ABSTRACT
Multi-tiered aviaries for laying hens are designed to provide resources, such as perches, that allow birds to perform natural behaviors, thus improving their welfare. This research examined nighttime roosting heights and substrates used by laying hens of 4 genetic strains (Dekalb White: W1, Hy-Line W36: W2, Hy-Line Brown: B1, Bovans Brown: B2), in multitier aviaries (144 hens/unit, 4 units/strain) at 25 to 28 wk of age (peak lay). Influence of litter provision on roosting patterns of the strains was also tested. Direct observations of hens’ nighttime roosting patterns on wire floors, ledges and perches across tiers were conducted before (PRE), immediately after (IMM), and 3 wk after (ACC) hens gained access to litter. During all periods, more W1 and W2 hens roosted on middle and upper ledges than B1 and B2 hens (all P≤0.05), while more B1 and B2 hens used perches throughout the aviary than W1 and W2 hens (all P≤0.05). W1 (15±1.9, 14±3.36) and W2 (19±2.1, 18±2.6) hens occupied perches in the upper tier in greater numbers than B1 (7±3.2, 3±4.6) and B2 (11±2.1, 5±3.36) hens during PRE (P = 0.01) and ACC (P = 0.02) periods, respectively. B1 and B2 hens occupied wire floors in larger numbers than W1 and W2 hens during PRE (P = 0.02) and IMM (P = 0.03) periods, though this difference disappeared in the ACC period. During the IMM period, more W1 and W2 roosted in the lower tier, while more B1 and B2 hens were observed in the middle and upper tiers (all P ≤ 0.05). These findings demonstrate the importance of perches for B1 and B2 hens and space to roost higher in aviary units for W1 and W2 hens during the night, and underscore the need to consider aviary design, management practices, and preferences of different hen strains to ensure good hen welfare in aviaries.
Keywords: aviary, laying hen, behavior, night, perch
INTRODUCTION
The behavioral need of the modern domestic chicken (Gallus gallus domesticus) to roost at night is partially influenced by the anti-predator behavioral repertoire of their ancestors (Gallus gallus) and partially by domestication (Wood-Gush and Duncan, 1976; Blokhuis, 1984; Duncan et al., 1992; Olsson and Keeling, 2000). Weeks and Nicol (2006) interpreted nighttime roosting on high perches as a behavioral priority, even in commercial indoor housing systems where laying hens are protected from predation. Experimental studies indicate that perch heights of more than 90 cm are preferred by Lohmann Selected Leghorn hens (LSL; Brendler et al., 2014) and that height matters more to LSL and Lohmann Brown hens than graspable perches over flat plastic grids for roosting (Schrader and Müller, 2009). In commercial aviaries, various white and brown strains of laying hens use higher perches for nighttime roosting to a greater extent than lower ones (Odén et al. 2002; Brendler and Schrader, 2016; Campbell et al., 2016). Thus, simply providing perches is not enough to meet hens’ behavioral roosting needs (Wall and Tauson, 2007; EFSA AHAW Panel, 2015). Additionally, there is conflicting evidence as to whether hens prefer perches that allow them to grasp with their feet versus flat surfaces that do not facilitate grasping (Schrader and Müller, 2009; Schrader et al., 2016). In some cases, solid metal ledges are provided in aviaries to help hens transition between levels, but it is unknown whether hens consider such structures suitable for roosting at night or are only used when more desirable sites are occupied.
Selection pressures on laying hens for other outcomes have resulted in differences among strains in behaviors such as foraging, pecking, and nest use (Klein et al., 2000; Albentosa et al., 2003; Singh et al., 2009). Such selection may also have impacted roosting behavior among modern laying hen strains, leading to variation among strains of hens in their preferences for roosting at different heights or on different types of substrates within aviary systems. In the few studies performed to date, behavioral variation has been found among white and brown hens (Braastad and Katle, 1989; Schütz and Jensen, 2001; Schütz et al., 2001) including differences in use of perches and height at which hens roost at night (Faure and Jones, 1982; Wall and Tauson, 2007; Ali et al., 2016).
Objective 1: Influence of Hen Strain on Nighttime Roosting
Recently the EFSA AHAW Panel (2015) addressed several factors that should be considered when providing perches to laying hens in order to fulfill their basic behavioral instinct for roosting. These factors include the height of the perches from the ground, surface type and depth, and the ability of hens to grasp perches with their feet when perching. Thus, the main objective of the current study was to investigate the influence of genetic strain on laying hens’ nighttime roosting behavior in a commercial-style aviary system with emphasis on the height and substrate of the roosting site. We demonstrated previously (Ali et al., 2016) that more W1 and W2 hens roosted throughout the highest tier of the aviary compared to B1 and B2 hens. In this paper, we describe the specific substrates hens are roosting upon and the interaction of substrate with aviary tier height. We predicted that all strains of hens would prefer to roost on round metal perches they can grasp with their feet versus on solid metal ledges or wire floors that permit no or only partial grasping. We also predicted that W1 and W2 hens would occupy substrates in the uppermost tier to a greater extent than substrates in lower tiers.
Objective 2: Influence of Litter Access on Nighttime Roosting
In addition to considering type of roosting substrate, it is also important to consider whether hens alter their nighttime roosting patterns in response to immediate changes in the environment, such as losing or gaining access to a floor litter area. Upon pullet arrival at a cage-free laying facility, litter access is routinely restricted for several weeks to help hens find water and food, and to minimize the number of floor-laid eggs (Alm et al., 2015; Lambton et al., 2015). For pullets reared with access to litter, this would represent a loss of a resource they were used to having. In one experimental study examining loss of litter access in hens of different strains, LSLs scratched less and feather pecked more than white Dekalb hens, which moved more and feather pecked to a lesser degree (Klein et al., 2000). As part of a larger study examining differences among strains of laying hens, we examined the effect of giving hens access to a floor litter area after a period of restriction to a tiered aviary on their distribution throughout the aviary before and after they gained access to the floor. Not surprisingly, we found hens altered their daytime distribution among the various levels in a multi-tier aviary when the floor litter area was available (Ali et al., 2016). Evidence that nighttime distribution of hens across the aviary levels changed as well, compelled us to examine further how hens were altering their roosting patterns at night in response to daytime litter access in addition to our main objective of understanding differences between strains of hens. Though hens were only active in the litter area during the day, opening the aviary doors represented a large environmental change, effectively doubling the amount of space per hen and providing additional areas for activity and rest during the day. Further, the initial novelty of accessing the floor litter area might be expected to affect hens differently depending on whether they, or their strain, were more fearful or more curious or were more or less physically able to access the area. Thus, a secondary objective of the present study was to examine the influence of giving laying hens access to floor litter areas on their nighttime roosting patterns. We predicted that all hens’ nighttime roosting distribution would be affected by this change, at least in the short term.
