Abstract
Optical coherence tomography angiography is a relatively new, noninvasive technology that has revolutionized imaging of the retinal and choroidal microvasculature. This technology is based on the detection of movement or changes that represent moving red cells in sequential optical coherence tomography scans. As with other established imaging technologies, it has unique benefits as well as certain disadvantages, which include a limited field of view and vulnerability to imaging artifacts. However, software and hardware improvements are continually evolving to mitigate these limitations. Optical coherence tomography angiography has been used to gain a better understanding of microvascular changes across a spectrum of ocular diseases including diabetic retinopathy, age-related macular degeneration, glaucoma, and retinal vein occlusions. In this article, we review algorithms and techniques commonly utilized in optical coherence tomography angiography systems and compare optical coherence tomography angiography to fluorescein angiography, the current gold standard for imaging the retinal vasculature. In addition, we provide an overview of important optical coherence tomography angiography findings in a variety of ocular diseases. Although the clinical role of this technology is still poorly defined, optical coherence tomography angiography has the potential to become an invaluable tool in the diagnosis and monitoring of vascular pathologies.
Keywords: age-related macular degeneration, diabetic retinopathy, optical coherence tomography angiography, retinal imaging, retinal segmentation, retinal vein occlusion
Introduction
Optical coherence tomography (OCT) is a technology that has revolutionized the field of ophthalmology. Introduced in the early 1990s, it has firmly established itself as one of the most prominent and useful imaging modalities over the last several decades. Using noninvasive low-coherence interferometry, OCT provides high-resolution two-dimensional (2D) images of the retinal structure in vivo.1,2 Along with developments in OCT technology such as spectral-domain OCT, improvements in acquisition speed and hardware have facilitated advanced imaging applications such as OCT angiography (OCTA), which would not have been possible with the original OCT technology, time-domain OCT.3
In general, OCTA images blood flow through detecting changes in reflectivity, thought to be related to red blood cell movement, facilitated by scanning the same location over time. Since it was first demonstrated in living patients,4 this promising concept has undergone significant improvements culminating in Food and Drug Administration (FDA) approval of the first commercial OCTA device in 2016.
Despite its infancy, OCTA has all the makings of a paradigm-changing technology for noninvasive ocular imaging. Since it is based on flow motion detection, there is no need for contrast dye injections that can be associated with rare but serious adverse reactions that range from nausea to full anaphylaxis.5,6 In addition, fast acquisition times allow OCTA imaging to be acquired in seconds compared to the 10–30 min required by traditional gold standard imaging such as fluorescein angiography (FA).7 Importantly, OCTA also offers the unique opportunity to resolve the individual retinal plexuses and thereby study the differential involvement of each capillary layer in a number of ocular and systemic diseases.8,9
The goals of this OCTA review article are to provide a brief overview of the various algorithms and techniques utilized in the OCTA devices currently available on the market and to discuss recent and notable OCTA findings in several diseases of the posterior pole.
Principles and techniques
Noninvasive imaging of ocular perfusion using Doppler OCT was first introduced in the mid 1990s.10 While it was a revolutionary concept, this technology suffered from inherent drawbacks including an inability to detect blood flow perpendicular to the OCT beam as well as vulnerability to sample motion.11 As a result, other OCT software algorithms for angiographic imaging were developed over the following decades, including split-spectrum amplitude-decorrelation angiography (SSADA) and optical microangiography (OMAG). These algorithms exploit differences in OCT signal phase, intensity, or a combination of the two in order to generate the angiograms.
SSADA is the algorithm currently utilized in the OCTA device RTVue XR Avanti manufactured by Optovue, Inc. (Fremont, CA, USA), which detects the differences in OCT signal intensity to represent flow.12 As with other OCTA techniques, SSADA relies on the comparison of sequential OCT B-scans for the detection of blood flow. Static tissues are highly correlated, whereas moving erythrocytes cause changes in reflectance between consecutive scans and are therefore highly decorrelated. Uniquely, SSADA splits the spectrum of light into multiple spectral bands, calculates the decorrelation of each band separately, and then averages the results. This leads to an increased signal-to-noise ratio and the ability to produce angiograms using only two consecutive OCT B-scans.3,13 As a result, SSADA has an axial resolution that is approximately three times lower than standard OCT (approximately 15 µm),11,14 but also has a decreased susceptibility to bulk motion.
OMAG is another algorithm that is implemented in the AngioPlex system by Carl Zeiss Meditec AG (Jena, Germany). This approach analyzes both the amplitude and phase differences in the OCT signal to generate the angiograms. One distinct advantage is the use of the entire spectrum without loss of axial resolution, but it has the disadvantage of requiring more than two sequential scans to generate the angiogram.15–17
Briefly, OCTA ratio analysis (OCTARA) is a separate algorithm developed by Topcon Corp (Tokyo, Japan), which uses a ratio method that is not based on amplitude decorrelation. Notably, this approach also does not require splitting of the full spectrum, resulting in preservation of axial resolution.18,19 This algorithm is paired with swept-source OCT (SS-OCT) in the Topcon DRI-OCT Triton SS-OCT system.
Artifact
An important limitation of OCTA is its unique susceptibility to imaging artifacts, which have been characterized by Spaide and colleagues.20 These artifacts include motion artifacts, a result of microsaccades and breathing, and projection artifacts, where flow in the superficial retinal vasculature is projected onto the underlying structures. Projection artifacts appear as long flow tails underneath the superficial vessels on cross-sectional OCTA images and can give the appearance of flow in areas where there should be none.21 These artifacts can often be magnified by hyperreflective material such as hard exudates or pigment migration.22
In general, platforms have integrated real-time eye tracking systems, such as FastTrac™ (Carl Zeiss Meditec AG (Jena, Germany)). and SMARTTrack™ (Topcon Corp.), that compensate for eye movement and blinking,19,23 a concept that was introduced over a decade ago.24 Optovue, Inc. instead utilizes a two-level approach with their DualTrac™ software, which incorporates eye tracking along with an orthogonal registration algorithm called motion correction technology (MCT).23 Their use of SSADA also mitigates effects of movement in the axial direction from microsaccades and pulsations during the cardiac cycle due to the lower axial resolution and subsequent higher signal-to-noise ratio.20
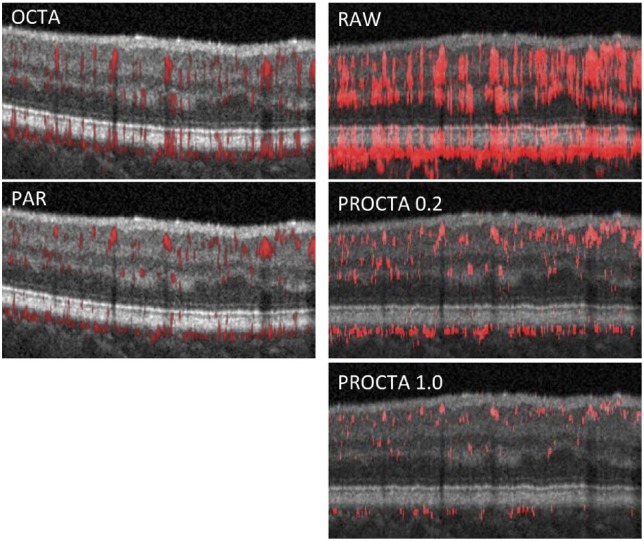
Projection artifact in commercial OCTA systems has typically been addressed using a slab-subtraction algorithm.19,25,26 This technique subtracts the superficial vascular pattern from the outer retina, thereby removing projection artifact but often causing an undesirable negative artifact.21 More recently, a new algorithm called projection-resolved OCTA (PR-OCTA) was introduced by Zhang and colleagues.27 This algorithm interprets high decorrelation-value peaks as real vessels while removing lower decorrelation-value signal as projection artifact and has successfully been used to study the retinal vasculature in healthy and diseased eyes.21,28–30 Larger values of an empiric parameter, α (range between 0 and 1), result in a higher threshold for decorrelation values for interpretation as flow and therefore less artifact. Subsequently, Optovue, Inc. incorporated their own proprietary three-dimensional (3D) projection artifact removal (PAR) technique into their AngioVue software. PAR is distinct from PR-OCTA and has also been shown to be effective in mitigating projection artifact in healthy eyes31 (Figure 1).
