Abstract
Patients with Spinal Muscular Atrophy (SMA) are at risk for poor bone health. The prevalence of fractures, low areal bone mineral density (aBMD; Z-score ≤−2.0) of the lateral distal femur and of osteoporosis by SMA subtype is not known. We aimed to describe the natural history of bone health in patients with SMA prior to bisphosphonate treatment. We reviewed data from 85 eligible patients with SMA ages 12 months to 18 years, seen at a single institution between January 2005 and July 2016. Fracture history was reported at annual clinic visits. aBMD was obtained from dual energy x-ray absorptiometry scans of the lumbar spine, total body, and lateral distal femur. 85% of patients had aBMD Z-scores ≤−2.0 SD and were progressively lower with worsening SMA severity. Longitudinal aBMD Z-scores of the lateral distal femur decreased with age. Fractures occurred in 38% (32/85) of patients with the femur being the most common location (25 of 57 fractures). Thirteen percent of patients fulfilled criteria for osteoporosis. Low aBMD and femur fractures are highly prevalent in all SMA subtypes from a young age; however, few patients met the criteria for osteoporosis. Poor bone health may be an under-recognized comorbidity of SMA.
Keywords: Osteoporosis, Children, Dual energy x-ray absorptiometry
1. Introduction
Spinal Muscular Atrophy (SMA), an autosomal recessive neuromuscular disease due to mutations in the survival motor neuron gene 1 (SMN1), affects 1 in 6000–10,000 live births and is the leading cause of death due to a genetic mutation in infants [1,2]. This degenerative disease of the spinal cord and lower brainstem motor neurons causes progressive proximal muscle weakness, resulting in varying degrees of hypotonic immobility and respiratory compromise. While there are no genotypephenotype correlations, clinical severity is associated with the number of copies of a rescue gene, SMN2 [3]. Patients are typically characterized by their clinical phenotype: patients with SMA Type 1 (SMA1) never sit independently; those with SMA Type 2 (SMA2) can sit but never stand or walk independently; and those with SMA Type 3 (SMA3) walk independently with a later loss of mobility [3,4]. SMA Type 4 is an adult-onset disease with mild muscle weakness [4].
Without intervention, survival of the most severely affected children is poor, with most patients dying before 24 months of life [5]. Advances in medical care have led to improved survival and quality of life [6]. However, these children now face complications due to chronic immobility that also impact those with milder SMA phenotypes.
A major complication of chronic immobility is poor bone health. Weight-bearing activity during growth is an important stimulus for bone mass accrual [7–9]. Children who have limited weight-bearing activity are at risk for poor bone accrual and a marked decrease in peak bone mineral density (BMD) [10–13]. Children with SMA also have low muscle mass, which may lead to lower mechanical loading forces on the osteocyte. Additionally, data from mouse models suggest a direct interaction of SMN with modulators of osteoclast activity, leading to altered bone remodeling and impaired bone mineralization [14,15]. Low BMD increases risk for all types of fractures and development of osteoporosis [16,17].
Despite these known risk factors, there is limited published literature on bone health in patients with SMA. Retrospective studies report a widely variable (9.3%–46%) fracture prevalence in pediatric patients with SMA, with the distal femur being the most common fracture location [18–21]. Analyses of BMD in these children have been inconclusive. Some studies report normal bone mineral parameters [22,23], whereas others found bone density in pediatric patients with SMA was lower than expected for age [24,25] and lower than in patients with other neuromuscular conditions [25]. No study has reported the prevalence of fractures or low BMD (Z-score ≤−2.0) by SMA subtype, prevalence of low BMD of the lateral distal femur (an important fracture location in non-ambulatory children), nor prevalence of osteoporosis in this population. Thus, the degree and extent of poor bone health among children and adolescents with SMA is not known.
In this study, we aimed to describe, by SMA subtype, the natural pattern of bone mineralization at multiple skeletal sites, and determine the prevalence of low BMD and fractures in pediatric patients with SMA. We also aimed to determine the prevalence of osteoporosis using the diagnostic criteria established in the 2013 International Society for Clinical Densitometry (ISCD) pediatric position statement.
2. Patients and methods
We conducted a retrospective chart review of patients with a confirmed diagnosis of SMA seen at the Neuromuscular Comprehensive Care Center at Cincinnati Children’s Hospital Medical Center between January 2005 and July 2016. The clinical protocol for medical care in this center includes anthropometric measurements at each visit with a segmental height used as a surrogate for standing height in non-ambulatory patients, nutritional counseling by a registered dietitian regarding appropriate dietary calcium intake and periodic monitoring of 25-hydroxyl vitamin D levels. DXA scans are ordered annually at age 3 and thereafter, whereas x-rays are ordered as needed if history or physical exam raises suspicion for fracture.
