Abstract
Background:
Radiofrequency ablation is commonly used in arthroscopic rotator cuff repair (RCR). New technology devices incorporating a plasma bubble may generate lower intra-articular temperatures and be more efficient.
Purpose:
To compare a plasma ablation device with a standard ablation device in arthroscopic RCR to determine which system is superior in terms of intra-articular heat generation and diathermy efficiency.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
This was a single-center randomized controlled trial. The inclusion criteria were adult patients undergoing primary RCR. Patients were randomized preoperatively to the standard ablation group (n = 20) or plasma ablation group (n = 20). A thermometer was inserted into the shoulder joint during surgery, and the temperature, surgery, and diathermy times of radiofrequency ablation were measured continually.
Results:
No significant differences were found between the standard ablation group and plasma ablation group for maximum temperature (38.20°C and 39.38°C, respectively; P = .433), mean temperature (31.66°C and 30.64°C, respectively; P = .757), minimum temperature (21.83°C and 23.45°C, respectively; P = .584), and baseline temperature (28.49°C and 29.94°C, respectively; P = .379). Similarly, no significant differences were found for surgery time (74 and 75 minutes, respectively; P = .866) and diathermy time (10 minutes for both; P = .678). Seven patients registered transient high temperatures greater than 45°C.
Conclusion:
There was no difference between plasma ablation and standard ablation in terms of intra-articular temperature in the joint and diathermy efficiency. Transient high intra-articular temperatures occurred in both groups.
Registration:
ACTRN1261300056970 (Australian New Zealand Clinical Trials Registry).
Keywords: diathermy, shoulder surgery, ablation, intra-articular temperature
Rotator cuff repair (RCR) is a common procedure performed on patients with a rotator cuff tear. Radiofrequency (RF) ablation is routinely used to clear soft tissue before RCR and in preparation for subacromial decompression. RF ablation produces heat in a confined space, which can potentially lead to tissue damage, in particular chondrocyte damage. In vitro chondrocyte damage occurs at temperatures near 55°C.9 This translates clinically, and case reports have been published connecting articular chondrolysis in patients to the use of RF ablation devices for arthroscopic procedures in both knee surgery3,4,10 and shoulder surgery.5,7,8 RF ablation instruments are considered safe, and their use in arthroscopic shoulder surgery is routine. Recent advances include devices that incorporate a plasma bubble that theoretically allows for more efficient debridement and reduced heat transmission to the joint fluid and surrounding tissue. A previous in vivo study found no difference between the 2 ablation techniques in the knee joint during arthroscopic anterior cruciate ligament reconstruction (ACLR).11 The shoulder, however, has a smaller articular volume than the knee, and the effect of RF in the context of RCR may not be the same as in ACLR.
The purpose of our study was to compare a plasma ablation device with a standard ablation device in shoulder RCR and determine which system is superior in terms of intra-articular heat generation and diathermy efficiency. We hypothesized that with the plasma ablation system, the plasma bubble would result in lower intra-articular temperatures and reduced diathermy times.
Methods
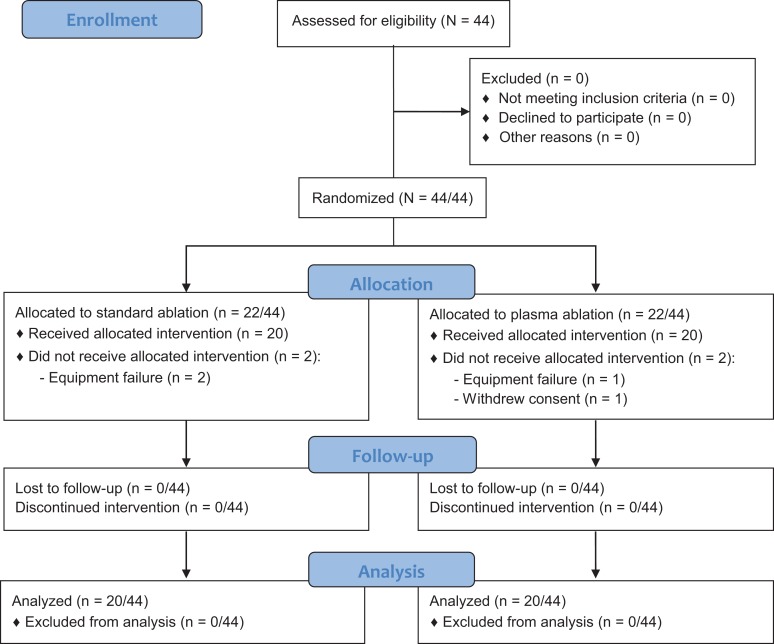
Institutional ethics committee approval was obtained before commencement of the study, and the trial was registered with the country of origin’s clinical trials registry. A summary of the study is outlined in the CONSORT (Consolidated Standards of Reporting Trials) flow diagram in Figure 1.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