MATERIALS AND METHODS
Ethics
All research protocols were approved by the Michigan State University Institutional Animal Care and Use Committee prior to the start of data collection (AUF #01/15–025-00).
Hens and Housing
A total of 2304 laying hens of 4 genetic strains (n = 576 each: Dekalb White (W1), Hy-Line W36 (W2), Hy-Line Brown (B1), and Bovans Brown (B2)) were used. These hens were part of a larger overall study, from which some results have been published regarding differences between strains in distribution throughout the aviary system during day and night (Ali et al., 2016). The details of hens, housing, system management, and mortality are identical to those already reported (Ali et al., 2016). The present study focuses specifically on nighttime roosting, describing heights and specific substrates used by hens, and details pertinent to understanding this study are presented below.
Pullets were reared in floor pens with litter and platforms (46 cm height) then placed in aviary units at 17 wk following UEP (2017) recommendations for cage-free egg layers with respect to litter and tiered enclosure space. Each of four replicate rooms had four discrete aviary units (Natura 60, Big Dutchman, Holland, MI USA). Each unit was stocked with 144 hens of a single strain. The four laying hen strains were allocated so all strains were present in each of the rooms (1 unit x 4 strains x 4 rooms = 16 units total). Each aviary unit was composed of a 3-tiered enclosure (each level with 61 cm internal ceiling height) and a litter area with wood shavings. The wire floor of the lower tier was 51 cm from the aviary floor, while the wire floor of the middle and the upper tiers were 112 and 173 cm from the aviary floor, respectively. Each tiered enclosure contained internal perches (round metal, 3.1 cm diameter) at all levels and one outer perch in the open litter area (Figure 1). Two solid, metal ledges, intended to help hens transition between tiers within the enclosure, ran the full width of the unit in front of the middle and upper tiers. Colony nests were provided in the upper tier. The lower and upper tiers contained drinkers and the lower and middle tiers had both internal and external feeders. Manure belts ran under each tier. Doors used to provide access to the litter area were located in the lower tiers to permit hen transition from enclosure to litter and vice versa. At 26 wk of age, the hens were given access to litter areas; this followed a period of litter restriction from 17 to 25 wk of age when hens were being trained to use nests after placement in the aviaries Aviary doors opened at 11:30 each morning, and a gradual 45-min dimming of lights in the evening was used to encourage hens to re-enter the aviary at night. Between 25 and 28 wk of age, the dark period was gradually reduced from 9 h 45 min to 9 h per night.
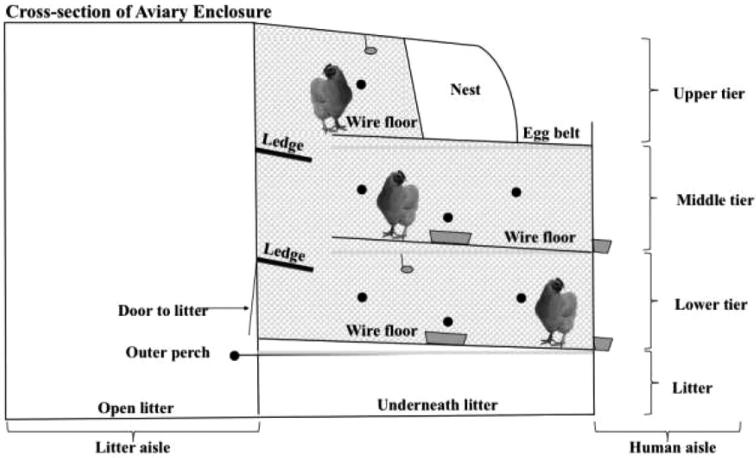
Figure 1.
An end view of the 3-tiered aviary unit, showing human and litter aisles and locations of litter areas, solid metal ledges, wire floors, the colony nest, manure belts (gray bars), perches (black circles), drinkers (gray ovals), and external and internal feeders (gray boxes). The stippled gray area shows the space available to hens at night.
Each hen was provided with 1132 cm2 of useable floor area divided between 581 cm2/hen of litter area and 551 cm2/hen of tiered enclosure space (439 cm2/hen wire flooring plus 112 cm2/hen solid metal ledges). There were 9 hens per pin-metered drinker (nipple). The system provided 5.08 cm feeder space per hen, 40.64 cm of perch space per hen, and 88 cm2 of nesting space per hen at the initial stocking of 144 hens/unit.
During the night, when observations for the present study were conducted, hens did not have litter access; therefore, litter areas were not included in our analysis. Similarly, colony nests, which ran the length of each unit in the upper tier, automatically closed approximately 2 h before lights turned off at the end of each day, thus hen occupancy of this space was not analyzed in the current study.
Data Collection
Direct observations of hen occupancy of round metal perches, solid surface ledges, and wire floors inside the tiered enclosures during the nighttime, when the lights were fully off, were conducted over 3 consecutive days at each of 3 time periods relative to hens gaining access to the floor litter area. Pre-opening observations (PRE: hens = 25 wk of age) occurred starting 3 d before hens first had daytime access to the litter area. Immediate post-opening observations (IMM: hens = 26 wk of age) started 1 d following initial daytime litter access to capture whether hens’ nighttime roosting patterns changed in the short term. As hens were reared on litter, this IMM period is actually a re-introduction of hens to litter following 9 wk without litter access rather than an entirely novel experience though the floor litter area of the aviary was a new location. Acclimated post-opening observations (ACC: hens = 28 wk of age) occurred 3 wk following initial litter access to examine whether daytime litter access affected distribution long term.