SMA subtypes were defined by classic criteria [3,4]. Patients were included in analyses if they had a clinic visit between ages 12 months and 18 years. We selected this age range to exclude congenital fractures and fractures secondary to delivery in order to refine fracture analyses while capturing the timeframe of bone accrual through childhood and adolescence. This also excluded the most severely affected children who did not survive to one year of age. Additional exclusion criteria included use of systemic glucocorticoids or valproic acid, or diagnosis of another chronic illness known to affect bone metabolism (e.g., malabsorption syndromes, inflammatory bowel disease, hypopituitarism). In order to study the natural history of bone health in this population, we excluded any data on bone health obtained 6 months or more after a bisphosphonate medication was prescribed as a change in BMD or fracture frequency would not be expected in this immediate time interval.
Data extracted from the medical records included sex, race, SMA subtype, age at SMA diagnosis, age at dual energy x-ray absorptiometry (DXA) scans, fracture history, bisphosphonate use, and age at clinical encounter. This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
2.1. Bone mineral density
Areal bone mineral density (aBMD) was measured by DXA. All DXA scans were obtained as part of routine clinical care where the standard protocol was to obtain annual DXA scans of the lumbar spine (LS), whole body (WB), and lateral distal femur (LDF), starting at three years of age. These sites were measured in accordance with ISCD recommendations [26,27]. DXA scans were obtained prior to age 3 years in specific clinical scenarios, such as a fragility fracture. Limitations in positioning and/or spinal rod instrumentation prevented DXA scans at all sites from being obtained on each patient. Scans were acquired on a Hologic densitometer (Delphi/Discovery/ Horizon) calibrated to a common manufacturer standard, and scans were analyzed using software with the same bone detection algorithms (version 12.3). LS and WB scans were acquired using standard positioning and analysis procedures. The coefficient of variation (CV) at our center is ≤1% for WB and LS BMD. LDF scans were obtained and three regions of interest (R1, R2 and R3) were identified as described by Henderson et al. [28] and Zemel et al. [29]. We included R1, composed of primarily trabecular bone, and R3, composed of primarily cortical bone, in our analyses. R2 is difficult to interpret given it is an admixture of both bony tissue types. The CV for the LDF BMD has not been reported. All scans were reviewed (by H.W.) for image quality, positioning and artifact (spinal instrumentation, ports and movement). LS aBMD Z-scores were calculated using reference data from Kalkwarf et al. [30] for ages 1–36 months, Kelly et al. [31] for ages 37–60 months, and Zemel et al. [32] for ages 5–20 years. WB BMC and aBMD Z-scores were calculated using reference data from Kelly et al. [31] for ages 37–60 months, and from Zemel et al. [32] for ages 5–20 years. LDF aBMD Z-scores were calculated for children ages ≥3 years using reference data from Henderson et al. [28]. We studied the outcomes of age-, sex-, and race-specific aBMD Z-scores for each skeletal region of interest.
2.2. Fracture history
Patients were asked about fracture history at each clinic visit and responses were recorded in the medical record. Data collected included age at fracture, number of fractures, and location of fractures. Reported fractures were confirmed when possible by review of radiographic images (by H.W.), with documentation of fracture by a radiologist or evidence of healing fracture on subsequent radiographic imaging. Fractures of the skull or of the digits were excluded as these do not usually constitute osteoporotic fractures.
2.3. Osteoporosis
We used the 2013 ISCD criteria for osteoporosis (vertebral compression fractures in the absence of high energy trauma or infiltrative disease; or a BMD Z-score ≤−2.0 SD, and two or more long bone fractures by 10 years of age, or three or more long bone fractures by 19 years of age) to determine the prevalence of osteoporosis in our study sample [33].