A single-center, double-blinded prospective randomized controlled trial was conducted. Inclusion criteria were patients undergoing arthroscopic RCR aged between 18 and 70 years; both men and women were included. Patients were excluded if they were younger than 18 years or were unable to attend the 6-month follow-up visit. All surgical procedures were performed by the 2 senior authors (P.M., M.W.), who are fellowship-trained consultant orthopaedic surgeons with 25 years’ combined experience in shoulder surgery. Upon recruitment, patients were randomly assigned to either the standard RF ablation group, utilizing the Stryker Endoscopy Radio Frequency Ablation System (SERFAS) probe (Stryker), or the plasma RF ablation group, utilizing the Super Turbovac 90 RF Wand IFS system (ArthroCare). The research assistant used a computer program to generate a random number list. Patients were blinded throughout, and the surgeon was blinded until immediately before the activation of the RF device; at this time, the device was opened, introduced to the sterile field, and used in the usual manner.
All patients were positioned in the lateral decubitus position with the arm in traction. Standard posterior and lateral portals were established, and a temperature probe (fiber optic temperature sensor [STB probe (part No. SQ10939 L)]; LumaSense Technologies) was inserted under vision into the glenohumeral joint from the posteroinferior direction. The probe was connected to a Luxtron 812 thermometer (LumaSense Technologies). The probe was positioned so that it was as close to the working area of the RF ablation probe as possible without interfering with the arthroscopic instrumentation. The probe was then connected to a computer-running Luxtron TrueTemp (version 2.0.0; LumaSense Technologies), and the temperature was recorded in real time every 0.5 seconds. During the procedure, normal saline at room temperature was used to irrigate the joint. The operating room temperature was set to 21°C. Fluid flow and delivery were achieved via a gravity-fed system using two 3-L bags elevated to 1.85 m or a pressure-regulated electric pump . The pump pressure was varied by the surgeon during the procedure to achieve adequate visualization. The pressure ranged between 35 and 60 mm Hg. This was true for both patient groups. The ablation devices were attached to low suction or free drainage according to surgeon preference.
The plasma RF ablation device has a built-in thermometer, which was set to activate an audible alarm at 45°C. The ablation devices have specific alarms that sound when being used. The start and stop times of these alarms were monitored and recorded by a research assistant using a stopwatch. The temperature probe was removed at the end before skin closure.
Interval data–type definitions previously published by Matthews et al11 were used and are as follows. The total diathermy time was defined as the total duration of active RF ablation within the intra-articular space during the procedure, calculated by the sum of all individual diathermy applications. The total temperature time was defined as the total duration for which the temperature probe was actively recording the temperature during the procedure. The total surgery time was defined as the time from knife to skin until the completion of wound closure. A port was defined as a stab incision to the skin for the passage of instruments, an accessory outflow portal, or the arthroscope; the number of ports was recorded.
All patients were evaluated in the surgeon’s office at 6 months postoperatively.
Statistical Analysis
To facilitate sample size calculations, we used a previous study by Barker et al1 that demonstrated a mean temperature of 32°C during arthroscopic measurements and a minimum increase to 40°C (mean difference of 8°C). This value was chosen, as 40°C to 45°C is a safe zone according to Barker et al.1 We expected approximately equal standard deviations of 9°C in both groups; this was demonstrated by McKeon et al.12 An a priori calculation was performed based on the magnitude of differences reported by Barker et al,1 with an anticipated effect size of 0.5, an α level of 0.05, and a power of 0.8 (G*Power 3.1.9.2; Heinrich-Heine-Universität Düsseldorf) for differences between 2 measures. The sample size was based on 2 groups (standard ablation and plasma ablation). According to these effects, a total sample size of 40 was required. The averages and measure of spread of the data are represented as means and standard deviations. The means were compared using the Student t test, with a level of significance established above .05. To determine the magnitude of differences between the 2 groups for all dependent variables, the effect size (Cohen d) was calculated, with 0.2, 0.5, and 0.8 classified as small, moderate, and large, respectively.2 All statistical analyses were conducted with SPSS software (v 20; IBM Corp).