Observations were performed 1) 30 min after full darkness (DARK PM: 20:45 for 25 wk, 21:15 for 26 wk, and 21:30 for 28 wk) and 2) 2 h before lights on (DARK AM: 3:15 for 25 wk and 26 wk, and 3:00 for 28 wk) over the 3 D for each period (PRE, IMM and ACC). During each observation time (AM and PM), 2 counts of hens were made for each of the 16 units with the second count made about 1 h after the first count. In total, each unit was observed 36 times across the study (2 counts/time x 2 times/night x 3 nights/period x 3 periods = 36 observations per unit). A count of all hens in unit took approximately 90 s. Disturbance of hens was minimized by using green headlamps, which allowed observers to see hens in the darkened room without rousing them to movement (Ali et al., 2016; Campbell et al., 2016).
Prior to the start of data collection, 3 observers were trained for 3 D to establish synchrony within observer pairs and ensure a high level of inter-observer reliability. All observations were performed by a pair of observers (composed of 2 of the 3 previously trained observers). Observations were completed by the pair of observers visiting each room in a different randomized order across the 3 D of each period (PRE, IMM, and ACC). One observer was located in the human aisle and the second was in the litter aisle of the tiered enclosure to allow for simultaneous recording of birds’ distribution from both perspectives (Figure 1). Each observer counted the number of hens per location throughout the aviary unit, starting from the bottom tier and working upward. Hens on the wire floor of the upper tier were recorded only by the observer in the human aisle, who climbed onto the aviary to look down on hens without disturbing them.
Data and Statistical Analyses
As described above, there were 4 aviary units for each of the 4 hen strains, and aviary unit was the subject of analysis for all statistical tests. Each observation time point (DARK PM and DARK AM) was composed of 2 counts of hen location within each unit. Statistical analyses were performed using R software (version 3.3.1), package “stats” (R Core Team, 2013). Descriptive statistics were calculated using the psych package, and data are presented as mean ± standard error of the mean (SEM). Since two observers collected data, inter-observer reliability was calculated using Cohen's kappa Agreement coefficient (K), following Landis and Koch (1977) and using the “cohen.kappa” function in the psych package. Inter-observer reliability was measured during the observer-training period, before data collection took place, when trainees observed the same areas of the aviary simultaneously. Inter-observer agreement was considered good (Kappa = 0.96 (P < 0.001), CI (0.90, 0.99)).
To describe the influence of different laying hen strains on the nighttime preference for roosting height and substrate within the aviary unit during different observational periods and all possible interactions, generalized linear mixed models were developed with family set to “Poisson”, with the “log” link function, using the lme4 package (Bates et al., 2015). Aviary unit, day, and time point (DARK PM and DARK AM) were included as random effects for all models, and P ≤ 0.05 was considered significant. The models included strain of laying hen (W1, W2, B1, B2), observational period (PRE, IMM and ACC), roosting substrate (wire floor, perch and ledge), and location of the roosting sites within aviary unit (lower tier, middle tier or upper tier) and their interactions were included as fixed effects. Statistically significant effects were further analyzed using Tukey's honestly significant difference multiple comparison procedures using the multcomp package (Hothorn et al., 2008); P ≤ 0.05 was considered significant.
To identify the potential overcrowding on roosting resources within aviaries (i.e., the perches, ledges and wire floors of each tier), the maximum numbers of hens observed on each resource in the current study were recorded. These maximums are presented in Table 1 with the estimated capacity of each resource as calculated based on kinematic analysis (Mench and Blatchford, 2014 and Riddle et al., 2018) and space recommendation standards (UEP, 2017).
Table 1.
Comparison of capacity of the aviary system to accommodate hens in the various levels with the maximum number of white (W1 or W2) and brown (B1 or B2) hens ever observed in those areas during nighttime observations.
| Maximum # hens observed | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated capacity in # hens1 | Wire floor | Perches | Ledges | ||||||
| Location | Wire floor | Perches | Ledges | White | Brown | White | Brown | White | Brown |
| Lower tier | 37 | 48 | - | 11a* | 49a‡ | 9a* | 31a† | - | - |
| (108) | (48) | 32b* | 21b‡ | 45b* | 7b‡ | - | - | ||
| [64] | [39] | 21c* | 69c‡ | 13c* | 30c† | - | - | ||
| Middle tier | 31 | 48 | 8 | 13a* | 27a† | 13a§ | 20a‡ | 25a§ | 14a‡ |
| (90) | (48) | (25) | 45b* | 55b† | 19b§ | 43b† | 37b* | 12b† | |
| [54] | [39] | [15] | 12c* | 41c‡ | 9c* | 36c‡ | 25c§ | 9c† | |
| Upper tier | 10 | 16 | 8 | 64a§ | 22a† | 25a* | 12a‡ | 30a§ | 14a‡ |
| (31) | (16) | (25) | 14b§ | 37b ‡ | 11b§ | 17b † | 16b* | 13b‡ | |
| [18] | [13] | [15] | 43 c * | 28c† | 24 c § | 6c‡ | 30c§ | 11c‡ | |
1Perching space was estimated at 15 cm/hen following the UEP (2017) guidelines. The space required to accommodate a hen on the floor and ledges was estimated at 929 cm2/hen as per UEP (2017) recommendations for multi-tier systems, at (the number presented in parentheses) at 318 cm2/lying hen following kinematic analysis of hen space requirements by Mench and Blatchford (2014), and at [the number presented in square brackets] 538.3 cm2/lying hen and 18.6 cm/hen perching space following space use analysis of Hy-line W36 hen space requirements by Riddle et al. (2018). Bold typeface indicates hen numbers above UEP (2017) recommended capacity. Different letter superscripts represent different periods of time relative to hens gaining access to the floor litter area: aPRE is the period before hens had litter access; bIMM is the period immediately after hens had litter access; and cACC is the period 3 wk after hens gained access to the floor litter area. Different symbol superscripts represent different strains of hens: *W1, §W2, †B1, and ‡B2.
RESULTS AND DISCUSSION
Impact of Strain of Hen on Nighttime Roosting
To the best of our knowledge, this is the first study to examine differences in nighttime roosting substrates between multiple strains of brown and white laying hens in aviary conditions. Overall, we found that both strains of brown hens (B1 and B2) were typically similar to each other in their nighttime patterns of occupancy of the various substrates and tiers in the aviary enclosure (Figures 2–5). In addition, in most cases, both strains of white hens (W1 and W2) also showed similar patterns to one another (Figures 2–5). However, in a few cases, the two white strains (W1 and W2) of hen differed in their substrate and location preferences; therefore, it should not be assumed that all brown hens will act similarly to each other and differently from all white hens. For example, in a previous study comparing perching between other strains of brown and white hens, more Lohmann selected white hens (86%) perched compared to Lohmann brown hens (81%) while more Hy-Line brown hens (87%) perched than Hy-Line white hens (84%; Wall and Tauson, 2007).