2.4. Statistical analyses
Data were analyzed using SAS®, version 9.3 (SAS Institute, Cary, NC). Due to small sample sizes and the distribution of variables, continuous data were summarized as medians with 25th and 75th percentiles, and categorical data were summarized as frequency counts and percentages. Chi-square and Fisher’s exact tests were used, as appropriate, for group comparisons of categorical variables. Nonparametric Kruskal– Wallis tests were used to compare continuous variables between groups at baseline. Generalized linear mixed models with random effects (to account for the longitudinal nature of multiple visits by the same subject) were used to assess trends in BMD Z-scores over time, differences by SMA subtype and group by time interaction. BMD Z-scores were analyzed with the three SMA types in the model, as well as each SMA type modeled separately. Since age at final measurement was greater in patients with SMA3 compared to SMA1 and SMA2 due to the nature of the disease, we performed a sensitivity analysis by restricting the sample to individuals ages <13 years to determine if having similar age ranges for all three SMA types altered conclusions. Because older children have more time to sustain a fracture, it was necessary to account for duration of follow-up to prevent biased comparisons among SMA subtypes regarding fracture risk. Thus, a survival analysis was performed using a Cox regression model to compare time to first fracture among SMA subtypes. Statistical significance was set a priori at α = 0.05. In order to account for multiple testing between groups, a False Discovery Rate-adjusted P-value was calculated for each group comparison tested.
3. Results
3.1. Demographics
A total of 101 patients with SMA were evaluated at our institution between January 2005 and July 2016, and 85 patients met the inclusion criteria (SMA1: n = 24; SMA2: n = 44; SMA3: n = 17). Table 1 shows the characteristics of the sample by SMA subtype. The majority of patients were Caucasian, and the sex distribution was similar across SMA subtypes. Age at initial clinic visit differed significantly by SMA subtype, youngest in SMA1 and oldest in SMA3 groups, consistent with the onset of symptoms leading to SMA diagnosis. Patients with a mild SMA subtype were also more likely to be older at the time of last encounter than those with a more severe subtype.
Table 1.
Demographics.
| SMA 1 (most severe) | SMA 2 (intermediate) | SMA 3 (mild) | p-value | |
|---|---|---|---|---|
| Number (n) | 24 | 44 | 17 | |
| Female (%) | 15 (63%) | 23 (52%) | 8 (47%) | 0.58 |
| Race, Caucasian | 20 (83%) | 39 (89%) | 14 (82%) | 0.77 |
| Age (y) at initial neuromuscular visit | 0.6 (0.3, 1.1) | 2.0 (0.9, 4.4) | 3.7 (2.1, 8.5) | 0.0005*,†,‡ |
| [n = 24] | [n = 44] | [n = 17] | ||
| Age (y) at loss of ambulation | N/A | N/A | 8.2 (6.4, 9.7) | – |
| [n = 9] | ||||
| Age (y) at last encounter | 9.2 (3.8, 13.5) | 6.8 (4.1, 13.6) | 12.6 (9.3, 18.0) | 0.04‡ |
| [n = 24] | [n = 44] | [n = 17] |
Data expressed as n (%) or median (25th, 75th percentile).
SMA1 vs. SMA2 between group difference adjusted p < 0.05 (p-value adjusted for multiple testing using False Discovery Rate adjustment).
SMA1 vs. SMA3 between group difference adjusted p < 0.05 (p-value adjusted for multiple testing using False Discovery Rate adjustment).
SMA2 vs. SMA3 between group difference adjusted p < 0.05 (p-value adjusted for multiple testing using False Discovery Rate adjustment).
3.2. Prevalence of low bone mineral density by SMA subtype
DXA data were available on 62 patients (73% of the study sample). Of these, 85% had an aBMD Z-score of ≤−2.0 at any skeletal site at the first DXA scan. Median Z-scores for the first DXA scan at each skeletal site for each SMA subtype are given in Table 2. Patients with SMA1 were likely to have low aBMD Z-score at all skeletal sites; SMA2 patients were likely to have low aBMD Z-scores at the LS and the LDF, whereas patients with SMA3 were only likely to have low aBMD Z-scores at the LDF. Patients with SMA1 had significantly lower aBMD Z-scores at all skeletal sites compared to those with SMA2 or SMA3.
Table 2.
Bone health outcomes.