Results
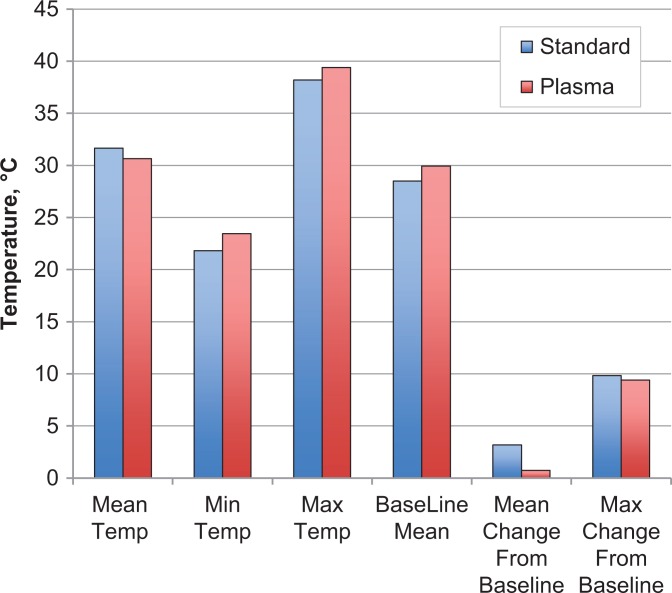
The trial was conducted between January 2014 and December 2016. A total of 44 patients were recruited. Three were excluded because of probe malfunction/breakage or computer system failure intraoperatively. One patient was excluded because the data file was unrecoverable. Of the remaining patients, 20 were randomized to the standard ablation group, and 20 were randomized to the plasma ablation group. There was no significant difference between the 2 groups in terms of surgery time, number of ports, diathermy time, or temperatures recorded by the probe (P > .05 for all) (Table 1). Furthermore, the magnitude of interindividual differences of all dependent measures was small, with effect size calculations ranging from 0.00 to 0.42 (Table 1). When the plasma ablation device alarm sounded, the intra-articular temperature was checked against real-time data on the Luxtron thermometer; the temperature was never greater than 45°C at that time. A graphical representation of the mean, range, baseline, mean change from baseline, and maximum change from baseline can be seen in Figure 2. The mean maximum temperatures recorded were 38.20°C ± 10.00°C and 39.38°C ± 12.10°C (P = .433) in the standard ablation and plasma groups, respectively.
TABLE 1.
Coefficient of Variation Between the 2 Groupsa
| Standard | Plasma | P Value | Effect Size | |
|---|---|---|---|---|
| No. of ports | 4.85 ± 1.04 | 5.25 ± 1.25 | .674 | 0.35 |
| Pressure, mm Hg | 37.00 ± 3.77 | 38.25 ± 6.13 | .502 | 0.25 |
| Surgery time, min | 74 ± 20 | 75 ± 19 | .866 | 0.05 |
| Temperature time, min | 59 ± 23 | 59 ± 19 | .530 | 0.00 |
| Diathermy time, min | 10 ± 5 | 10 ± 6 | .678 | 0.00 |
| Temperature, °C | ||||
| Mean | 31.66 ± 2.47 | 30.64 ± 2.63 | .757 | 0.40 |
| Minimum | 21.83 ± 8.86 | 23.45 ± 6.09 | .584 | 0.22 |
| Maximum | 38.20 ± 10.00 | 39.38 ± 12.10 | .433 | 0.11 |
| Baseline | 28.49 ± 3.88 | 29.94 ± 3.06 | .379 | 0.42 |
| Mean change from baseline | 3.17 ± 0.64 | 3.06 ± 0.73 | .638 | 0.16 |
| Maximum change from baseline | 9.83 ± 9.23 | 9.41 ± 12.96 | .630 | 0.04 |
aData are shown as mean ± SD.
Figure 2.
Comparison of mean temperatures in the standard and plasma ablation groups.