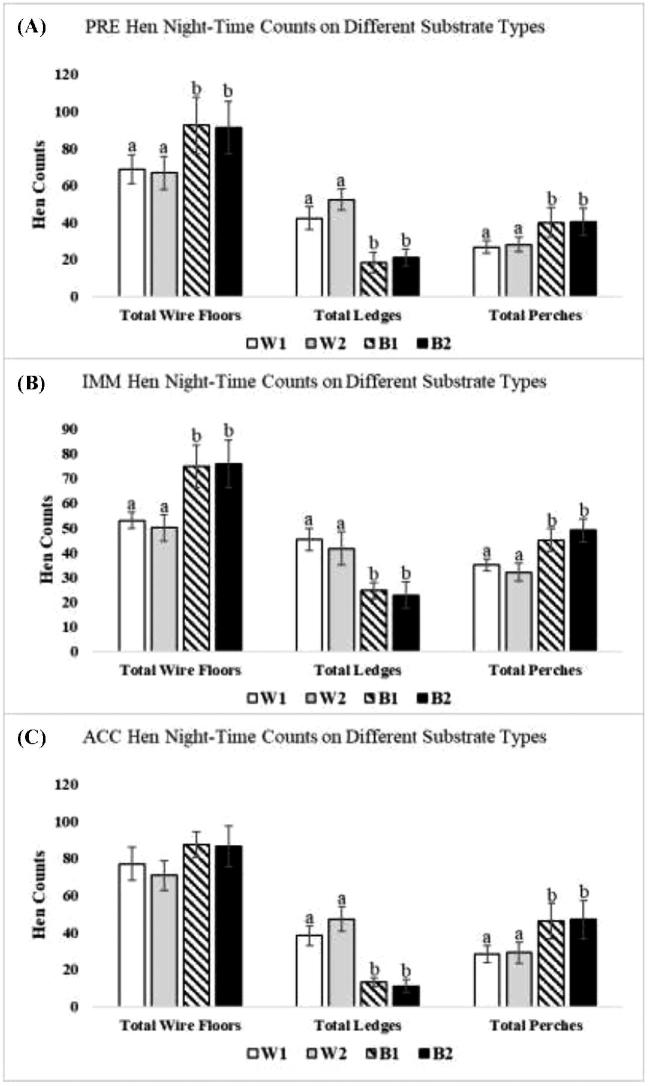
Figure 2.
Counts of hens of the 4 strains occupying perch, ledge and wire floor space throughout the enclosure (W1 = DeKalb White, W2 = Hy-Line W36, B1 = Hy-Line Brown, and B2 = Bovans Brown). All parameters are expressed as the mean counts of hens ± SEM. Different superscripts indicate differences (P < 0.05) among different strains for that substrate.
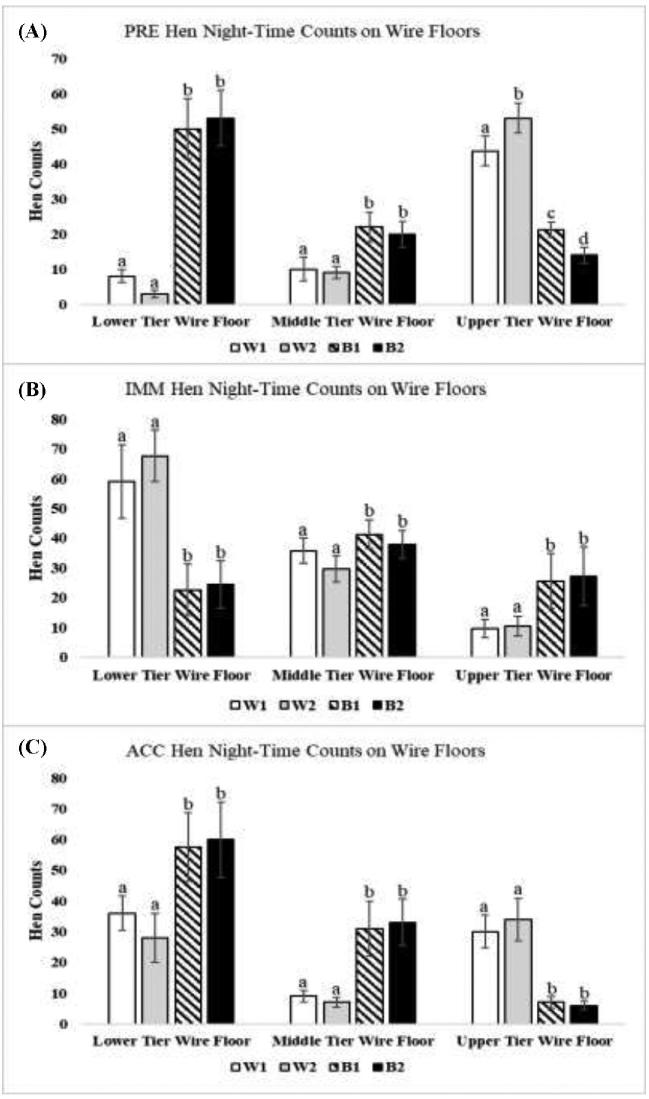
Figure 5.
Counts of hens of the 4 strains occupying wire floor presented by tier of the aviary enclosure (W1 = DeKalb White, W2 = Hy-Line W36, B1 = Hy-Line Brown, and B2 = Bovans Brown). All parameters are expressed as the mean counts of hens ± SEM. Different superscripts indicate differences (P < 0.05) among different strains for that floor location.
