries: one of the most successful zoonotic viral diseases in human history? J Virus Erad 2015; 1: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol 2004; 19: 778–784. [DOI] [PubMed] [Google Scholar]
- 127. Dreier J, Juhl D. Autochthonous hepatitis E virus infections: a new transfusion-associated risk? Transfus Med and Hemother 2014; 41: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Loyrion E, Trouve-Buisson T, Pouzol P, et al. Hepatitis E virus infection after platelet transfusion in an immunocompetent trauma patient. Emerg Infect Dis 2017; 23: 146–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Matsubayashi K, Kang JH, Sakata H, et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion 2008; 48: 1368–1375. [DOI] [PubMed] [Google Scholar]
- 130. Yamazaki Y, Naganuma A. Clinical and virological features of acute hepatitis E in Gunma prefecture, Japan between 2004 and 2015. Hepatol Res 2017; 47: 435–445. [DOI] [PubMed] [Google Scholar]
- 131. Hoad VC, Gibbs T, Ravikumara M, et al. First confirmed case of transfusion-transmitted hepatitis E in Australia. Med J Aus 2017; 206: 289–290. [DOI] [PubMed] [Google Scholar]
- 132. Juhl D, Baylis SA, Blumel J, et al. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion 2014; 54: 49–56. [DOI] [PubMed] [Google Scholar]
- 133. Al-Sadeq DW, Majdalawieh AF, Nasrallah GK. Seroprevalence and incidence of hepatitis E virus among blood donors: a review. Rev Med Virol 2017; 27: e1937. [DOI] [PubMed] [Google Scholar]
- 134. Sauleda S, Ong E, Bes M, et al. Seroprevalence of hepatitis E virus (HEV) and detection of HEV RNA with a transcription-mediated amplification assay in blood donors from Catalonia (Spain). Transfusion 2015; 55: 972–979. [DOI] [PubMed] [Google Scholar]
- 135. Cleland A, Smith L, Crossan C, et al. Hepatitis E virus in Scottish blood donors. Vox Sang 2013; 105: 283–289. [DOI] [PubMed] [Google Scholar]
- 136. Hogema BM, Molier M, Sjerps M, et al. Incidence and duration of hepatitis E virus infection in Dutch blood donors. Transfusion 2016; 56: 722–728. [DOI] [PubMed] [Google Scholar]
- 137. Roth NJ, Schafer W, Alexander R, et al. Low hepatitis E virus RNA prevalence in a large-scale survey of United States source plasma donors. Vox Sang 2017; 57: 2958–2964. [DOI] [PubMed] [Google Scholar]
- 138. Hoad VC, Seed CR, Fryk JJ, et al. Hepatitis E virus RNA in Australian blood donors: prevalence and risk assessment. Vox Sang 2017; 112: 614–621. [DOI] [PubMed] [Google Scholar]
- 139. Vollmer T, Diekmann J, Johne R, et al. Prevalence of hepatitis E virus RNA and progress of asymptomatically hepatitis E infection in German blood donors. Int J Med Microbiol 2012; 302: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Fearon MA, O’Brien SF, Delage G, et al. Hepatitis E in Canadian blood donors. Transfusion 2017; 57: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 141. Goel A, Aggarwal R. Advances in hepatitis E-II: epidemiology, clinical manifestations, treatment and prevention. Expert Rev Gastroenterol Hepatol 2016; 10: 1065–1074. [DOI] [PubMed] [Google Scholar]
- 142. Dreier J, Knabbe C, Vollmer T. Transfusion-transmitted hepatitis E: NAT screening of blood donations and infectious dose. Front Med (Lausanne) 2018; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pavio N, Meng XJ, Renou C. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res 2010; 41: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sonoda H, Abe M, Sugimoto T, et al. Prevalence of hepatitis E virus (HEV) infection in wild boars and deer and genetic identification of a genotype 3 HEV from a boar in Japan. J Clin Microbiol 2004; 42: 5371–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tei S, Kitajima N, Takahashi K, et al. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003; 362: 371–373. [DOI] [PubMed] [Google Scholar]
- 146. Izopet J, Dubois M, Bertagnoli S, et al. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg Infect Dis 2012; 18: 1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Grierson S, Heaney J, Cheney T, et al. Prevalence of hepatitis E virus infection in pigs at the time of slaughter, United Kingdom, 2013. Emerg Infect Dis 2015; 21: 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Halbur PG, Kasorndorkbua C, Gilbert C, et al. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol 2001; 39: 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Berto A, Martelli F, Grierson S, et al. Hepatitis E virus in pork food chain, United Kingdom, 2009–2010. Emerg Infect Dis 2012; 18: 1358–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Grodzki M, Schaeffer J, Piquet JC, et al. Bioaccumulation efficiency, tissue distribution, and environmental occurrence of hepatitis E virus in bivalve shellfish from France. Appl Environ Microbiol 2014; 80: 4269–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kokkinos P, Kozyra I, Lazic S, et al. Harmonised investigation of the occurrence of human enteric viruses in the leafy green vegetable supply chain in three European countries. Food Environ Virol 2012; 4: 179–191. [DOI] [PubMed] [Google Scholar]