| SMA 1 (most severe) | SMA 2 (intermediate) | SMA 3 (mild) | p-value | |
|---|---|---|---|---|
| Number (n) | 24 | 44 | 17 | |
| Patients ≥1 fracture | 12 (50%) | 13 (30%) | 7 (41%) | 0.24 |
| Age (y) at 1st reported fracture* | 3.2 (2.3, 6.9) | 7.7 (3.8, 11.6) | 10.7 (9.5, 11.8) | 0.01‡ |
| Fractures at femur (%) | 13/21 (62%) | 7/25 (28%) | 5/11 (45%) | 0.07 |
| Age (y) at 1st DXA (any site) | 3.6 (2.7, 4.4) | 5.3 (4.0, 6.4) | 7.9 (4.7, 11.3) | 0.004†,‡ |
| [n = 16] | [n = 30] | [n = 16] | ||
| LS BMD Z-score | −5.1 (−6.3, −3.6) | −2.5 (−3.3, −0.7) | −0.2 (−1.8, 0.2) | <0.0001†,‡,§ |
| LS DXA age (y) | 3.8 (2.6, 4.8) | 5.2 (4.0, 5.7) | 7.5 (4.3, 8.5) | 0.02‡ |
| [n=15] | [n=22] | [n=13] | ||
| WB BMD Z-score | −2.9 (−4.3, −2.6) | −1.8 (−2.6, −0.2) | −1.9 (−2.9, −1.3) | 0.01† |
| WB DXA age (y) | 4.5 (4.0, 4.9) | 5.4 (4.6, 6.8) | 7.6 (4.8, 8.7) | 0.03 |
| [n=13] | [n=22] | [n=12] | ||
| LDF BMD Z-scores | [n = 16] | [n = 29] | [n = 14] | |
| Region 1 | −5.0 (−5.7, −4.4) | −4.1 (−4.7, −2.8) | −3.4 (−3.8, −1.1) | <0.0001†,‡,§ |
| Region 3 | −4.4 (−5.6, −3.6) | −3.0 (−3.9, −2.4) | −1.8 (−3.9, −1.2) | 0.0005†,‡ |
| LDF DXA age (y) | 4.6 (4.0, 6.4) | 5.4 (4.3, 7.4) | 8.7 (4.3, 11.3) | 0.12 |
| Prevalence of BMD ≤−2.0 SD at 1st DXA (any site) | 16/16 (100%) | 27/30 (90%) | 10/16 (63%) | 0.01 |
| Osteoporosis by ISCD criteria by last encounter | 3/16 (19%) | 3/30 (10%) | 2/16 (13%) | 0.72 |
Data expressed as n (%) or median (25th, 75th percentile).
Summary statistics only for those patients with a reported fracture.
SMA1 vs. SMA2 between group difference adjusted p < 0.05 (p-value adjusted for multiple testing using False Discovery Rate adjustment).
SMA1 vs. SMA3 between group difference adjusted p < 0.05 (p-value adjusted for multiple testing using False Discovery Rate adjustment).
SMA2 vs. SMA3 between group difference adjusted p < 0.05 (p-value adjusted for multiple testing using False Discovery Rate adjustment).
Age-related longitudinal trajectories in aBMD Z-scores for individual patients by skeletal site are given in Fig. 1. When including all SMA subtypes in mixed effect regression models, LS aBMD Z-scores significantly increased with age (p = 0.03), whereas there was no change in WB aBMD Z-score with age. There was no significant interaction between age and SMA subtype on aBMD Z-scores of the LS and WB.
Fig. 1.
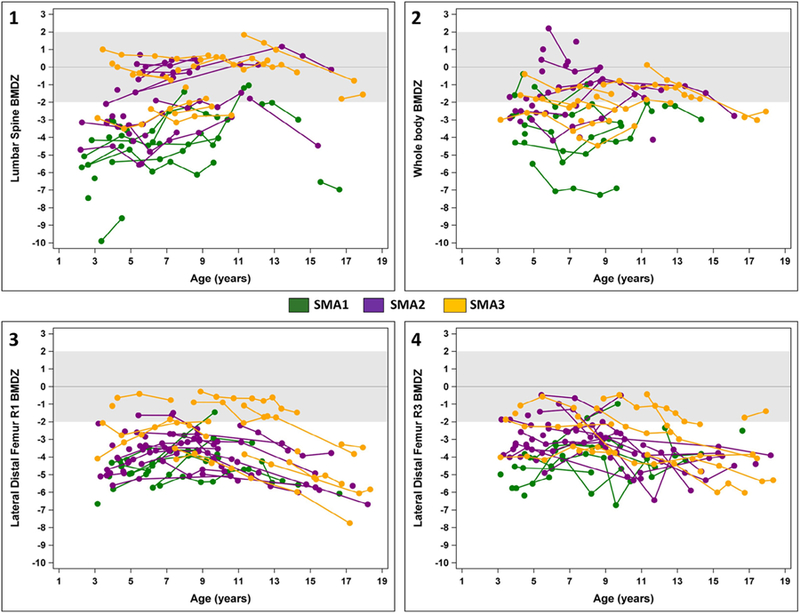
(1–4) Change in areal bone mineral density over time by skeletal site. BMDZ: Areal Bone Mineral Density Z-score
In contrast, the age-related trajectories for LDF aBMD Z-scores for R1 and R3 differed statistically by SMA subtype (SMA subtype by age interaction p < 0.002). Thus, we examined these age-related trajectories separately by SMA subtype. For patients with SMA1, age-related trajectories in aBMD Z-scores at R1 were non-linear, with a slight increase followed by decline (p = 0.002). There was no age-related change in aBMD Z-scores at R3 (p = 0.12). For patients with SMA2 and SMA3, age-related trajectories in aBMD Z-scores at R1 were also non-linear, with a slight increase followed by decline (p < 0.0001 for both subgroups), whereas aBMD Z-scores at R3 declined in a linear manner with age (p = 0.0004 and p = 0.007 respectively). R3, an area associated with increased fracture risk in non-ambulatory children [34], showed the greatest overall decline over time in aBMD Z-scores for patients with both SMA2 and SMA3 compared to other regions of interest. Among patients with SMA3, loss of ambulation was significantly associated with lower aBMD Z-scores at R3 compared to children who maintained the ability to walk (p = 0.002). There were no differences in aBMD Z-scores by ambulatory status at R1. Conducting a sensitivity analysis including only individuals <13 years of age did not change any results at the LDF.