Roosting Substrate Matters
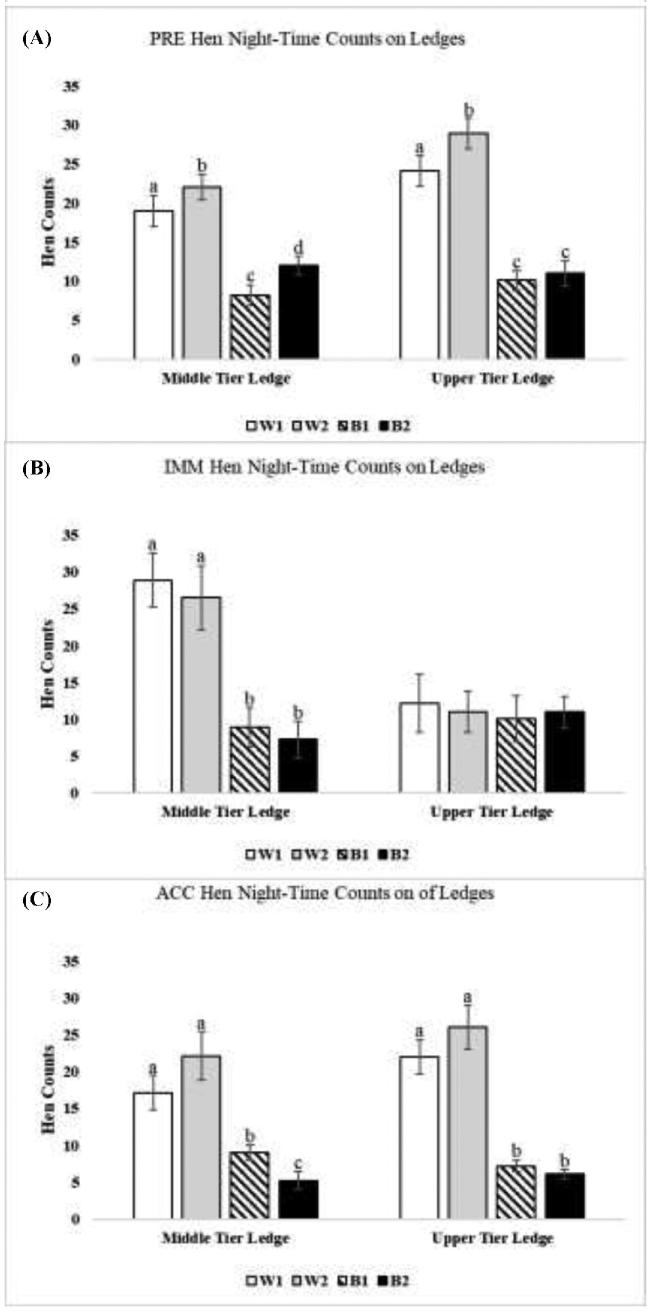
At all periods, more W1 and W2 hens roosted on the solid metal ledge space than B1 and B2 hens (Figure 2; PRE: Z = 8.26, P = 0.001; IMM: Z = 6.98, P = 0.011; ACC: Z = 7.89, P = 0.021), and this was true for ledges in both the upper and middle tiers (Figure 3; PRE: Z = 10.23, P = 0.001, IMM: Z = 4.65, P = 0.041, ACC: Z = 9.36, P = 0.01).
Figure 3.
Counts of hens of the 4 strains occupying solid metal ledge space in middle and upper tiers (W1 = DeKalb White, W2 = Hy-Line W36, B1 = Hy-Line Brown, and B2 = Bovans Brown). All parameters are expressed as the mean counts of hens ± SEM. Different superscripts indicate differences (P < 0.05) among different strains for that ledge location.
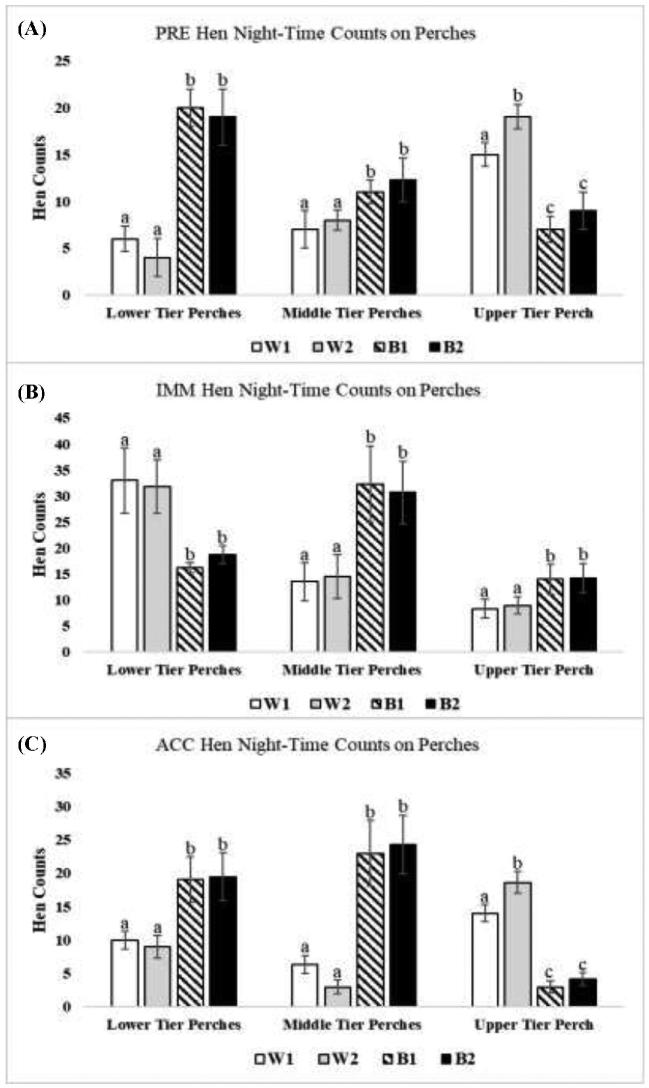
At night, more B1 and B2 hens used perches throughout the aviary enclosure than W1 and W2 hens (Figure 2; PRE: Z = 3.56, P = 0.031; IMM: Z = 5.26, P = 0.021; ACC: Z = 4.02, P = 0.027). Specifically, more B1 and B2 hens were generally found perching in the lower and middle tiers. However, W1 and W2 hens occupied perches in the upper tier at higher counts during PRE (Figure 4A; Z = 7.63, P = 0.001) and ACC (Figure 4C; Z = 5.85, P = 0.021).
Figure 4.