- 152. Maunula L, Kaupke A, Vasickova P, et al. Tracing enteric viruses in the European berry fruit supply chain. Int J Food Microbiol 2013; 167: 177–185. [DOI] [PubMed] [Google Scholar]
- 153. Brassard J, Gagne MJ, Genereux M, et al. Detection of human food-borne and zoonotic viruses on irrigated, fieldgrown strawberries. Appl Environ Microbiol 2012; 78: 3763–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mansuy JM, Gallian P, Dimeglio C, et al. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology 2016; 63: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 155. Zaaijer HL. No artifact, hepatitis E is emerging. Hepatology 2015; 62: 654. [DOI] [PubMed] [Google Scholar]
- 156. Muller B, Koch H, Pichl L. PCR-Screening of blood donations for hepatitis E with the cobas HEV test performed on the new Roche cobas 8800 platform in minipools of 6. Transfus Med Hemother 2015; 42: 1–66. [Google Scholar]
- 157. Bura M, Łagiedo M, Michalak M, et al. Hepatitis E virus IgG seroprevalence in HIV patients and blood donors, west-central Poland. Int J Infect Dis 2017; 61: 20–22. [DOI] [PubMed] [Google Scholar]
- 158. Lucarelli C, Spada E, Taliani G, et al. High prevalence of anti-hepatitis E virus antibodies among blood donors in central Italy, February to March 2014. Euro Surveill 2016; 21(30):pii=30299. [DOI] [PubMed] [Google Scholar]
- 159. Ijaz S, Vyse AJ, Morgan D, et al. Indigenous hepatitis E virus infection in England: more common than it seems. J Clin Virol 2009; 44: 272–276. [DOI] [PubMed] [Google Scholar]
- 160. Holm DK, Moessner BK, Engle RE, et al. Declining prevalence of hepatitis E antibodies among Danish blood donors. Transfusion 2015; 55: 1662–1667. [DOI] [PubMed] [Google Scholar]
- 161. Thom K, Gilhooly P, McGowan K, et al. HEV in Scotland: evidence of recent increase in viral circulation in humans. Euro Surveill 2018; 23: pii: 17-00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ijaz S, Said B, Boxall E, et al. Indigenous hepatitis E in England and Wales from 2003 to 2012: evidence of an emerging novel phylotype of viruses. J Infect Dis 2014; 209: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 163. Tillmann H, Patel K, McHutchison J. Hepatitis B virus viral load and treatment decision. Hepatology 2009; 49: 699. [DOI] [PubMed] [Google Scholar]
- 164. Wiegand J, Wedermeyer H, Franke A, et al. Treatment of severe, nonfulminant acute hepatitis B with lamivudine versus placebo: a prospective randomized double-blinded multicentre trial. J Viral Hepat 2014; 21: 744–750. [DOI] [PubMed] [Google Scholar]
- 165. Deterding K, Spinner CD, Schott E, et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis 2017; 17: 215–222. [DOI] [PubMed] [Google Scholar]
- 166. Peron JM, Abravanel F, Guillaume M, et al. Treatment of autochthonous acute hepatitis E with short-term ribavirin: a multicenter retrospective study. Liver Int 2016; 36: 328–333. [DOI] [PubMed] [Google Scholar]
- 167. Gerolami R, Borentain P, Raissouni F, et al. Treatment of severe acute hepatitis E by ribavirin. J Clin Virol 2011; 52: 60–62. [DOI] [PubMed] [Google Scholar]
- 168. Pischke S, Hardtke S, Bode U, et al. Ribavirin treatment of acute and chronic hepatitis E: a single-centre experience. Liver Int 2013; 33: 722–726. [DOI] [PubMed] [Google Scholar]
- 169. Goyal R, Kumar A, Panda SK, et al. Ribavirin therapy for hepatitis E virus-induced acute on chronic liver failure: a preliminary report. Antivir Ther 2012; 17: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 170. Kamar N, Izopet J, Tripon S, et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med 2014; 370: 1111–1120. [DOI] [PubMed] [Google Scholar]
- 171. Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol 2006; 16: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Todt D, Meister TL, Steinmann E. Hepatitis E virus treatment and ribavirin therapy: viral mechanisms of nonresponse. Curr Opin Virol 2018; 32: 80–87. [DOI] [PubMed] [Google Scholar]
- 173. Domingo E, Sheldon J, Perales C. Viral quasispecies evolution. Microbiol Mol Biol Rev 2012; 76: 159–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Debing Y, Ramière C, Dallmeier K, et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J Hepatol 2016; 65: 499–508. [DOI] [PubMed] [Google Scholar]
- 175. Lhomme S, Kamar N, Nicot F, et al. Mutation in the hepatitis E virus polymerase and outcome of ribavirin therapy. Antimicrob Agents Chemother 2015; 60: 1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Debing Y, Gisa A, Dallmeier K, et al. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology 2014; 147: 1008–1011.e7. [DOI] [PubMed] [Google Scholar]