3.3. Fracture frequency by SMA subtype
All 85 patients provided fracture information; 32 (38%) reported at least one fracture. The proportion of patients with at least one long bone fracture did not differ significantly by SMA subtype (Table 2). A survival analysis accounting for the differing follow-up intervals showed SMA1 patients had a trend (p = 0.066) toward greater risk of fracture than the other sub-types (Fig. 2). Among patients that had a fracture, the age at first fracture was significantly younger among SMA1 patients than among SMA3 patients (Table 2).
Fig. 2.
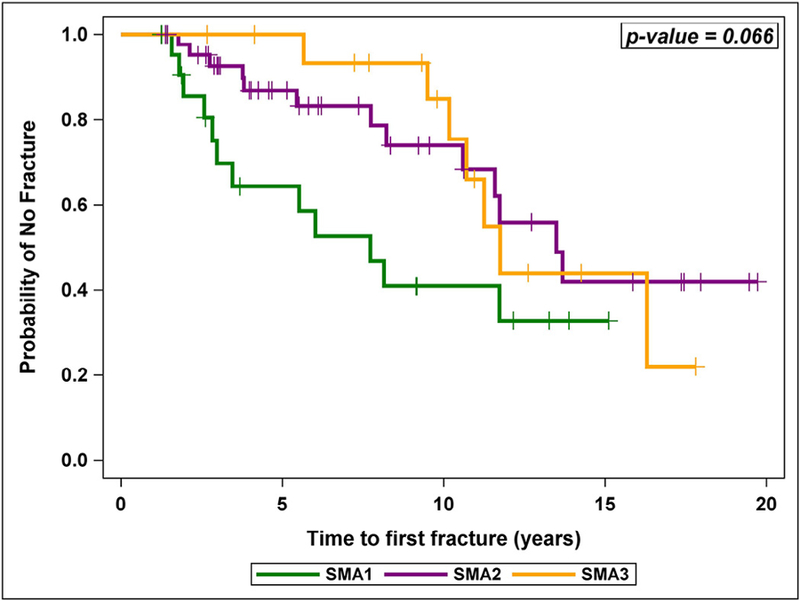
Probability of remaining fracture free by SMA subtype.
Fracture of the distal femur accounted for 44% of all fractures and was the most common fracture location in all SMA subtypes. Twelve patients (14%) had multiple fractures (SMA1: n = 4, SMA2: n = 6, SMA3: n = 2). We radiographically verified the fracture in 70% of all reported fracture occurrences.
3.4. Osteoporosis prevalence
The overall prevalence of osteoporosis according to the 2013 ISCD pediatric criteria was 12.9% at the end of the study period. The percentage of patients with either SMA1 or SMA3 meeting criteria for osteoporosis appeared to be higher than for patients with SMA2; however, this was not statistically significant.
4. Discussion
To our knowledge, this is the first study to assess both aBMD and fracture prevalence in a sample of patients with SMA inclusive of all subtypes of childhood disease. We found that children with SMA had a high prevalence of low BMD and fractures at a young age. Importantly, low BMD was common at the lateral distal femur, a site where fractures often occur in these patients. Despite the high prevalence of low BMD and fracture among children with SMA, only 12.9% met the criteria for osteoporosis in children.