Counts of hens of the 4 strains occupying perch space presented by tier of the aviary enclosure (W1 = DeKalb White, W2 = Hy-Line W36, B1 = Hy-Line Brown, and B2 = Bovans Brown). All parameters are expressed as the mean counts of hens ± SEM. Different superscripts indicate differences (P < 0.05) among different strains for that perch location.
More B1 and B2 hens occupied the wire floor space overall in the aviary enclosure at night than W1 and W2 hens during PRE (Figure 2A; Z = 6.02, P = 0.021) and IMM (Figure 2B; Z = 5.98, P = 0.027), though this difference disappeared in the ACC period (Figure 2C). However, more W1 and W2 hens typically occupied the wire floor space in the upper tier than B1 and B2 hens (Figure 5A; PRE: Z = 11.85, P = 0.001 and Figure 5C; ACC: Z = 9.89, P = 0.003).
Overall, the patterns seen with respect to roosting substrates could reflect W1 and W2 hens’ prioritizing roosting at height above type of substrate upon which they roost (see below and also Schrader and Müller, 2009). Both strains of brown hens (B1 and B2) on the other hand may be driven to maintain more inter-bird distance, and previous observations in litter-based systems found that ISA Brown hens dispersed across the space provided to them (Channing et al., 2001; Odén et al., 2002). Fewer B1 and B2 hens roosted in areas with limited space, such as ledges and the upper tier, which may also be a reflection of their larger bodies occupying more space when they perch or lie down (Riddle et al., 2018). (Average body weights for hens in the current study were: W1: 1.6 ± 0.31, W2: 1.5 ± 0.34 kg, B1: 2.0 ± 0.52, and B2: 1.9 ± 0.42 kg.) Another possible explanation for B1 and B2 hens’ higher occupancy of perch and wire floors and lower use of solid metal ledges could be that these hens prefer to roost on substrates they can grasp with their toes. Anecdotally, B1 and B2 hens in the present study appeared to roost on the edges of the wire floors and wrap their toes around the edge, which would support recommendations to consider graspable edges of elevated slatted or grid platforms as part of the perching allowance in a system (EFSA AHAW Panel, 2015). However, Schrader and Müller (2009) reported that Lohmann Brown hens showed weaker preference for a perch they could grasp than for an elevated resting area. Differences between our findings and previous studies could indicate that not all strains of brown hens have the same roosting preferences.
Roosting Height Matters
In this study, W1 and W2 hens than by B1 and B2 hens occupied the upper tier at night to a greater degree during the stable PRE and ACC periods (Figures 3-5; and see Ali et al., 2016). During PRE and ACC periods, W2 hens also occupied the roosting substrates of the upper tier in greater numbers than W1 hens (i.e., upper tier perch: Figures 4A (PRE) and 4c (ACC); middle and upper tier ledges: Figures 3A (PRE); and upper tier wire floor: Figure 5A (PRE); all P ≤ 0.05).
Due to the complex configuration of multi-tier aviaries, it is still not clear how hens perceive height of perches within these systems (EFSA AHAW Panel, 2015). Hens may perceive perch height as distance from the house floor, or they may view perch height as distance from the wire tier floor. In experimental studies, LSL hens preferred perches at or greater than 90 cm above the ground (Brendler et al., 2014), and all elements of the middle and upper tiers in the present study were above these heights. Height of the nighttime roosting site from the house floor appeared to be important to both W1 and W2 hens in ours study, suggesting that they do perceive height from the floor despite the complexity of the system. However, the B1 and B2 hens did not display a clear preference for roosting based on height of substrates from the house floor. It is unclear whether this is due to a difference in their recognition of the tiers as being at different heights, or whether, as discussed below, other factors were more important in their selection of nighttime roosting sites.
Access to Litter has Short-term Effects on Distribution at Night
Daytime litter access resulted in changes in use of perches and wire floors for nighttime roosting in the IMM period (Figure 2). Both strains of white hens (W1 and W2) occupied these substrates to a greater extent on the lower tier in the IMM than B1 and B2 hens. In contrast, B1 and B2 hens shifted to occupy these substrates to higher degrees in the middle and upper tiers (Figure 4B; middle tier perches: Z = 4.63, P = 0.031; upper tier perch: Z = 5.02, P = 0.019; and Figure 5B; upper tier wire floor: Z = 6.02, P = 0.021). The highest counts of W1 and W2 hen strains on ledges were recorded during the IMM period (Figure 2B), resulting from increased occupancy of the middle ledge (Figure 3B). However, this occurred in conjunction with a decrease in the number of W1 and W2 hens on the upper ledge, which became similar to numbers of B1 and B2 hens during IMM (Figure 3B).
Daytime access to litter initially impacted all strains’ nighttime roosting heights in the aviary at night. During the IMM period, W2 and W1 hens, which had previously occupied the upper tier at high numbers at night, instead occupied the lower tiers to a greater degree while B1 and B2 hens, which had occupied the lower tier at the highest counts in the PRE period, occupied both middle and upper tiers at higher numbers (Ali et al., 2016). However, 3 wk later, in the ACC period hens reverted to their PRE patterns, and more W1 and W2 hens were again found in the upper tier at night and more B1 and B2 hens in the lower tier (Ali et al., 2016).
Klein and colleagues (2000) also reported that different strains of laying hens (in their case LSLs and Dekalb White hens) displayed different responses to changes in their environment. As W2 and W1 hens in our study initially entered and occupied the litter in greater numbers in the IMM period (as reported by Ali et al., 2016), it may be that these white hens remained in the lower tiers at night in order to access the litter more quickly the next day. However, it is not clear from either our work or studies of others why the B1 and B2 hens would have initially moved higher into the aviary; the likeliest explanation is that they were avoiding the litter. However, research linking fear responses to genetic strains of hens has found either no difference or greater fear responses in white Leghorn strains than in brown hens derived from Rhode Island Red origins (Murphy, 1977; Jones and Faure, 1981; Albentosa et al., 2003; Uitdehaag et al., 2008).