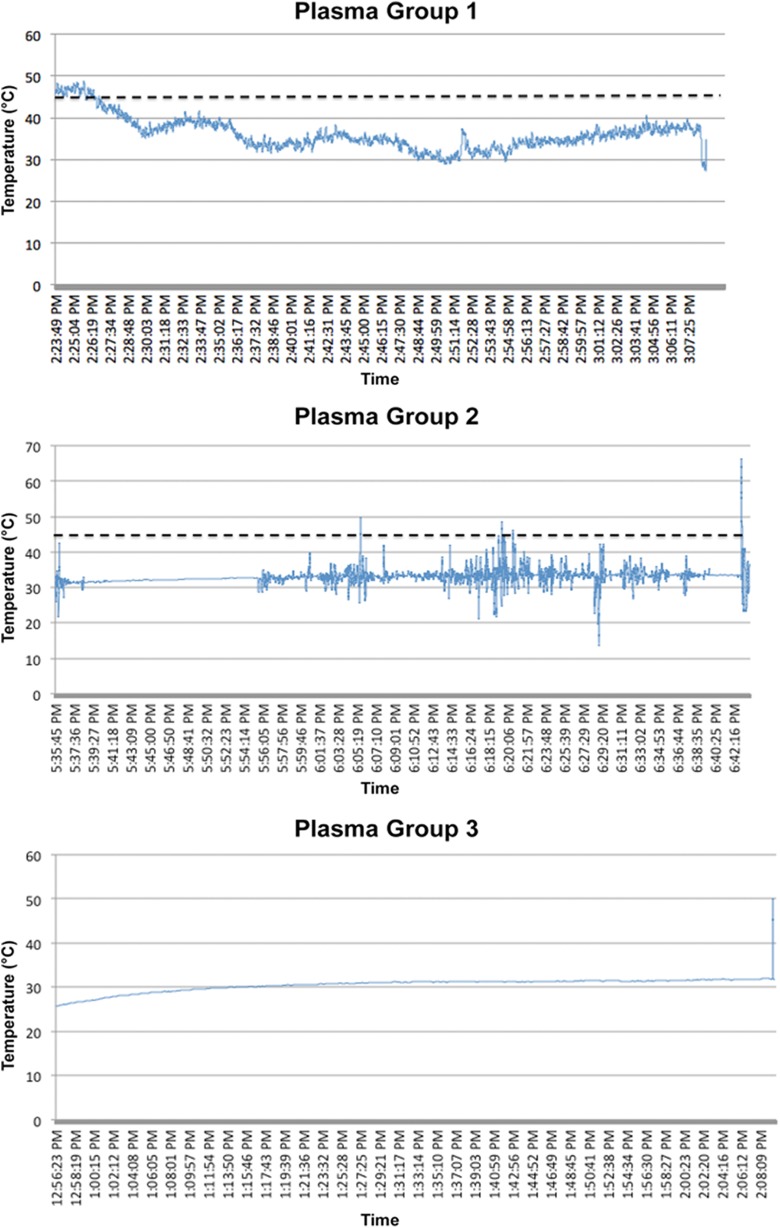
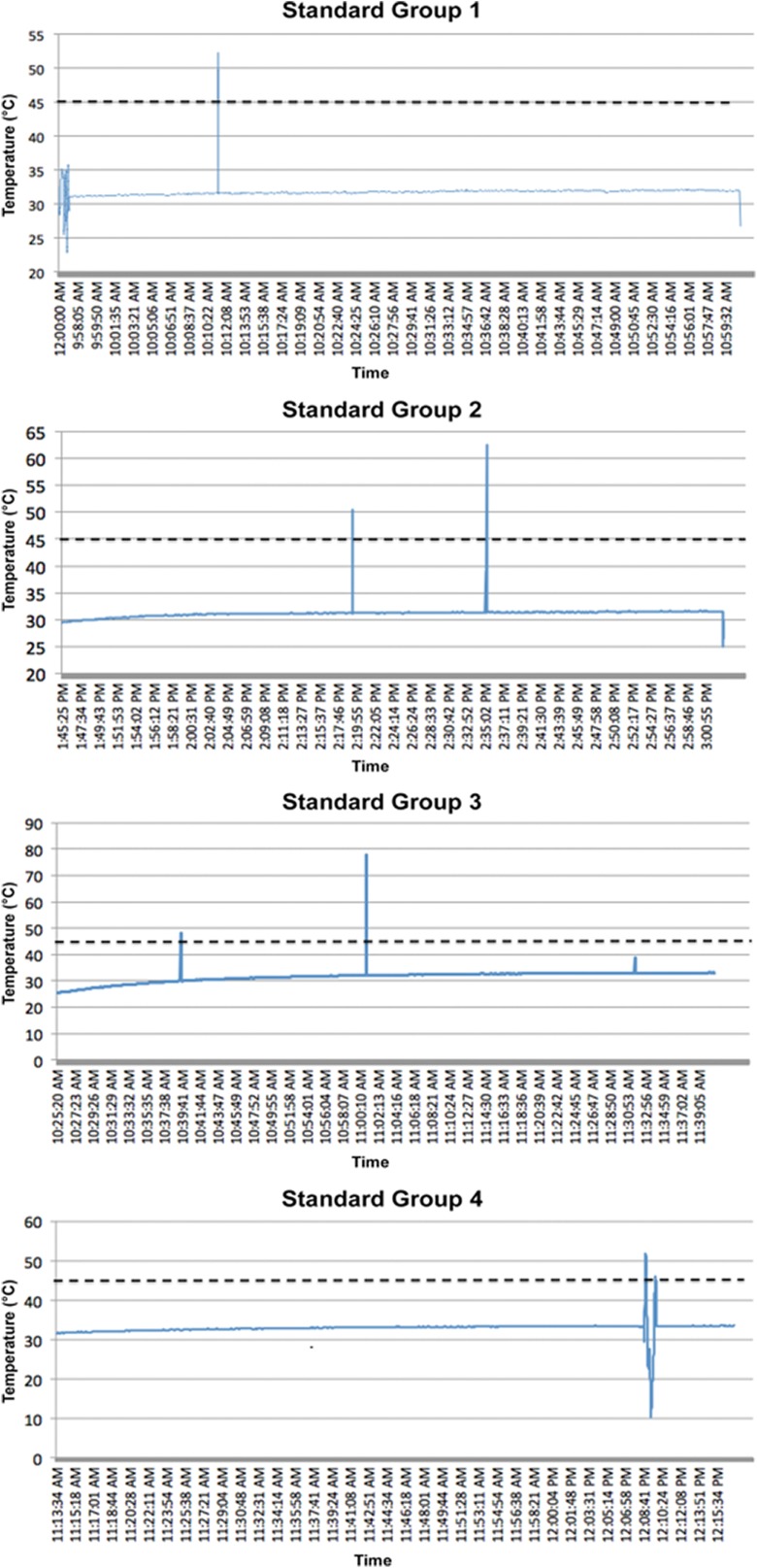
More concerning, 3 patients in the plasma ablation group recorded temperatures higher than 45°C (52.3°C, 62.7°C, and 77.7°C); however, the alarm did not sound, and there were only 2 recordings of these temperatures (ie, 0.5-second intervals × 2). In the standard ablation group, 4 patients recorded temperatures above 45°C (54.1°C, 51.7°C, 49.0°C, and 66.5°C). During the study, a probe had to be changed because of malfunctioning. The cases with concerning high temperature readings were analyzed closely and temperatures plotted over the entire length of the surgical procedure of these patients. These temperature spikes were transient, lasting less than 0.2 seconds (Appendix Figures A1 and A2). One patient in the plasma ablation group (patient 2) demonstrated persistent temperatures approaching 40°C early in the procedure, which normalized in the middle of surgery.
No patient reported symptoms of or demonstrated signs of significant chondrolysis on clinical review at 6 months.
Discussion
Our study demonstrated no significant difference between the standard RF ablation device and plasma RF ablation device in terms of intra-articular temperature or diathermy efficiency. This is consistent with previously published comparisons in arthroscopic surgery.6,11
Of concern, we detected temperatures greater than 45°C; this had not previously occurred in a similar study on ACLR. These high temperatures were transient, lasting less than a few seconds. They may represent the diathermy tip being in close proximity to the temperature probe during activation. While this was not observed in the ACLR study, it