Figure 1.
Projection artifact and correction with projection artifact removal (PAR) and projection-resolved OCTA (PR-OCTA). (Top left) Optovue AngioVue OCTA B-scan and (top right) OCTA B-scan with raw angiographic overlay. Note the significant projection artifact visible as long tails of apparent flow projecting from areas of real flow located superficially. (Middle left) default PAR. (Middle right and bottom right) PR-OCTA with increasingly stringent removal of projection artifact (α = 0.2 and 1.0).
OCTA: optical coherence tomography angiography.
Segmentation
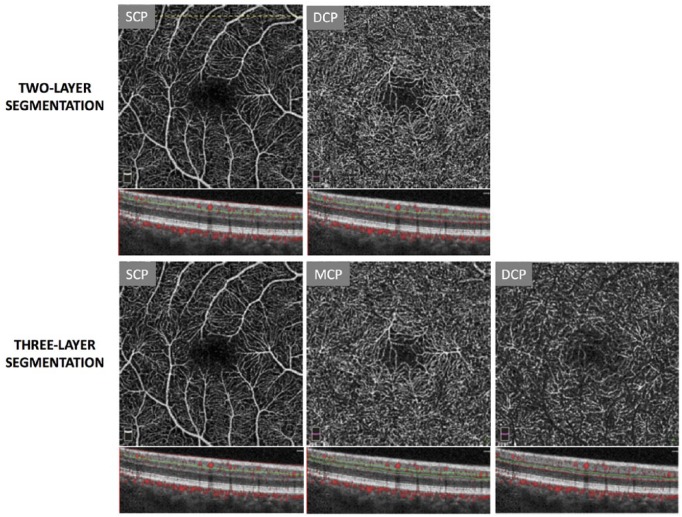
OCTA has sparked interest in the retinal capillary layers because of its unique ability to optically section the distinct networks. More recently, studies using segmentation schemes to separate the inner retinal circulation into three layers have become commonplace (Figure 2). This approach is based on histologic reports that have demonstrated the existence of four distinct capillary planes in the perifoveal retina32 that form three capillary plexuses: the superficial capillary plexus (SCP), middle capillary plexus (MCP), and deep capillary plexus (DCP). The SCP is found in the nerve fiber and ganglion cell layers, the MCP is located between the inner plexiform layer (IPL) and inner nuclear layer (INL), and the DCP is present between the INL and outer plexiform layer (OPL). OCTA has successfully and consistently been used to visualize these three capillary layers,8,21,31,33,34 leading to deeper insights into their structure and connectivity. For example, while there has been debate regarding previously proposed theories of parallel or serial connections between the layers,28,35 Nesper and colleagues36 have found evidence supporting a hybrid model of connectivity using OCTA.
Figure 2.
Inner retinal segmentation schemes in a healthy eye using the Optovue OCTA system. The top row displays en face images and B-scans from default segmentation of the inner retina into the superficial capillary plexus (SCP) and deep capillary plexus (DCP). The dashed line demarcates the location of the B-scans used in the figure. The bottom row displays en face images and B-scans from inner retinal segmentation into the SCP, middle capillary plexus (MCP), and DCP using manufacturer-recommended custom boundaries. Figures are created with images using default Optovue Projection Artifact Removal (PAR; AngioVue Analytics Version 2017.1.0.151).
OCTA: optical coherence tomography angiography.
Other common predefined OCTA segmentation slabs include the outer retina, which is normally an avascular area with any apparent flow being the result of pathology or projection artifact,28 and the choriocapillaris. OCTA offers a rare opportunity to visualize the choriocapillaris in vivo, as it is more difficult to study in detail using FA and indocyanine green angiography (ICGA).37 However, image quality of the choriocapillaris remains limited by low lateral resolution as well as susceptibility to projection artifact and shadowing artifact from overlying pathology such as large drusen.20,38
While automated segmentation is a helpful starting point, manual adjustment of the segmentation lines is often necessary to correct for segmentation errors that are relatively common in the setting of retinal pathology.39
Comparison to FA
Major advantages of OCTA over FA include its quick imaging time, noninvasiveness, and lower cost.14,40 However, FA is a well-established imaging modality that is still the gold standard for multiple conditions including choroidal neovascularization (CNV) and retinal neovascularization (NV).41–43 Benefits of this technology include a wide field of view, visualization of flow from retinal arterioles to venules, and accurate identification of exudates or leakage (which OCTA is unable to detect).
The field of view in FA (50°, 120°, or 200°) is significantly larger than the 3 × 3 mm2 area (~7°) traditionally used in OCTA.44 However, these larger fields of view have lower resolution than 3 × 3 mm2 OCTA scans, making identification of microvasculature dysfunction in conditions such as diabetes more difficult.45,46 In addition, with developments in OCTA hardware and software, larger imaging areas up to 12 × 12 mm2 have become possible with commercial OCTA systems. Techniques like extended field imaging using 20 diopter lenses, as well as image montaging, can increase the field of view even further while preserving detail to become comparable if not superior to that of FA.40,47,48 Furthermore, OCTA has been found to be superior to FA for visualization of the deeper retinal microvasculature.49,50
Study of posterior pole disease
Diabetic retinopathy
Numerous OCTA measures including vessel density, perfusion density, and vessel diameter index have been developed and studied for diabetic retinopathy (DR). Changes in these measures of vascular function have almost universally been shown to correlate with increasing severity of DR and worsening visual acuity, thereby suggesting a promising role for OCTA in the monitoring of disease progression.9,33,45,51–54 In addition, OCTA parameters such as foveal avascular zone (FAZ) area have been reported to be enlarged in diabetic eyes prior to the development of clinically apparent DR, thereby indicating value as a potential screening tool.55 Of note, the derivation of many of these measures requires the use of third-party software such as ImageJ (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) or MATLAB (MathWorks, Natick, MA, USA).
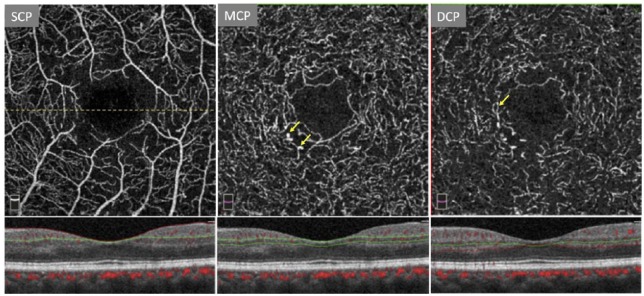
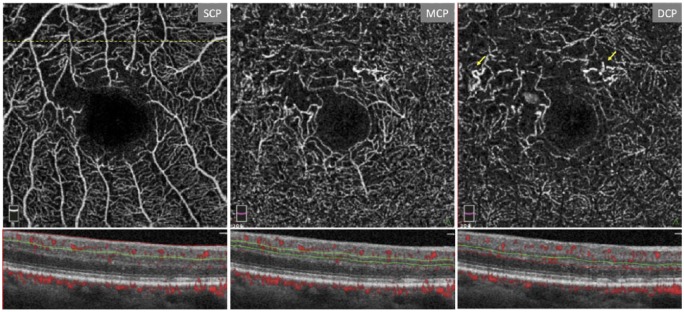
OCTA has been found to be superior to FA in delineating the microvasculature, allowing an improved visualization of capillary dropout and changes in the FAZ.56 However, while Ishibazawa and colleagues have reported that OCTA can be useful in identifying microaneurysms and retinal perfusion deficits, La Mantia and colleagues57 have shown FA to be more effective in visualizing microaneurysms relative to OCTA. This is more consistent with other OCTA reports58,59 and can be explained by the relative insensitivity of OCTA to slow flow60 (Figure 3).