- 177. Todt D, Gisa A, Radonic A, et al. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut 2016; 65: 1733–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ikram A, Hakim MS, Zhou JH, et al. Genotype-specific acquisition, evolution and adaptation of characteristic mutations in hepatitis E virus. Virulence 2018; 9: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kamar N, Rostaing L, Abravanel F, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis 2010; 50: e30–e33. [DOI] [PubMed] [Google Scholar]
- 180. Haagsma EB, Riezebos-Brilman A, van den Berg AP, et al. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl 2010; 16: 474–477. [DOI] [PubMed] [Google Scholar]
- 181. Kamar N, Abravanel F, Garrouste C, et al. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant 2010; 25: 2792–2795. [DOI] [PubMed] [Google Scholar]
- 182. Tavitian S, Peron JM, Huguet F, et al. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies. Emerg Infect Dis 2015; 21: 1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Alric L, Bonnet D, Laurent G, et al. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-α therapy. Ann Intern Med 2010; 153: 135–136. [DOI] [PubMed] [Google Scholar]
- 184. Alric L, Bonnet D, Beynes-Rauzy O, et al. Definitive clearance of a chronic hepatitis E virus infection with ribavirin treatment. Am J Gastroenterol 2011; 106: 1562–1563. [DOI] [PubMed] [Google Scholar]
- 185. Neukam K, Barreiro P, Macias J, et al. Chronic hepatitis E in HIV patients: rapid progression to cirrhosis and response to oral ribavirin. Clin Infect Dis 2013; 57: 465–468. [DOI] [PubMed] [Google Scholar]
- 186. Hajji H, Gerolami R, Solas C, et al. Chronic hepatitis E resolution in a human immunodeficiency virus (HIV)-infected patient treated with ribavirin. Int J Antimicrob Agents 2013; 41: 595–597. [DOI] [PubMed] [Google Scholar]
- 187. Nelson KE, Kmush B, Labrique AB. The epidemiology of hepatitis E virus infections in developed countries and among immunocompromised patients. Expert Rev Anti Infect Ther 2011; 9: 1133–1148. [DOI] [PubMed] [Google Scholar]
- 188. Haque F, Banu SS, Ara K, et al. An outbreak of hepatitis E in an urban area of Bangladesh. J Viral Hepat 2015; 22: 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Naik SR, Aggarwal R, Salunke PN, et al. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ 1992; 70: 597–604. [PMC free article] [PubMed] [Google Scholar]
- 190. Feagins AR, Opriessnig T, Guenette DK, et al. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int J Food Microbiol 2008; 123: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Barnaud E, Rogee S, Garry P, et al. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol 2012; 78: 5153–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Schielke A, Ibrahim V, Czogiel I, et al. Hepatitis E virus antibody prevalence in hunters from a district in Central Germany, 2013: a cross-sectional study providing evidence for the benefit of protective gloves during disembowelling of wild boars. BMC Infect Dis 2015; 15: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010; 376: 895–902. [DOI] [PubMed] [Google Scholar]
- 194. Zhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med 2015; 372: 914–922. [DOI] [PubMed] [Google Scholar]
- 195. World Health Organization. Global advisory committee on vaccine safety, 11–12 June 2014. Wkly Epidemiol Rec 2014; 89: 325–336.25045773 [Google Scholar]
- 196. Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet 1995; 345: 1025–1026. [DOI] [PubMed] [Google Scholar]
- 197. Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat 2009; 16: 519–523. [DOI] [PubMed] [Google Scholar]
- 198. Hartl J, Wehmeyer MH, Pischke S. Acute hepatitis E: two sides of the same coin. Viruses 2016; 8: E299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Cossaboom CM, Cordoba L, Dryman BA, et al. Hepatitis E virus in rabbits, Virginia, USA. Emerg Infect Dis 2011; 17: 2047–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ahn HS, Han SH, Kim YH, et al. Adverse fetal outcomes in pregnant rabbits experimentally infected with rabbit hepatitis E virus. Virology 2017; 512: 187–193. [DOI] [PubMed] [Google Scholar]
- 201. Xia J, Liu L, Wang L, et al. Experimental infection of pregnant rabbits with hepatitis E virus demonstrating high mortality and vertical transmission. J Viral Hepat 2015; 22; 850–857. [DOI] [PubMed] [Google Scholar]
- 202. Giakoumelou S, Wheelhouse N, Cuschieri K, et al. The role of infection in miscarriage. Hum Reprod Update 2015; 22: 116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Frøen JF, Amestad M, Frey K, et al. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986-1995. Am J Obstet Gynecol 2001; 184: 694–702. [DOI] [PubMed] [Google Scholar]