Lack of ambulation and decreased mechanical loading forces are significant risk factors for poor bone mineral accrual. Multiple studies have documented low BMD in non-ambulatory children [11,13,34,35]. Few studies, however, have reported BMD in patients with SMA, and the extent of bone mineral deficits has been inconclusive. Khatri et al. found LS aBMD Z-scores were lower in children with SMA (n = 8, mean −2.25 ± 0.31) as compared to children with Duchenne Muscular Dystrophy (n = 53, mean −1.72 ± 0.1) [25]. Neither steroid use nor vertebral fractures were controlled for in the Duchenne Muscular Dystrophy group, factors that could decrease or falsely increase aBMD at the LS respectively. In a cross-sectional study of twelve patients with SMA, young children had WB aBMD Z-scores in the normal range, whereas Z-scores were low in teenagers [23]. The authors concluded that age had a more significant impact on BMD than did disease severity or ambulatory status. However, the head region was not excluded from the WB results, which may falsely increase the WB aBMD, as the head makes a relatively large contribution in young children but is not responsive to physical activity [36]. In addition to small sample sizes, these previous reports are limited by primarily including children with SMA2 or SMA3. Our study includes a much larger sample of children, enabling us to examine children with SMA1, SMA2 and SMA3 separately. We found that children with SMA1 had the lowest aBMD at all skeletal sites as compared to the milder phenotypes, even at a younger age at presentation. Importantly, all patients, regardless of subtype, demonstrated deficits in aBMD at the LDF, a site that has not been previously reported in the literature in children with SMA.
The LDF site is practical for measuring aBMD in children with contractures and spinal instrumentation, and LDF aBMD has been shown to be associated with fracture risk in other non-ambulatory patients [34]. In our SMA cohort, the initial median LDF aBMD Z-score was low at R1 in all subtypes, and low at R3 for SMA1 and SMA2. Our data suggest that patients with SMA1 may experience an initial increase in LDF aBMD Z-score between 3 and 10 years of age followed by a subsequent decline during adolescence; however, at no point was the aBMD Z-score in the normal range. Many patients with SMA3 initially had a LDF aBMD Z-score in the normal range; however, there was a significant decline with age, especially at R3 where correlation with fracture risk is greatest [34]. LDF R3 is composed of primarily cortical bone, a tissue that is responsive to mechanical stimulation in early childhood [37]. Thus, lack of ambulation and low muscle forces may explain why initially patients with SMA1 and SMA2 had lower aBMD Z-scores at this region than those with SMA3, but patients with SMA3 showed subsequent decreases in aBMD as weight-bearing declined. Patients with SMA3 were significantly older than those with SMA1 or SMA2 at the time of last encounter, and thus it remains unknown if more severely affected patients show similar rates of decline in late adolescence when peak bone mass accrual should occur.
Multiple studies report higher fracture prevalence in children with neuromuscular disorders as compared to healthy children. This is of concern as fractures may lead to worsening contractures and loss of remaining mobility in these patients. Among patients with SMA, Granata et al. reported 16 fractures in 10 children out of a cohort of 93 patients (10.7%) [18]. This study did not categorize SMA patients by subtype; however, as data were collected between 1974 and 1988, it is likely that most children had SMA3 or mild SMA2. Fracture rates were higher at 45.8% in a more recent study, with 53% occurring at the distal femur [21]. Most of those fractures occurred in children with SMA2 or SMA3. Selection bias limits the generalizability of this study as only children referred for orthopedic evaluation were included. Our findings from a more generalizable cohort of patients with SMA are consistent with these prior studies and suggest a high prevalence of fractures specifically at the femur. While fracture risk among SMA subtypes did not reach statistical significance in our study, there was a trend with SMA1 patients demonstrating a higher risk of fractures at a younger age as compared to the other SMA subtypes. Studies are needed following these patients into the second decade of life and after bisphosphonate treatment to better describe their long-term bone health.
We found 12.9% of patients with SMA fulfilled the current ISCD guidelines for the diagnosis of osteoporosis in children. These fracture criteria are stringent and were designed to apply to an otherwise healthy child. Fracture patterns in our SMA cohort differed considerably from the general pediatric population. Fractures tended to occur at a younger age and affect lower rather than upper extremities. Thus, while many children with SMA did not meet the ISCD criteria for osteoporosis, these patients had significantly low BMD Z-scores and a high prevalence of clinically important femur fractures. Using ISCD guidelines may underestimate the extent of bone disease in patients with SMA.