Figure 3.
Three-layer segmentation in a patient with proliferative diabetic retinopathy. The dashed line denotes location of B-scan used in the figure. Note the enlarged foveal avascular zone (FAZ). Arrows mark vascular abnormalities such as microaneurysms and dilated capillary loops. Figure created with images using default Optovue Projection Artifact Removal (PAR; AngioVue Analytics Version 2017.1.0.151).
DCP: deep capillary plexus; MCP: middle capillary plexus; SCP: superficial capillary plexus.
Additional benefits of wide-field OCTA are still being explored by numerous researchers. Sawada and colleagues61 and Hirano and colleagues40 have directly compared wide-field OCTA to FA, showing a high sensitivity and specificity of OCTA for retinal nonperfusion areas (NPAs) and retinal NV. Both studies utilized 12 × 12 mm2 SS-OCTA scans, although the study by Hirano and colleagues was enhanced with extended field imaging to offer an even greater field of view. In the report by Sawada and colleagues, the sensitivity and specificity of OCTA for NPA were 0.98 and 0.82, respectively, with the sensitivity and specificity for NV being 1.0 and 0.97, respectively. Hirano and colleagues reported a sensitivity and specificity of 0.96 and 1.0 for NPA and a sensitivity and specificity of 0.79 and 0.96 for NV, respectively. Separately, Schaal and colleagues62 compared widefield OCTA imaging to color fundus photography and reported an increased detection rate for intraretinal microvascular abnormalities with OCTA.
Age-related macular degeneration
OCTA is an effective tool for the identification of CNV associated with neovascular age-related macular degeneration (AMD),26,63 although reported sensitivities and specificities of OCTA have varied.64 This may be due to differing study distributions of type 1 and type 2 CNV, which appear to be more readily apparent on OCTA than Type 3 CNV and polypoidal complexes.64
Given its high level of image detail, OCTA has been advantageous in the study of the structure of CNV and its changes in response to antiangiogenic agents. Different morphologic appearances of CNV have been described, including ‘medusa’ and ‘sea-fan’ forms, which generally consist of smaller vessels radiating from a larger feeder vessel.65 These larger vessels remain stable despite changes in lesion size with antiangiogenic treatment.64
Multiple studies have been conducted to identify OCTA biomarkers for CNV activity. Souied and colleagues found that active CNV lesions requiring treatment were more likely to have a combination of high-risk features including a well-defined shape (lacy-wheel or sea-fan shaped), branching of many small capillaries, the appearance of anastomoses and loops, the presence of a peripheral arcade at the vessel termini, and the presence of a hypointense halo surrounding the lesion on OCTA.66 In their study of type 1 CNV, Al-Sheikh and colleagues67 similarly found that branching of small vessels and the presence of peripheral arcades were more prevalent in active versus quiescent lesions and also reported that fractal dimension (a measure of complexity of CNV lesions) was significantly different between active and quiescent lesions. Other OCTA indicators of long-term growth of type 1 CNV lesions include the presence of a network of tiny capillaries at the border of the CNV lesion, as well as a lack of mature dilated feeder vessels.68
The ability of OCTA to analyze lesions three-dimensionally may be important in the study of neovascular AMD as well. Recent work by Nesper and colleagues69 demonstrated the novel use of 3D volume-rendered PR-OCTA in the characterization of complex CNV lesions. After examining parameters such as the number of CNV flow layers, they found that increasing complexity of the lesions was associated with poor treatment response to antiangiogenic agents. Further investigation using OCTA could potentially lead to the development of accurate predictors of treatment response in CNV and could validate the use of similar 3D approaches in the study of other diseases.
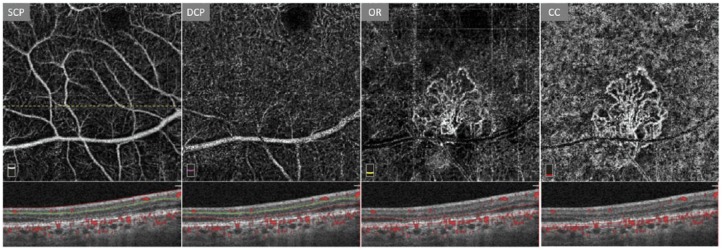
Prior to the availability of OCTA, imaging of subclinical NV (defined as asymptomatic CNV prior to the development of exudation) in dry AMD using ICGA was limited by cost, invasiveness, and an overall limited understanding of the significance of these lesions. More recently, de Oliveira Dias and colleagues70 found that 21.1% of patients with subclinical CNV found on baseline OCTA imaging demonstrated exudation within 1 year, compared to 3.6% of those eyes without subclinical NV at baseline. In general, eyes with subclinical CNV had an approximately 15 times greater risk of exudation than those eyes without NV. As a result, this noninvasive and time-efficient technology may be critical as a tool for screening and monitoring subclinical CNV (Figure 4).
Figure 4.
Default segmentation of an eye with age-related macular degeneration displaying a subclinical choroidal neovascular membrane in the outer retinal slab (OR). Dashed line indicates location of B-scan used in the figure. Note the lack of edema or exudate as well as the overlaid angiographic flow signal within the boundaries of the B-scan. Figures are created with images using default Optovue Projection Artifact Removal (PAR; AngioVue Analytics Version 2017.1.0.151).
CC: choriocapillaris; DCP: deep capillary plexus; SCP: superficial capillary plexus.
OCTA has also been used to study drusen-associated atrophy and geographic atrophy (GA) in dry AMD. Imaging of the choriocapillaris is inherently more difficult due to factors including its location beneath the highly reflective retinal pigment epithelium (RPE).71 However, OCTA reports have consistently demonstrated significant impairment of choriocapillaris perfusion in areas of GA, as well as diffuse choriocapillaris dysfunction outside the margins of atrophy.72,73 Interestingly, using OCTA, Pellegrini and colleagues74 found that the choriocapillaris is still present in regions of RPE loss in GA, but is rarefied.
Retinal vein occlusion
Retinal vein occlusion (RVO) has been a highly active area of research using OCTA. Reports have shown significant changes in vascular measures such as fractal dimension and vessel density in eyes with RVO compared to healthy eyes, as well as correlations with increasing severity of RVO.75 Other parameters such as FAZ area have been found to be enlarged in RVO (as well as in fellow unaffected eyes) compared to healthy controls on OCTA,76 and changes in these markers have been demonstrated to be associated with worsening visual acuity.77,78 Derivation of the aforementioned parameters requires postacquisition image analysis using third-party software such as ImageJ and Matlab.