can be explained by the smaller size of the shoulder joint and the tighter structures in the shoulder compared with the knee joint, thus leading to a smaller volume of irrigation fluid for the heat to dissipate into along with a probe forced into closer proximity to the RF ablation device.
Matthews et al11 performed a complex statistical analysis in a similar study and found that the mean intra-articular temperature correlated closely with the maximum temperature in the joint, a similar finding to that observed by Barker et al.1 In this study, we opted for a simple statistical analysis to make the data more intuitive for the reader. While instances of catastrophic chondrolysis have been published in case reports and previous studies,7–10,13 no instances of chondrolysis were detected in the current study, despite high transient temperatures. These temperature spikes most likely reflected proximity to the active probe tip, as noted by Good et al.6
There is no convincing evidence to support the routine use of an intra-articular temperature probe in RCR; in the setting of an intact rotator cuff in which the potential space is smaller, this may theoretically contribute to increased working temperatures. When the rotator cuff is intact, it may shield the intra-articular chondrocytes from the heat generated during ablation, helping to protect against chondrolysis; this is an area that could be investigated further. Huynh et al7 examined the temperature of the subacromial space in vivo, comparing 2 types of RF ablation systems, and found that at no point during the 13 cases did a 50-second application of the devices lead to a temperature above the safe working limit of 45°C.