Although our study reports a comprehensive view of aBMD and fracture history across the pediatric SMA phenotypes, there are some limitations. First, the study was retrospective, and we were unable to ensure that the frequency of bone health monitoring was consistent in all patients. DXA data were available on 73% of our study sample, which could bias results toward patients who had a greater clinical suspicion of poor bone mineral density. However, in the patients lacking DXA data, 57% were <3 years of age at the last encounter; therefore, the main reason for missing BMD data was lack of available clinical reference data. We were able to standardize BMD measurements for age, sex and race by use of appropriate reference data, but were unable to further correct for skeletal size, such as calculating height-adjusted Z-scores. Height measurements in non-ambulatory patients are difficult to obtain, and consistent surrogate height measures (segmental arm span, ulnar length, or supine length) were not available in this retrospective study. Furthermore, height-adjusted reference ranges are neither available for children <5 years nor for BMD measures at the LDF at any age. Nutrition plays a key role in bone health. Although we did not have detailed information on dietary intake in this retrospective study, all patients with SMA seen at our institution have dietary reviews by a registered dietician at each neuromuscular clinic visit, minimizing the likelihood of nutritional deficiencies in these patients. Some patients receive medical care at local hospitals and only travel to our institution for subspecialty care, thus we were able to radiographically confirm only 70% of fractures. We did not assess vertebral compression fractures as spine x-rays were not part of routine clinical care. The overall slight increase of LS aBMD with age in this cohort may be due to vertebral compression fractures. While there were no symptomatic vertebral compression fractures reported in this cohort, recently published data suggest that asymptomatic fractures may be more prevalent than previously thought [24]. Finally, although it is tempting to speculate on the relationship between LDF DXA findings and the high prevalence of femur fractures, it was not possible to determine if LDF aBMD Z-score was predictive of fracture risk as too few patients in this study had a LDF DXA scan prior to their first femur fracture.
5. Conclusions
Fracture risk is high for children with SMA. Low aBMD may be the first indicator of this increased risk, and deficits in aBMD are apparent even at a very young age. Further work is necessary to determine the natural trajectory of aBMD changes at different skeletal sites, especially in adolescent and young adult patients with SMA, and to determine if low aBMD and propensity to fracture are related to immobility, muscle weakness, or direct action of SMN on bone turnover. Importantly, more work is needed to identify effective interventions to delay the decline in BMD and prevent fractures in children with SMA.
Acknowledgments
Dr. Wasserman received funding support for this research from the National Institutes of Health T32 Grant ES10957.
We would like to thank Jean Bange for her help with data management and our patients and their families for participation in the database.
References
- [1].Wirth B An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat 2000;15:228–37. [DOI] [PubMed] [Google Scholar]
- [2].Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995;80:155–65. [DOI] [PubMed] [Google Scholar]
- [3].D’Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis 2011;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol 2007;22:1027–49. [DOI] [PubMed] [Google Scholar]
- [5].Farrar MA, Vucic S, Johnston HM, du Sart D, Kiernan MC. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J Pediatr 2013;162:155–9. [DOI] [PubMed] [Google Scholar]
- [6].Gregoretti C, Ottonello G, Chiarini Testa MB, et al. Survival of patients with spinal muscular atrophy type 1. Pediatrics 2013;131:e1509–14. [DOI] [PubMed] [Google Scholar]
- [7].Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res 2014;29:467–78. [DOI] [PubMed] [Google Scholar]
- [8].Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 2007;40: 14–27. [DOI] [PubMed] [Google Scholar]
- [9].Ireland A, Rittweger J, Schonau E, Lamberg-Allardt C, Viljakainen H. Time since onset of walking predicts tibial bone strength in early childhood. Bone 2014;68:76–84. [DOI] [PubMed] [Google Scholar]
- [10].Henderson RC, Kairalla JA, Barrington JW, Abbas A, Stevenson RD. Longitudinal changes in bone density in children and adolescents with moderate to severe cerebral palsy. J Pediatr 2005;146:769–75. [DOI] [PubMed] [Google Scholar]