While FA is still the gold standard imaging modality for RVO,79,80 OCTA has been found to be comparable to FA in identifying capillary nonflow and changes such as dilations and telangiectasias.81 Dysfunction of retinal peripheral perfusion on FA has also been shown to be significantly associated with changes in OCTA quantitative parameters.82 Using OCTA to resolve the different capillary plexuses, Adhi and colleagues76 identified more severe perfusion deficits at the level of the DCP in central and branch RVO, which is consistent with the data from other studies.83,84 Another interesting finding is that FAZ areas are significantly enlarged only at the level of the DCP in branch RVO compared to nonaffected fellow eyes78 (Figure 5).
Figure 5.
Three-layer capillary segmentation of a case of long-standing superotemporal branch retinal vein occlusion (BRVO) of the right eye. The dashed line indicates location of B-scan used in the figure. Enlargement of the foveal avascular zone (FAZ) is visible. Arrows demarcate capillary abnormalities. Note that these abnormalities and capillary dropout appear to be more severe in the deeper capillary layers. Figures are created with images using Optovue Projection Artifact Removal (PAR; AngioVue Analytics Version 2017.1.0.151).
DCP: deep capillary plexus; MCP: middle capillary plexus; SCP: superficial capillary plexus.
OCTA has also demonstrated possible utility in the clinical setting as an imaging modality for the follow-up of treatment of RVO. In their study, Sellam and colleagues85 used OCTA in RVO patients before and after intravitreal antivascular endothelial growth factor injections and found that significant decreases in retinal fluid, capillary disruption, and capillary cysts could be visualized post intervention.
Glaucoma
Although the exact pathophysiologic process of glaucoma is unknown, there is convincing evidence that vascular dysfunction contributes to the progression of the disease.86–89 For that reason, OCTA has been a popular tool for the study of the microvasculature in glaucoma, with the majority of these reports examining the peripapillary region. Generally, these studies have found vascular dysfunction in glaucomatous eyes compared to healthy controls.90–93 Optic disk flow index, the average decorrelation signal in OCTA, has previously been shown to have high sensitivity and specificity for glaucoma and to be highly correlated with visual field pattern standard deviation.94 Peripapillary perfusion deficits have also been shown to be associated with structural changes in glaucoma on OCTA.93 Those OCTA studies examining vascular changes in the macula have identified similar dysfunction in retinal blood flow and perfusion.95–98
OCTA reports have suggested that quantitative OCTA parameters such as vessel density could eventually be used for the diagnosis of glaucoma. Takusagawa and colleagues96 demonstrated a high area under the receiver operating characteristic curve (AUC) of 0.961 using superficial macular vessel density for glaucoma, consistent with a similar report by Richter and colleagues95 who identified an AUC of 0.83 for the macular microvasculature. However, there has been some controversy as to the area of choice as Rao and colleagues99 found that peripapillary and inside the optic disk vessel densities had higher AUC than macular vessel density.
One important unresolved question in the field relates to whether vascular changes precede structural changes or whether loss of the retinal ganglion cells and their decreased metabolic need leads to the reduced blood flow observed with OCTA.100 In their longitudinal OCTA study, Shoji and colleagues101 demonstrated that the average rate of decline in macular vessel density over the course of at least 1 year was significantly higher in glaucomatous eyes than healthy or glaucoma suspect eyes. Interestingly, these authors found no significant decline in ganglion cell layer thickness in any of the three groups over the same time period. However, they could not definitively conclude that vascular changes preceded structural changes because it is possible that neural structural changes might occur earlier in the course of glaucoma and thus would not be captured in their population of mainly moderate-severity glaucoma. Further carefully detailed longitudinal and multimodal structure and function studies involving OCTA will be required to settle this chicken-or-egg debate.
Acute macular neuroretinopathy, paracentral acute middle maculopathy, and disorganization of the retinal inner layers
OCTA offers an exciting opportunity for further investigation into acute macular neuroretinopathy (AMN), paracentral acute middle maculopathy (PAMM), and disorganization of the retinal inner layers (DRIL), which manifest on OCT as disruption of the retinal architecture, given their postulated relationship with inner retinal ischemia. However, due to the rarity of these entities as well as the difficulties of segmentation in these conditions, the quality of evidence of the OCTA studies discussed in this section is relatively low and consists mainly of small descriptive studies and case reports.
AMN is a rare retinal disease that classically presents with initial OPL and outer nuclear layer (ONL) hyperreflectivity on spectral domain-OCT. These changes are followed by eventual disruption of the outer segment/RPE lines correlating to hyporeflective lesions on infrared (IR) imaging and further progression to long-term findings such as thinning of the ONL.102 PAMM is a similar but distinct condition and presents with middle retinal involvement manifesting as initial IPL and INL hyperreflectivity followed ultimately by INL thinning.103
AMN and PAMM are both thought to be associated with retinal capillary ischemia, although there is still controversy over the specific plexuses affected. Fawzi and colleagues102 originally postulated that DCP ischemia was responsible for the adjacent lesions of AMN, which has since been demonstrated in several studies.104,105 Choriocapillaris nonperfusion has also been reported in AMN,106,107 although these findings are potentially confounded by choriocapillaris artifact as well as the use of larger OCTA scan sizes with limited density and resolution of the retinal capillaries.108 More recently, Casalino and colleagues109 have reported focal DCP involvement correlating with AMN lesions as well as decreased global choriocapillaris flow. While MCP and DCP nonflows have been hypothesized to cause PAMM,110 there have been disagreements due to reports of perfusion abnormalities in the SCP and DCP.111
OCTA imaging is particularly difficult in AMN and PAMM given focal thinning and hyperreflectivity of the retinal layers, which can, respectively, lead to segmentation errors and exacerbation of projection artifact and can potentially explain the previously reported conflicting findings. Using PR-OCTA to mitigate these artifacts, Chu and colleagues112 have shown that AMN is associated with DCP ischemia, while PAMM is associated with MCP and DCP ischemia with occasional SCP perfusion deficits. Improved software algorithms are needed to lead to better consensus in the field regarding inner retinal blood flow changes in AMN and PAMM.
DRIL is a retinal biomarker first described by Sun and colleagues113 that also appears to be associated with inner retinal ischemia.114 DRIL is defined as the absence of distinguishable boundaries between the ganglion cell–IPL junction, INL, and OPL and is usually reported in eyes with macular edema.113,115,116 Moien and colleagues117 have used OCTA with a two-layer segmentation scheme and found enlargement of the FAZ as well as ischemia in the superficial, deep, and full retina in eyes with DRIL. Using PR-OCTA as well as a three-layer segmentation scheme, Onishi and colleagues30 were able to further localize these perfusion deficits to the SCP or MCP in addition to underlying DCP ischemia present in areas of DRIL.
The complexity of perfusion deficits at multiple capillary levels on a 3D basis in these entities emphasizes the importance of OCTA and algorithms such as PR-OCTA in the study of macular ischemia at the capillary level.