Uneven Distribution of Hens
It is important to highlight that the observed preferences for different nighttime roosting heights and substrates of white (W2 and W1) and brown (B1 and B2) strains of laying hens resulted in an uneven distribution of hens throughout the aviary tiers and resources. To illustrate whether preference for roosting sites resulted in overcrowding of some aviary locations, we created Table 1 to present the maximum number of hens observed on the perches, ledges, and floors of each tier in comparison with the number of hens estimated to be able to occupy those spaces based on kinematic analysis and space recommendation standards. The number of hens estimated to fit on the round metal perches was calculated using perching space of 15 cm per hen, a common recommendation (e.g., EFSA AHAW Panel, 2015; UEP, 2017). This recommendation has a basis in the scientific literature (Appleby, 1995), though there is evidence that hens, including of these strains, may in fact both need (Riddle et al., 2018) and prefer more space to perch (Duncan et al., 1992; Newberry et al., 2001; Cook et al., 2011). The number of hens estimated to fit on floor and ledge space was estimated and presented in Table 1 using both UEP’s (2017) recommendation of 929 cm2/hen as well as using the amount of static space occupied by a lying hen using kinematic analysis (Mench and Blatchford, 2014; Riddle et al., 2018).
Hens on the floor or ledges (i.e., those not roosting on perches) lie down to sleep and are essentially stationary at night, thus UEP’s (2017) recommendation to provide each hen in an aviary with 929 cm2/hen overestimates the amount of space an individual hen would need at night. Conversely, Mench and Blatchford's (2014) estimate of 318 cm2/hen is likely too conservative for several reasons. First, the Mench and Blatchford (2014) estimate of physical space was made using W2 hens; thus, it is likely that the larger B1 and B2 hens require more space to lie down and would be relatively more crowded at the 318 cm2/hen estimates. Further, though their hens were of similar weight to our W2 hens (1.6 ± 0.7 kg in their study vs. 1.5 ± 0.34 kg in ours), the birds they studied were 1.5 yr old (i.e., ∼78 wk of age), and may have had reduced feather cover, resulting in a smaller physical outline. Our kinematic analysis of the space required by these strains of hens (at 28 wk of age) to perform lying suggests that even W2 hens may need more than 318 cm2/hen to lie down (538 ± 23 cm2/hen; Riddle et al., 2018).
When the observed number of hens in a certain location was higher than the number of hens calculated to fit in that space, hens could potentially be crowded (Table 1). Crowding in the upper tier of this aviary style is not surprising given that this level had less floor area and perch space than the other two tiers (Table 1). The lower tiers, in contrast, were never found to be over-occupied (Figure 3–5 and Table 1; and see Ali et al., 2016; ). Together our results agree with findings from Odén and colleagues (2002) that hens only occupied lower perches when the upper ones were filled as hens prefer higher roosting sites at night (Olsson and Keeling, 2000; Brendler et al., 2014). In the present study, W1 and W2 hens were observed tightly packed together in the top tier, in some cases lying on top of each other, which made it visually difficult to distinguish between individual hens in the dark (Ali, personal observation).
In the current study, during the PRE-period, more hens were counted on the upper tier than would be estimated to fit there in 93% of observations of W1 hens and in 88% of observations of W2 hens. In the ACC period, this happened less often with more W1 hens recorded on the upper tier in 74% of observations and in 60% of observations of W2 hens. The frequent incidence of crowding in the upper tier during the night seen in this study is also similar to findings of an observational study examining perch and ledge use conducted with the same aviary style on a commercial farm (Campbell et al., 2016). In that study, more LSLs roosted in the upper tier at night, sometimes at occupancy rates over 100%, while the lower tier was the least occupied (Campbell et al., 2016). Similarly, recent observations in two other types of commercial aviary systems housing multiple different hen strains, showed hens perched higher at night, including using higher perches within lower tiers more, but direct comparisons among the various strains were not made (Brendler and Schrader, 2016).
CONCLUSION
The top tier of the aviary enclosure was generally occupied in the greatest numbers by both strains of white hens (W1 and W2). The degree of occupancy seems to suggest that these strains of white hens use higher nighttime roosting sites to such a degree that they become crowded. Other aviary designs with limited upper level perch space may also be overcrowded during the night when stocked with these hens. The B1 and B2 hens in this study, on the other hand, roosted more evenly across areas of the aviary, occupying perches and wire floor space in greater numbers than W1 and W2 hens including those in the lower and middle levels of the aviary. These findings suggest that B1 and B2 hens either rely on factors beyond height when selecting a nighttime roosting site or that they have stronger preferences for grasping or greater inter-bird distances.
Introduction of a litter area disrupted nighttime roosting patterns in the short term, despite the fact that hens could use this resource only during the day. However, after 3 wk of daytime access to the litter, the hens largely returned to previous patterns of nighttime roosting. In total, this study indicates the importance matching aviary configurations with the predilections of the strain in question. This study also highlights the potential impact of a management practice on hen behavior and welfare, even when it seems unlikely that the management action would influence a particular behavior or type of resource use, such as could reasonably be expected when considering the impact of granting daytime litter access on nighttime roosting site choice.
ACKNOWLEDGMENTS
The authors thank Nicholas Newsome and Silvia Villanueva for their assistance with onsite data collection. We would also like to thank Angelo Napolitano and the Michigan State University Poultry Teaching and Research Center personnel for their assistance with and contribution to this research. This study was supported by the Michigan Alliance for Animal Agriculture (East Lansing, MI) and from the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch projects #1002990 and #1010765. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Footnotes
1This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch projects #1002990 and #1010765, and by a grant from the Michigan Alliance for Animal Agriculture (East Lansing, MI).