- [11].Kilpinen-Loisa P, Paasio T, Soiva M, et al. Low bone mass in patients with motor disability: prevalence and risk factors in 59 Finnish children. Dev Med Child Neurol 2010;52:276–82. [DOI] [PubMed] [Google Scholar]
- [12].Chen CL, Ke JY, Wang CJ, Wu KP, Wu CY, Wong AM. Factors associated with bone density in different skeletal regions in children with cerebral palsy of various motor severities. Dev Med Child Neurol 2011;53:131–6. [DOI] [PubMed] [Google Scholar]
- [13].Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 2000;20:71–4. [PubMed] [Google Scholar]
- [14].Kurihara N, Menaa C, Maeda H, Haile DJ, Reddy SV. Osteoclast-stimulating factor interacts with the spinal muscular atrophy gene product to stimulate osteoclast formation. J Biol Chem 2001;276:41035–9. [DOI] [PubMed] [Google Scholar]
- [15].Shanmugarajan S, Tsuruga E, Swoboda KJ, Maria BL, Ries WL, Reddy SV. Bone loss in survival motor neuron (Smn(−/−) SMN2) genetic mouse model of spinal muscular atrophy. J Pathol 2009;219:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 2006;21:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? J Bone Miner Res 2006;21:501–7. [DOI] [PubMed] [Google Scholar]
- [18].Granata C, Giannini S, Villa D, Bonfiglioli Stagni S, Merlini L. Fractures in myopathies. Chir Organi Mov 1991;76:39–45. [PubMed] [Google Scholar]
- [19].Vestergaard P, Glerup H, Steffensen BF, Rejnmark L, Rahbek J, Moseklide L. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J Rehabil Med 2001;33:150–5. [PubMed] [Google Scholar]
- [20].Febrer A, Vigo M, Rodriguez N, Medina J, Colomer J, Nascimento A. [Fractures in spinal muscular atrophy]. Rev Neurol 2013;57:207–11. [PubMed] [Google Scholar]
- [21].Fujak A, Kopschina C, Forst R, Gras F, Mueller LA, Forst J. Fractures in proximal spinal muscular atrophy. Arch Orthop Trauma Surg 2010;130: 775–80. [DOI] [PubMed] [Google Scholar]
- [22].Crabtree NJ, Kibirige MS, Fordham JN, et al. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone 2004;35:965–72. [DOI] [PubMed] [Google Scholar]
- [23].Kinali M, Banks LM, Mercuri E, Manzur AY, Muntoni F. Bone mineral density in a paediatric spinal muscular atrophy population. Neuropediatrics 2004;35:325–8. [DOI] [PubMed] [Google Scholar]
- [24].Vai S, Bianchi ML, Moroni I, et al. Bone and spinal muscular atrophy. Bone 2015;79:116–20. [DOI] [PubMed] [Google Scholar]
- [25].Khatri IA, Chaudhry US, Seikaly MG, Browne RH, Iannaccone ST. Low bone mineral density in spinal muscular atrophy. J Clin Neuromuscul Dis 2008;10:11–17. [DOI] [PubMed] [Google Scholar]
- [26].Gordon CM, Leonard MB, Zemel BS, International Society for Clinical Densitometry. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom 2014;17:219–24. [DOI] [PubMed] [Google Scholar]
- [27].Rauch F, Plotkin H, DiMeglio L, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2007 Pediatric Official Positions. J Clin Densitom 2008;11:22–8. [DOI] [PubMed] [Google Scholar]
- [28].Henderson RC, Lark RK, Newman JE, et al. Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur. AJR Am J Roentgenol 2002;178:439–43. [DOI] [PubMed] [Google Scholar]
- [29].Zemel BS, Stallings VA, Leonard MB, et al. Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy X-ray absorptiometry. J Clin Densitom 2009;12:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kalkwarf HJ, Zemel BS, Yolton K, Heubi JE. Bone mineral content and density of the lumbar spine of infants and toddlers: influence of age, sex, race, growth, and human milk feeding. J Bone Miner Res 2013;28: 206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kelly J, Damron T, Grant W, et al. Cross-sectional study of bone mineral density in adult survivors of solid pediatric cancers. J Pediatr Hematol Oncol 2005;27:248–53. [DOI] [PubMed] [Google Scholar]
- [32].Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 2011;96:3160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bishop N, Arundel P, Clark E, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom 2014;17:275–80. [DOI] [PubMed] [Google Scholar]
- [34].Henderson RC, Berglund LM, May R, et al. The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy. J Bone Miner Res 2010;25:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Apkon SD, Fenton L, Coll JR. Bone mineral density in children with myelomeningocele. Dev Med Child Neurol 2009;51:63–7. [DOI] [PubMed] [Google Scholar]
- [36].Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom 2014;17:225–42. [DOI] [PubMed] [Google Scholar]
- [37].Baptista F, Barrigas C, Vieira F, et al. The role of lean body mass and physical activity in bone health in children. J Bone Miner Metab 2012;30:100–8. [DOI] [PubMed] [Google Scholar]