Posterior uveitis
OCTA has been shown to be a useful research tool and potential clinical adjunct in inflammatory eye disease. FA can provide valuable information about active areas of inflammation in retinal vasculitis by displaying vessel wall staining and leakage, but this leakage may prevent visualization of the adjacent vasculature that may therefore only be assessed with OCTA.118 In addition, OCTA can help identify complications such as inflammatory CNV, which mostly affects the macula and can lead to severe vision loss.119 While dye-based imaging is often helpful for identification of these neovascular membranes, there is also overlap with inflammatory lesions.118,119 Additional OCT imaging may be useful as it can show subretinal hyperreflective tissue that is consistent with CNV, but can also be seen with other conditions like fibrosis.118
The highly detailed images and depth-resolved quantitative data regarding retinal perfusion contributed by OCTA provides information that may not be possible with other imaging modalities. For example, a case report by Pichi and colleagues120 was the first to report NV within a foci of retinochoroiditis secondary to Bartonella henselae with OCTA that was not seen on FA. With their cohort of uveitic eyes, Kim and colleagues121 used a two-layer segmentation scheme to show that parafoveal vessel density and fractal dimension in the SCP and DCP were lower in uveitic eyes compared to healthy eyes. In the same study, these authors found that uveitic eyes with macular edema had significantly lower DCP vessel densities (with no significant difference in SCP vessel densities) compared to uveitic eyes without edema, thus potentially implicating DCP ischemia in the development of cystoid macular edema.
OCTA has been helpful in the study of conditions such as birdshot chorioretinopathy (BSCR). de Carlo and colleagues122 used OCTA to study eight eyes of patients with BSCR and identified abnormalities such as inner retinal telangiectatic vessels and increased intercapillary space. These areas of decreased SCP perfusion have been found to correlate with central retinal thinning,123 raising the possibility that ischemia could contribute to ganglion cell damage and the macular thinning that has been observed in BSCR,118 although prior studies have shown that the majority of thinning is in the outer retina.124 In a patient with BSCR complicated by retinal NV and vitreous hemorrhage, Sarraf and colleagues found more profoundly impaired vessel densities in the DCP than the SCP compared to age-matched normal eyes, indicating that both ischemia and inflammation may contribute to the development of retinal NV in BSCR.125
Value of OCTA beyond the role of FA and ICGA
Given the ease and noninvasive nature of OCTA imaging in relation to conventional angiography, OCTA can be used to study changes in the retinal vasculature in conditions and procedures where angiographic imaging would not traditionally be indicated. For example, multiple authors have examined changes in the FAZ in vitreomacular interface disorders such as macular holes and epiretinal membranes (ERM). In their retrospective study, Baba and colleagues126 reported that superficial FAZ area was significantly reduced in eyes following pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling, as well as in relation to unaffected companion eyes. This decrease in superficial FAZ area was significantly associated with a higher central foveal thickness (CFT), suggesting that the process of closing the macular hole results in a movement of macular tissue centrally. These quantitative changes are consistent with subsequent studies,127,128 although a report by Kim and colleagues127 interestingly demonstrated an additional significant association between the FAZ area and 6-month postoperative best-corrected visual acuity (BCVA) that was not previously described (possibly due to differences in baseline subject characteristics or follow-up periods). This could indicate that eyes with smaller FAZ may have more neural tissue filling in the macular hole, thus leading to improved central visual outcomes.127
In their study of FAZ changes following PPV with ERM and ILM peeling, Yoon and colleagues129 found that preoperative FAZ area was significantly smaller than in unaffected fellow eyes and that preoperative FAZ area was highly negatively correlated with CFT. Following the intervention, postoperative FAZ area was significantly increased and CFT was significantly decreased compared to preoperative measurements, providing further evidence that ERMs cause substantial foveal architectural changes that are somewhat restored by surgery. These changes in FAZ area and CFT are consistent with the results of other studies.130,131
Importantly for ERM patients, preoperative parameters involving the FAZ area may also be predictive of vision postoperatively. For patients with a unilateral idiopathic ERM, the preoperative FAZ area interocular ratio has been found to be significantly correlated with the degree of aniseikonia following surgical intervention.132 The authors postulated that this ratio compensates for variability in the FAZ area between individuals and is an indicator of centripetal contraction, which may lead to the changes in INL thickness that have been shown to be significantly associated with metamorphopsia. Postoperative FAZ area (along with CFT) has also been shown to be negatively correlated (CFT is positively correlated) with postoperative LogMAR BCVA, suggesting that restoration of the normal foveal morphology is important for the recovery of visual acuity.131 These quantitative OCTA measures should be studied further as they may provide valuable prognostic information for surgical interventions in the future.
Future directions and conclusion
OCTA is an innovative game-changing technology that has already contributed to a greater understanding of the vascular pathologies of a variety of retinal disease. However, a clearly defined role for OCTA in the clinic is yet to be determined, and there are numerous steps that must be taken to reach that point. While normative data for healthy and diseased eyes in multiple ocular diseases have been published, more extensive databases, including racial and sex databases, are needed for the various OCTA parameters. In addition, given the increasing number of OCTA systems, cross-platform agreements are needed for sublayer segmentation.19 Advances in software and hardware are necessary to better correct for motion and projection artifacts. The utility of SS-OCTA systems, which promise faster imaging with greater field of view and enhanced visualization of deeper retinal structures, still needs to be validated.
Since its inception, OCTA has evolved into the promising tool it is today and is continuing to improve at an incredible rate. It is positioned to establish itself as the first-line angiographic imaging modality over the course of the next decade, revolutionizing the field of ophthalmology much as the technology of OCT has done over the past few decades.133
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alex C. Onishi  https://orcid.org/0000-0001-8488-3602
https://orcid.org/0000-0001-8488-3602
Contributor Information
Alex C. Onishi, Department of Ophthalmology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Amani A. Fawzi, Department of Ophthalmology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
References
- 1. Adhi M, Duker JS. Optical coherence tomography—current and future applications. Curr Opin Ophthalmol 2013; 24: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991; 254: 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao SS, Jia Y, Zhang M, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2016; 57: OCT27–OCT36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yazdanfar S, Rollins AM, Izatt JA. Imaging and velocimetry of the human retinal circulation with color Doppler optical coherence tomography. Opt Lett 2000; 25: 1448–1450. [DOI] [PubMed] [Google Scholar]
- 5. Kwan AS, Barry C, McAllister IL, et al. Fluorescein angiography and adverse drug reactions revisited: the Lions Eye experience. Clin Exp Ophthalmol 2006; 34: 33–38. [DOI] [PubMed] [Google Scholar]
- 6. Marcus DF, Bovino JA, Williams D. Adverse reactions during intravenous fluorescein angiography. Arch Ophthalmol 1984; 102: 825. [DOI] [PubMed] [Google Scholar]