Limitations
In an otherwise well-designed study, we concede that we did not standardize or measure fluid irrigation pressure intraoperatively, nor did we control the number of ports; however, bias as a result of design was minimized by randomization and reflects real practice. Our study only investigated 2 RF ablation devices; there is scope to perform this simple study on other RF ablation systems and on other joints. Our study used only 1 temperature probe in the posterior lateral aspect of the glenohumeral joint. An improved study design would use multiple temperature probes spread out through the joint so that high temperature readings could be correlated between probes, although at an increased risk of probe entanglement and damage.
Conclusion
Plasma ablation and standard ablation devices are comparable in terms of their effect on intra-articular temperature and diathermy efficiency. Transient high temperatures can occur during the use of these devices in arthroscopic RCR.
APPENDIX
Figure A1.
Temperature recordings that exceeded 45°C in the plasma ablation group (3 patients).
Figure A2.
Temperature recordings that exceeded 45°C in the standard ablation group (4 patients).
Footnotes
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Human Research Ethics Committee of Mater Health Services.
References
- 1. Barker SL, Johnstone AJ, Kumar K. In vivo temperature measurement in the subacromial bursa during arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2012;21(6):804–807. [DOI] [PubMed] [Google Scholar]
- 2. Cohen J, ed. Statistical Power Analysis for the Behavioural Sciences. 2nd ed Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 3. Edwards RB, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339–346. [DOI] [PubMed] [Google Scholar]
- 4. Enochson L, Sönnergren HH, Mandalia VI, Lindahl A. Bipolar radiofrequency plasma ablation induces proliferation and alters cytokine expression in human articular cartilage chondrocytes. Arthroscopy. 2012;28(9):1275–1282. [DOI] [PubMed] [Google Scholar]
- 5. Ficklscherer A, Loitsch T, Serr M, et al. Does footprint preparation influence tendon-to-bone healing after rotator cuff repair in an animal model? Arthroscopy. 2014;30(2):188–194. [DOI] [PubMed] [Google Scholar]
- 6. Good CR, Shindle MK, Kelly BT, Wanich T, Warren RF. Glenohumeral chondrolysis after shoulder arthroscopy with thermal capsulorrhaphy. Arthroscopy. 2007;23(7):797. [DOI] [PubMed] [Google Scholar]
- 7. Huynh V, Barbier O, Bajard X, Bouchard A, Ollat D, Verier G. Subacromial temperature profile during bipolar radiofrequency use in shoulder arthroscopy: comparison of Coblation® vs. VAPR® . Orthop Traumatol Surg Res. 2017;103(4):489–491. [DOI] [PubMed] [Google Scholar]
- 8. Jerosch J, Aldawoudy A. Chrondrolysis of the glenohumeral joint following arthroscopic capsular release for adhesive capsulitis: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(3):292–294. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan LD, Ionescu D, Ernsthausen JM, Bradley JP, Fu FH, Farkas DL. Temperature requirements for altering the morphology of osteoarthritic and nonarthritic articular cartilage: in vitro thermal alteration of articular cartilage. Am J Sports Med. 2004;32:688–692. [DOI] [PubMed] [Google Scholar]
- 10. Lee CH, Ha CW. Rapid chondrolysis of knee joint after arthroscopy using radiofrequency device [in Korean]. Korean Orthop Assoc. 2011;46(1):88–94. [Google Scholar]
- 11. Matthews B, Wilkinson M, McEwen P, et al. In vivo arthroscopic temperatures: a comparison between 2 types of radiofrequency ablation systems in arthroscopic anterior cruciate ligament reconstruction. A randomized controlled trial. Arthroscopy. 2017;33(1):165–172. [DOI] [PubMed] [Google Scholar]
- 12. McKeon B, Baltz MS, Curtis A, Scheller A. Fluid temperatures during radiofrequency use in shoulder arthroscopy: a cadaveric study. J Shoulder Elbow Surg. 2007;16(1):107–111. [DOI] [PubMed] [Google Scholar]
- 13. Zoric BB, Horn N, Braun S, Millett PJ. Factors influencing intra-articular fluid temperature profiles with radiofrequency ablation. J Bone Joint Surg Am. 2009;91:2448–2454. [DOI] [PubMed] [Google Scholar]