REFERENCES
- Albentosa M. J., Kjaer J. B., Nicol C. J.. 2003. Strain and age differences in behaviour, fear response and pecking tendency in laying hens. Br. Poult. Sci. 44:333–344. [DOI] [PubMed] [Google Scholar]
- Ali A. B. A., Campbell D. L. M., Karcher D. M., Siegford J. M.. 2016. Influence of genetic strain and access to litter on spatial distribution of 4 strains of laying hens in an aviary system. Poult. Sci. 95:2489–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle E. R., Ali A. B. A., Campbell D. L. M., Siegford J. M.. 2018. Space use by 4 strains of laying hens to perch, wing flap, dust bathe, stand and lie down. PLoS One 13:e0190532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm M., Wall H., Holm L., Wichman A., Palme R., Tauson R.. 2015. Welfare and performance in layers following temporary exclusion from the litter area on introduction to the layer facility. Poult. Sci. 94:565–573. [DOI] [PubMed] [Google Scholar]
- Appleby M. C. 1995. Perch length in cages for medium hybrid laying hens. Br. Poult. Sci. 36:23–31. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B. M., Walker S. C.. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67:1–48. [Google Scholar]
- Blokhuis H., 1984. Rest in poultry. Appl. Anim. Behav. Sci 12:289–303. [Google Scholar]
- Braastad B., Katle J.. 1989. Behavioural differences between laying hen populations selected for high and low efficiency of food utilisation. Br. Poult. Sci. 30:533–544. [DOI] [PubMed] [Google Scholar]
- Brendler C., Schrader L.. 2016. Perch use by laying hens in aviary systems. Appl. Anim. Behav. Sci 182:9–14. [Google Scholar]
- Brendler C., Kipper S., Schrader L.. 2014. Vigilance and roosting behaviour of laying hens on different perch heights. Appl. Anim. Behav. Sci 157:93–99. [Google Scholar]
- Campbell D. L. M., Makagon M. M., Swanson J. C., Siegford J. M.. 2016. Perch use by laying hens in a commercial aviary. Poult. Sci. 95:1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channing C., Hughes B., Walker A.. 2001. Spatial distribution and behaviour of laying hens housed in an alternative system. Appl. Anim. Behav. Sci. 72:335–345. [DOI] [PubMed] [Google Scholar]
- Cook N., Schaefer A., Korver D., Haley D., Feddes J., Church J.. 2011. Minimally-invasive assessments of the behavioural and physiological effects of enriched colony cages on laying hens. TOASJ 5:10–18. [Google Scholar]
- Duncan E., Appleby M., Hughes B.. 1992. Effect of perches in laying cages on welfare and production of hens. Br. Poult. Sci. 33:25–35. [Google Scholar]
- EFSA AHAW Panel 2015. Scientific Opinion on welfare aspects of the use of perches for laying hens. EFSA Journal 13:4131. [Google Scholar]
- Faure J. M., Jones R. B.. 1982. Effects of sex, strain and type of perch on perching behaviour in the domestic fowl. Appl. Anim. Ethol. 8:281–293. [Google Scholar]
- Hothorn T., Bretz F., Westfall P.. 2008. Simultaneous inference in general parametric models. Biom. J. 50:346–363. [DOI] [PubMed] [Google Scholar]
- Jones R. B., Faure J. M.. 1981. Sex and strain comparisons of tonic immobility (“righting time”) in the domestic fowl and the effects of various methods of induction. Behav. Processes 6:47–55. [DOI] [PubMed] [Google Scholar]
- Klein T., Zeltner E. B., Huber-Eicher B.. 2000. Are genetic differences in foraging behaviour of laying hen chicks paralleled by hybrid-specific differences in feather pecking? Appl. Anim. Behav. Sci. 70:143–155. [DOI] [PubMed] [Google Scholar]
- Lambton S. L., Knowles T. G., Yorke C., Nicol C. J.. 2015. The risk factors affecting the development of vent pecking and cannibalism in free-range and organic laying hens. Anim. Welf. 24:101–111. [Google Scholar]
- Landis J. R., Koch G. G.. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. [PubMed] [Google Scholar]
- Mench J. A., Blatchford R. A.. 2014. Determination of space use by laying hens using kinematic analysis. Poult. Sci. 93:794–798. [DOI] [PubMed] [Google Scholar]
- Murphy L. B., 1977. Responses of domestic fowl to novel food and objects. Appl. Anim. Ethol 3:335–349. [Google Scholar]
- Newberry R. C., Estevez I., Keeling L. J.. 2001. Group size and perching behaviour in young domestic fowl. Appl. Anim. Behav. Sci 73:117–129. [DOI] [PubMed] [Google Scholar]
- Odén K., Keeling L., Algers B.. 2002. Behaviour of laying hens in two types of aviary systems on 25 commercial farms in Sweden. Br Poult Sci. 43:169–181. [DOI] [PubMed] [Google Scholar]
- Olsson I. A. S., Keeling L. J.. 2000. Night-time roosting in laying hens and the effect of thwarting access to perches. Appl. Anim. Behav. Sci 68:243–256. [DOI] [PubMed] [Google Scholar]
- R Core Team 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/. [Google Scholar]
- Schrader L., Müller B.. 2009. Night-time roosting in the domestic fowl: the height matters. Appl. Anim. Behav. Sci 121:179–183. [Google Scholar]
- Schrader L., Dippel S., Nicol N.. 2016. Do laying hens have a motivation to grasp while nighttime roosting? Proceedings of the 50th Congress of the International Society for Applied Ethology. 50:340. [Google Scholar]
- Schütz K. E., Jensen P.. 2001. Effects of resource allocation on behavioural strategies: A comparison of red junglefowl (Gallus gallus) and two domesticated breeds of poultry. Ethology 107:753–765. [Google Scholar]
- Schütz K. E., Forkman B., Jensen P.. 2001. Domestication effects on foraging strategy, social behaviour and different fear responses: A comparison between the red junglefowl (Gallus gallus) and a modern layer strain. Appl. Anim. Behav. Sci. 74:1–14. [Google Scholar]
- Singh R., Cheng K. M., Silversides F. G.. 2009. Production performance and egg quality of four strains of laying hens kept in conventional cages and floor pens. Poult Sci 88:256–264. [DOI] [PubMed] [Google Scholar]
- Uitdehaag K., Komen H., Rodenburg T. B., Kemp B., van Arendonk J.. 2008. The novel object test as predictor of feather damage in cage-housed Rhode Island red and white leghorn laying hens. Appl. Anim. Behav. Sci. 109:292–305. [Google Scholar]
- United Egg Producers 2017. Animal Husbandry Guidelines for U.S. Laying Flocks 2017 Edn https://uepcertified.com/uep-certified-resources/2017-uep-animal-welfare-cage-free-guidelines-11-01-2017-final/. Accessed October 2017. [Google Scholar]
- Wall H., Tauson R.. 2007. Perch arrangements in small-group furnished cages for laying hens. J. Appl. Poult. Res. 16:322–330. [Google Scholar]
- Weeks C., Nicol C.. 2006. Behavioural needs, priorities and preferences of laying hens. Worlds Poult. Sci. J. 62:296–307. [Google Scholar]
- Wood-Gush D., Duncan I.. 1976. Some behavioural observations on domestic fowl in the wild. Appl. Anim. Ethol 2:255–260. [Google Scholar]