- 7. Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation 1961; 24: 82–86. [DOI] [PubMed] [Google Scholar]
- 8. Park JJ, Soetikno BT, Fawzi AA. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina 2016; 36: 2039–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nesper PL, Roberts PK, Onishi AC, et al. Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2017; 58: BIO307–BIO315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Z, Milner TE, Srinivas S, et al. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Opt Lett 1997; 22: 1119–1121. [DOI] [PubMed] [Google Scholar]
- 11. Kashani AH, Chen C-L, Gahm JK, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res 2017; 60: 66–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012; 20: 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao SS, Liu G, Huang D, et al. Optimization of the split-spectrum amplitude-decorrelation angiography algorithm on a spectral optical coherence tomography system. Opt Lett 2015; 40: 2305–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalam KV, Sambhav K. Optical coherence tomography angiography in retinal diseases. J Ophthalmic Vis Res 2016; 11: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang RK, An L, Francis P, et al. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Opt Lett 2010; 35: 1467–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang RK, An L, Saunders S, et al. Optical microangiography provides depth-resolved images of directional ocular blood perfusion in posterior eye segment. J Biomed Opt 2010; 15: 020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express 2008; 16: 11438–11452. [DOI] [PubMed] [Google Scholar]
- 18. Stanga PE, Tsamis E, Papayannis A, et al. Swept-source optical coherence tomography angio (Topcon Corp, Japan): technology review. Dev Ophthalmol 2016; 56: 13–17. [DOI] [PubMed] [Google Scholar]
- 19. Munk MR, Giannakaki-Zimmermann H, Berger L, et al. OCT-angiography: a qualitative and quantitative comparison of 4 OCT-A devices. PLoS ONE 2017; 12: e0177059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina 2015; 35: 2163–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang TS, Zhang M, Bhavsar K, et al. Visualization of 3 distinct retinal plexuses by projection-resolved optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol 2016; 134: 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang Y, Zhang Q, Wang RK. Efficient method to suppress artifacts caused by tissue hyper-reflections in optical microangiography of retina in vivo. Biomed Opt Express 2015; 6: 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camino A, Zhang M, Gao SS, et al. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed Opt Express 2016; 7: 3905–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferguson RD, Hammer DX, Paunescu LA, et al. Tracking optical coherence tomography. Opt Lett 2004; 29: 2139–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang A, Zhang Q, Wang RK. Minimizing projection artifacts for accurate presentation of choroidal neovascularization in OCT micro-angiography. Biomed Opt Express 2015; 6: 4130–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu L, Gao SS, Bailey ST, et al. Automated choroidal neovascularization detection algorithm for optical coherence tomography angiography. Biomed Opt Express 2015; 6: 3564–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang M, Hwang TS, Campbell JP, et al. Projection-resolved optical coherence tomographic angiography. Biomed Opt Express 2016; 7: 816–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep 2017; 7: 42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhavsar KV, Jia Y, Wang J, et al. Projection-resolved optical coherence tomography angiography exhibiting early flow prior to clinically observed retinal angiomatous proliferation. Am J Ophthalmol Case Rep 2017; 8: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Onishi AC, Ashraf M, Soetikno BT, et al. Multilevel ischemia in disorganization of the retinal inner layers on projection-resolved optical coherence tomography angiography. Retina. Epub ahead of print 10 April 2018. DOI: 10.1097/IAE.0000000000002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garrity ST, Iafe NA, Phasukkijwatana N, et al. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2017; 58: 5548–5555. [DOI] [PubMed] [Google Scholar]
- 32. Chan G, Balaratnasingam C, Yu PK, et al. Quantitative morphometry of perifoveal capillary networks in the human retina. Invest Ophthalmol Vis Sci 2012; 53: 5502–5514. [DOI] [PubMed] [Google Scholar]
- 33. Onishi AC, Nesper PL, Roberts PK, et al. Importance of considering the middle capillary plexus on OCT angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci 2018; 59: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christopher S, Chung PLN, Park JJ, et al. Comparison of Zeiss Cirrus and Optovue RTvue OCT angiography systems: a quantitative and qualitative approach examining the three capillary networks in diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina 2018; 49: e198–e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrity ST, Paques M, Gaudric A, et al. Considerations in the understanding of venous outflow in the retinal capillary plexus. Retina 2017; 37: 1809–1812. [DOI] [PubMed] [Google Scholar]
- 36. Nesper PL, Fawzi AA. Human parafoveal capillary vascular anatomy and connectivity revealed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2018; 59: 3858–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Q, Zheng F, Motulsky EH, et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Invest Ophthalmol Vis Sci 2018; 59: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uji A, Balasubramanian S, Lei J, et al. Choriocapillaris imaging using multiple en face optical coherence tomography angiography image averaging. JAMA Ophthalmol 2017; 135: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lauermann JL, Woetzel AK, Treder M, et al. Prevalences of segmentation errors and motion artifacts in OCT-angiography differ among retinal diseases. Graefes Arch Clin Exp Ophthalmol 2018; 256: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 40. Hirano T, Kakihara S, Toriyama Y, et al. Wide-field en face swept-source optical coherence tomography angiography using extended field imaging in diabetic retinopathy. Br J Ophthalmol 2017; 102: 1199–1203. [DOI] [PubMed] [Google Scholar]
- 41. Do DV. Detection of new-onset choroidal neovascularization. Curr Opin Ophthalmol 2013; 24: 244–247. [DOI] [PubMed] [Google Scholar]
- 42. Kotsolis AI, Killian FA, Ladas ID, et al. Fluorescein angiography and optical coherence tomography concordance for choroidal neovascularization in multifocal choroiditis. Br J Ophthalmol 2010; 94: 1506–1508. [DOI] [PubMed] [Google Scholar]
- 43. Do DV, Gower EW, Cassard SD, et al. Detection of new-onset choroidal neovascularization using optical coherence tomography: the AMD DOC study. Ophthalmology 2012; 119: 771–778. [DOI] [PubMed] [Google Scholar]
- 44. Nesper PL, Soetikno BT, Zhang HF, et al. OCT angiography and visible-light OCT in diabetic retinopathy. Vision Res 2017; 139: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirano T, Kitahara J, Toriyama Y, et al. Quantifying vascular density and morphology using different swept-source optical coherence tomography angiographic scan patterns in diabetic retinopathy. Br J Ophthalmol 2019; 103: 216–221. [DOI] [PubMed] [Google Scholar]
- 46. Lei J, Durbin MK, Shi Y, et al. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol 2017; 135: 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uji A, Yoshimura N. Application of extended field imaging to optical coherence tomography. Ophthalmology 2015; 122: 1272–1274. [DOI] [PubMed] [Google Scholar]
- 48. De Carlo TE, Salz DA, Waheed NK, et al. Visualization of the retinal vasculature using wide-field montage optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina 2015; 46: 611–616. [DOI] [PubMed] [Google Scholar]
- 49. Weinhaus RS, Burke JM, Delori FC, et al. Comparison of fluorescein angiography with microvascular anatomy of macaque retinas. Exp Eye Res 1995; 61: 1–16. [DOI] [PubMed] [Google Scholar]
- 50. Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 2015; 133: 45–50. [DOI] [PubMed] [Google Scholar]
- 51. Samara WA, Shahlaee A, Adam MK, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology 2017; 124: 235–244. [DOI] [PubMed] [Google Scholar]
- 52. Kim AY, Chu Z, Shahidzadeh A, et al. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2016; 57: OCT362–OCT370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu Y, Simonett JM, Wang J, et al. Evaluation of automatically quantified foveal avascular zone metrics for diagnosis of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2018; 59: 2212–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lei J, Yi E, Suo Y, et al. Distinctive analysis of macular superficial capillaries and large vessels using optical coherence tomographic angiography in healthy and diabetic eyes. Invest Ophthalmol Vis Sci 2018; 59: 1937–1943. [DOI] [PubMed] [Google Scholar]
- 55. Takase N, Nozaki M, Kato A, et al. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 2015; 35: 2377–2383. [DOI] [PubMed] [Google Scholar]
- 56. Soares M, Neves C, Marques IP, et al. Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br J Ophthalmol 2017; 101: 62–68. [DOI] [PubMed] [Google Scholar]
- 57. La Mantia A, Kurt RA, Mejor S, et al. Comparing fundus fluorescein angiography and swept-source optical coherence tomography angiography in the evaluation of diabetic macular perfusion. Retina. Epub ahead of print 16 January 2018. DOI: 10.1097/IAE.0000000000002045. [DOI] [PubMed] [Google Scholar]
- 58. Couturier A, Mane V, Bonnin S, et al. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina 2015; 35: 2384–2391. [DOI] [PubMed] [Google Scholar]
- 59. Salz DA, deCarlo TE, Adhi M, et al. Select features of diabetic retinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol 2016; 134: 644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. De Carlo TE, Romano A, Waheed NK, et al. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous 2015; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sawada O, Ichiyama Y, Obata S, et al. Comparison between wide-angle OCT angiography and ultra-wide field fluorescein angiography for detecting non-perfusion areas and retinal neovascularization in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2018; 256: 1275–1280. [DOI] [PubMed] [Google Scholar]
- 62. Schaal KB, Munk MR, Wyssmueller I, et al. Vascular abnormalities in diabetic retinopathy assessed with swept-source optical coherence tomography angiography widefield imaging. Retina 2019; 39: 79–87. [DOI] [PubMed] [Google Scholar]
- 63. Jia Y, Bailey ST, Wilson DJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 2014; 121: 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perrott-Reynolds R, Cann R, Cronbach N, et al. The diagnostic accuracy of OCT angiography in naive and treated neovascular age-related macular degeneration: a review. Eye (Lond) 2019; 33: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma J, Desai R, Nesper P, et al. Optical coherence tomographic angiography imaging in age-related macular degeneration. Ophthalmol Eye Dis 2017; 9: 86075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Coscas GJ, Lupidi M, Coscas F, et al. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: A new diagnostic challenge. Retina 2015; 35: 2219–2228. [DOI] [PubMed] [Google Scholar]
- 67. Al-Sheikh M, Iafe NA, Phasukkijwatana N, et al. Biomarkers of neovascular activity in age-related macular degeneration using optical coherence tomography angiography. Retina 2018; 38: 220–230. [DOI] [PubMed] [Google Scholar]
- 68. Xu D, Dávila JP, Rahimi M, et al. Long-term progression of type 1 neovascularization in age-related macular degeneration using optical coherence tomography angiography. Am J Ophthalmol 2018; 187: 10–20. [DOI] [PubMed] [Google Scholar]
- 69. Nesper PL, Soetikno BT, Treister AD, et al. Volume-rendered projection-resolved OCT angiography: 3D lesion complexity is associated with therapy response in wet age-related macular degeneration. Invest Ophthalmol Vis Sci 2018; 59: 1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology 2018; 125: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Unterhuber A, Povazay B, Hermann B, et al. In vivo retinal optical coherence tomography at 1040 nm—enhanced penetration into the choroid. Opt Express 2005; 13: 3252–3258. [DOI] [PubMed] [Google Scholar]
- 72. Kvanta A, CasselholmdeSalles M, Amren U, et al. Optical coherence tomography angiography of the foveal microvasculature in geographic atrophy. Retina 2017; 37: 936–942. [DOI] [PubMed] [Google Scholar]
- 73. Moult EM, Waheed NK, Novais EA, et al. Swept-source optical coherence tomography angiography reveals choriocapillaris alterations in eyes with nascent geographic atrophy and drusen-associated geographic atrophy. Retina 2016; 36(Suppl. 1): S2–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pellegrini M, Acquistapace A, Oldani M, et al. Dark atrophy: an optical coherence tomography angiography study. Ophthalmology 2016; 123: 1879–1886. [DOI] [PubMed] [Google Scholar]
- 75. Koulisis N, Kim AY, Chu Z, et al. Quantitative microvascular analysis of retinal venous occlusions by spectral domain optical coherence tomography angiography. PLoS ONE 2017; 12: e0176404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adhi M, Filho MA, Louzada RN, et al. Retinal capillary network and foveal avascular zone in eyes with vein occlusion and fellow eyes analyzed with optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2016; 57: OCT486–OCT494. [DOI] [PubMed] [Google Scholar]
- 77. Balaratnasingam C, Inoue M, Ahn S, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology 2016; 123: 2352–2367. [DOI] [PubMed] [Google Scholar]
- 78. Samara WA, Shahlaee A, Sridhar J, et al. Quantitative optical coherence tomography angiography features and visual function in eyes with branch retinal vein occlusion. Am J Ophthalmol 2016; 166: 76–83. [DOI] [PubMed] [Google Scholar]
- 79. Glacet-Bernard A, Coscas G, Chabanel A, et al. Prognostic factors for retinal vein occlusion: prospective study of 175 cases. Ophthalmology 1996; 103: 551–560. [DOI] [PubMed] [Google Scholar]
- 80. Jonas J, Paques M, Mones J, et al. Retinal vein occlusions. Dev Ophthalmol 2010; 47: 111–135. [DOI] [PubMed] [Google Scholar]
- 81. NobreCardoso J, Keane PA, Sim DA, et al. Systematic Evaluation of Optical Coherence Tomography Angiography in Retinal Vein Occlusion. Am J Ophthalmol 2016; 163: 93–107.e106. [DOI] [PubMed] [Google Scholar]
- 82. Seknazi D, Coscas F, Sellam A, et al. Optical coherence tomography angiography in retinal vein occlusion: correlations between macular vascular density, visual acuity, and peripheral nonperfusion area on fluorescein angiography. Retina 2018; 38: 1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kashani AH, Lee SY, Moshfeghi A, et al. Optical coherence tomography angiography of retinal venous occlusion. Retina 2015; 35: 2323–2331. [DOI] [PubMed] [Google Scholar]
- 84. Coscas F, Glacet-Bernard A, Miere A, et al. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol 2016; 161 e161162: 160–71e1. [DOI] [PubMed] [Google Scholar]
- 85. Sellam A, Glacet-Bernard A, Coscas F, et al. Qualitative and quantitative follow-up using optical coherence tomography angiography of retinal vein occlusion treated with anti-VEGF: optical coherence tomography angiography follow-up of retinal vein occlusion. Retina 2017; 37: 1176–1184. [DOI] [PubMed] [Google Scholar]
- 86. Cherecheanu AP, Garhofer G, Schmidl D, et al. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol 2013; 13: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Flammer J, Orgul S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res 1998; 17: 267–289. [DOI] [PubMed] [Google Scholar]
- 88. Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol 2005; 16: 79–83. [DOI] [PubMed] [Google Scholar]
- 89. Harris A, Rechtman E, Siesky B, et al. The role of optic nerve blood flow in the pathogenesis of glaucoma. Ophthalmol Clin North Am 2005; 18: 345–353v. [DOI] [PubMed] [Google Scholar]
- 90. Lee EJ, Lee KM, Lee SH, et al. OCT angiography of the peripapillary retina in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 2016; 57: 6265–6270. [DOI] [PubMed] [Google Scholar]
- 91. Rao HL, Kadambi SV, Weinreb RN, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol 2017; 101: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 92. Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci 2016; 57: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen CL, Bojikian KD, Wen JC, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in eyes with glaucoma and single-hemifield visual field loss. JAMA Ophthalmol 2017; 135: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014; 121: 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Richter GM, Madi I, Chu Z, et al. Structural and functional associations of macular microcirculation in the ganglion cell-inner plexiform layer in glaucoma using optical coherence tomography angiography. J Glaucoma 2018; 27: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Takusagawa HL, Liu L, Ma KN, et al. Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology 2017; 124: 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Akil H, Chopra V, Al-Sheikh M, et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br J Ophthalmol 2017; 102: 2017. [DOI] [PubMed] [Google Scholar]
- 98. Xu H, Yu J, Kong X, et al. Macular microvasculature alterations in patients with primary open-angle glaucoma: A cross-sectional study. Medicine (Baltimore) 2016; 95: e4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rao HL, Pradhan ZS, Weinreb RN, et al. Regional comparisons of optical coherence tomography angiography vessel density in primary open-angle glaucoma. Am J Ophthalmol 2016; 171: 75–83. [DOI] [PubMed] [Google Scholar]
- 100. Daneshvar R, Nouri-Mahdavi K. Optical coherence tomography angiography: a new tool in glaucoma diagnostics and research. J Ophthalmic Vis Res 2017; 12: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shoji T, Zangwill LM, Akagi T, et al. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol 2017; 182: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fawzi AA, Pappuru RR, Sarraf D, et al. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina 2012; 32: 1500–1513. [DOI] [PubMed] [Google Scholar]
- 103. Sarraf D, Rahimy E, Fawzi AA, et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol 2013; 131: 1275–1287. [DOI] [PubMed] [Google Scholar]
- 104. Nemiroff J, Sarraf D, Davila JP, et al. Optical coherence tomography angiography of acute macular neuroretinopathy reveals deep capillary ischemia. Retin Cases Brief Rep 2018; 12(Suppl. 1): S12–S15. [DOI] [PubMed] [Google Scholar]
- 105. Liu JC, Nesper PL, Fawzi AA, et al. Acute macular neuroretinopathy associated with influenza vaccination with decreased flow at the deep capillary plexus on OCT angiography. Am J Ophthalmol Case Rep 2018; 10: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thanos A, Faia LJ, Yonekawa Y, et al. Optical coherence tomographic angiography in acute macular neuroretinopathy. JAMA Ophthalmol 2016; 134: 1310–1314. [DOI] [PubMed] [Google Scholar]
- 107. Lee SY, Cheng JL, Gehrs KM, et al. Choroidal features of acute macular neuroretinopathy via optical coherence tomography angiography and correlation with serial multimodal imaging. JAMA Ophthalmol 2017; 135: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ashraf M, Goldstein D, Fawzi A. optical coherence tomography angiography: potential artifacts in acute macular neuroretinopathy. JAMA Ophthalmol 2017; 135: 675–676. [DOI] [PubMed] [Google Scholar]
- 109. Casalino G, Arrigo A, Romano F, et al. Acute macular neuroretinopathy: pathogenetic insights from optical coherence tomography angiography. Br J Ophthalmol 2018; 6: 202–203. [DOI] [PubMed] [Google Scholar]
- 110. Chen X, Rahimy E, Sergott RC, et al. Spectrum of retinal vascular diseases associated with paracentral acute middle maculopathy. Am J Ophthalmol 2015; 160: 26–34.e21. [DOI] [PubMed] [Google Scholar]
- 111. Sridhar J, Shahlaee A, Rahimy E, et al. Optical coherence tomography angiography and en face optical coherence tomography features of paracentral acute middle maculopathy. Am J Ophthalmol 2015; 160: 1259–1268.e1252. [DOI] [PubMed] [Google Scholar]
- 112. Chu S, Nesper PL, Soetikno BT, et al. Projection-resolved OCT angiography of microvascular changes in paracentral acute middle maculopathy and acute macular neuroretinopathy. Invest Ophthalmol Vis Sci 2018; 59: 2913–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol 2014; 132: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 114. Nicholson L, Ramu J, Triantafyllopoulou I, et al. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin Exp Ophthalmol 2015; 43: 735–741. [DOI] [PubMed] [Google Scholar]
- 115. Grewal DS, O’Sullivan ML, Kron M, et al. Association of disorganization of retinal inner layers with visual acuity in eyes with uveitic cystoid macular edema. Am J Ophthalmol 2017; 177: 116–125. [DOI] [PubMed] [Google Scholar]
- 116. Mimouni M, Segev O, Dori D, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol 2017; 182: 160–167. [DOI] [PubMed] [Google Scholar]
- 117. Moein HR, Novais EA, Rebhun CB, et al. Optical coherence tomography angiography to detect macular capillary ischemia in patients with inner retinal changes after resolved diabetic macular edema. Retina 2018; 38: 2277–2284. [DOI] [PubMed] [Google Scholar]
- 118. Pichi F, Sarraf D, Arepalli S, et al. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog Retin Eye Res 2017; 59: 178–201. [DOI] [PubMed] [Google Scholar]
- 119. Invernizzi A, Cozzi M, Staurenghi G. Optical coherence tomography and optical coherence tomography angiography in uveitis: a review. Clin Exp Ophthalmol. Epub ahead of print 11 February 2019. DOi: 10.1111/ceo.13470. [DOI] [PubMed] [Google Scholar]
- 120. Pichi F, Srivastava SK, Levinson A, et al. A focal chorioretinal bartonella lesion analyzed by optical coherence tomography angiography. Ophthalm Surg Lasers Imag Retina 2016; 47: 585–588. [DOI] [PubMed] [Google Scholar]
- 121. Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol 2016; 171: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. de Carlo TE, BoniniFilho MA, Adhi M, et al. Retinal and choroidal vasculature in birdshot chorioretinopathy analyzed using spectral domain optical coherence tomography angiography. Retina 2015; 35: 2392–2399. [DOI] [PubMed] [Google Scholar]
- 123. Pichi F, Lowder CY, Sharma S, et al. Macular ischemia in inactive birdshot chorioretinopathy evaluated by OCT angiography. Poster Presented at AAO 2016; 42: PO436. [Google Scholar]
- 124. Birch DG, Williams PD, Callanan D, et al. Macular atrophy in birdshot retinochoroidopathy: an optical coherence tomography and multifocal electroretinography analysis. Retina 2010; 30: 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Phasukkijwatana N, Iafe N, Sarraf D. Optical coherence tomography angiography of A29 birdshot chorioretinopathy complicated by retinal neovascularization. Retin Cases Brief Rep 2017; 11(Suppl. 1): S68–S72. [DOI] [PubMed] [Google Scholar]
- 126. Baba T, Kakisu M, Nizawa T, et al. Superficial foveal avascular zone determined by optical coherence tomography angiography before and after macular hole surgery. Retina 2017; 37: 444–450. [DOI] [PubMed] [Google Scholar]
- 127. Kim YJ, Jo J, Lee JY, et al. Macular capillary plexuses after macular hole surgery: an optical coherence tomography angiography study. Br J Ophthalmol 2018; 102: 966–970. [DOI] [PubMed] [Google Scholar]
- 128. Cho JH, Yi HC, Bae SH, et al. Foveal microvasculature features of surgically closed macular hole using optical coherence tomography angiography. BMC Ophthalmol 2017; 17: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yoon YS, Woo JM, Woo JE, et al. Superficial foveal avascular zone area changes before and after idiopathic epiretinal membrane surgery. Int J Ophthalmol 2018; 11: 1711–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kitagawa Y, Shimada H, Shinojima A, et al. Foveal avascular zone area analysis using optical coherence tomography angiography before and after idiopathic epiretinal membrane surgery. Retina 2019; 39: 339–346. [DOI] [PubMed] [Google Scholar]
- 131. Chen H, Chi W, Cai X, et al. Macular microvasculature features before and after vitrectomy in idiopathic macular epiretinal membrane: an OCT angiography analysis. Eye (Lond). Epub ahead of print 22 November 2018. DOI: 10.1038/s41433-018-0272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hirata A, Nakada H, Mine K, et al. Relationship between the morphology of the foveal avascular zone and the degree of aniseikonia before and after vitrectomy in patients with unilateral epiretinal membrane. Graefes Arch Clin Exp Ophthalmol 2019; 257: 507–515. [DOI] [PubMed] [Google Scholar]
- 133. Windsor MA, Sun SJJ, Frick KD, et al. Estimating public and patient savings from basic research: a study of optical coherence tomography in managing antiangiogenic therapy. Am J Ophthalmol 2018; 185: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]