Abstract
Study Design:
Systematic review and meta-analysis.
Objectives:
To determine the efficacy of intrawound treatments in reducing deep surgical site infections (SSIs) in instrumented spinal surgery.
Methods:
The electronic databases MEDLINE, EMBASE, and Cochrane were systematically searched for intrawound treatments for the prevention of SSIs in clean instrumented spine surgery. Both randomized controlled trials and comparative cohort studies were included. The results of included studies were pooled for meta-analysis.
Results:
After full text- and reference screening, 20 articles were included. There were 2 randomized controlled trials and 18 observational studies. Sixteen studies investigated the use of intrawound antibiotics, and 4 studies investigated the use of intrawound antiseptics. The relative risk of deep SSI for any treatment was 0.26 (95% confidence interval [CI] 0.16-0.44, P < .0001), a significant reduction compared with controls receiving no treatment. For patients treated with local antibiotics the relative risk was 0.29 (95% CI 0.17-0.51, P < .0001), and patients treated with local antiseptics had a relative risk of 0.14 (95% CI 0.05-0.44, P = .0006).
Conclusions:
Both the use of antibiotic and antiseptic intrawound prophylactics was associated with a significant 3 to 7 times reduction of deep SSIs in instrumented spine surgery. No adverse events were reported in the included studies.
Keywords: surgical site infection, postoperative infection, prophylaxis, prevention, intrawound, povidone-iodine, vancomycin
Introduction
Surgical site infections (SSIs) are serious adverse events with substantial patient morbidity and increased mortality.1 The incidence of SSIs is highly dependent on the type of surgery; in spinal surgery, the overall incidence is around 4%.2 The incidence is substantially higher in implant-related surgery, with SSIs developing in 9.4% of patients undergoing instrumented spinal surgery for traumatic fractures and in up to 19.2% of patients undergoing pediatric deformity surgery.3,4 SSIs in instrumented spinal surgery are a challenge to treat.5 Besides having a profound impact on patients, SSIs are a substantial financial burden on the health care system as well, costing up to $30 000 per patient for patients undergoing orthopedic surgery.6,7 With the increasing focus on preventing complications and limiting health care costs, finding new ways to avert SSIs is of critical importance. Aseptic surgical techniques and perioperative intravenous antibiotic prophylaxis have proven to be effective.8–11 For other measures like nasal Staphylococcus aureus decontamination, preoperative chlorhexidine baths, and many forms of surgical attire, the effect has not been shown unequivocally.12–14 In the past years, there has been an increased interest in additional decontamination of the surgical wound before closure. One of the strategies involves the application of antibiotics, like vancomycin, directly into the wound.15–17 Alternatively, antiseptic irrigation solutions are used, like povidone-iodine and hydrogen peroxide.18 The former antiseptic is most often used, in varying concentrations.18 Antiseptics have the advantage of not inducing bacterial resistance. They are, however, cytotoxic when used in high concentrations.19,20 Current evidence regarding efficacy and side effects associated with the use of intrawound antibiotics and antiseptics is still limited. Therefore, their use is not generally adopted in clinical practice.
The aim of this study was to determine the efficacy and potential side effects of intrawound prophylactic treatments in instrumented spinal surgery. A secondary goal was to compare the different methods used as intrawound treatment. Since meta-epidemiological research has shown that for surgical research questions, both randomized controlled trials (RCTs) and well-designed observational comparative study designs should be analyzed,21–24 we included both to make this review as representative and comprehensive as possible.
Methods
This systematic review and meta-analysis was performed in accordance with the items outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement25 and the Cochrane Handbook for Systematic Reviews of Interventions.26 The electronic databases MEDLINE, EMBASE, and Cochrane were systematically searched for articles that investigated the use of intrawound treatments for the prevention of SSIs in all types of clean instrumented spinal surgery. We searched for all possible types of prophylactic wound treatment and all phrases that were synonymous with SSI. The complete syntax used for each database can be found in the appendix.
Eligibility Criteria and Study Selection
Studies were limited to articles published in English, German, or French until April 16, 2018, with no restriction on publication date. Articles were screened for eligibility by 2 independent reviewers (JVCL and WB). Any disagreement between the reviewers was resolved through discussion or, if no consensus was reached, through consultation of a third reviewer (MCK). Reference screening and citation tracking was performed to find additional relevant articles. Human, comparative studies that investigated clean, instrumented spinal surgery were included. Treatment had to be given peroperatively, inside the wound before closure, with the intention to prevent infection. Studies with a reported mean follow-up time of less than 3 months, studies from which deep SSI rates in instrumented patients could not be extracted, studies with treatments that were applied onto the implants instead of into the wound, and studies with treatments in which a prolonged effect was intended (eg, antibiotic bone cement) were excluded. To minimize the apparent risk of bias as a result of selection by indication (treatment allocation based on surgeons judgment), these studies were also excluded.27
Data Collection and Study Quality Evaluation
Relevant study data was collected by one reviewer (JVCL) and checked by a second reviewer (SPJW). Disagreements were resolved through discussion. Deep SSI rates in instrumented patients were extracted from the article or were calculated by using the information reported in the article. We assessed the presence and extent of heterogeneity between studies based on data extracted from each article. Study quality for observational studies and randomized trials was determined using the Methodological Index for Non-Randomized Studies (MINORS) grading tool.28 The articles were independently graded by 2 reviewers (JVCL and SPJW).
Statistical Analysis
We combined the studies in a random-effects meta-analysis to calculate the relative risk and the 95% confidence intervals (CIs) by using the restricted maximum likelihood estimator.29 Due to the expected few cases in either group, a relative outcome measure was chosen in order to better illustrate differences. The Mantel-Haenszel method with a fixed-effects model was used to provide an unbiased pooled estimate. To gauge the effect of heterogeneity (ie, the different clinical settings and study methodologies), Tau2 was used as an estimate of the total amount of statistical heterogeneity. The I 2 index was used to quantify the influence of heterogeneity on the final result. Heterogeneity was considered relevant when I 2 was >50%. Publication bias, based on standard error, was explored with a funnel plot with random-effects pseudo–confidence limits.
To assess the effect of the different intrawound prophylactic methods, a subgroup analysis of both antibiotics and antiseptics was done. Furthermore, to assess the effect of study quality, a sensitivity analysis was done based on study quality. We arbitrarily divided the included studies in 3 groups, based on their MINORS score. Low-quality studies were defined as a MINORS score ≤12 (out of a maximum of 24). Medium-quality studies were defined as a MINORS score between 12 and 16, and high-quality studies were defined as a score ≥16.
Since the effect of prophylactic treatment was compared with historical control groups in many of the retrospective studies, the bias of a potential time-related effect caused by improved infection prevention over time was studied with a weighted regression analysis, by plotting the incidence of SSIs in the control groups against the year of operation. The Metafor package (R Foundation for Statistical Computing, Vienna, Austria, 2012) was used for all statistical analyses. A P < .05 was considered to be significant.
Results
Search
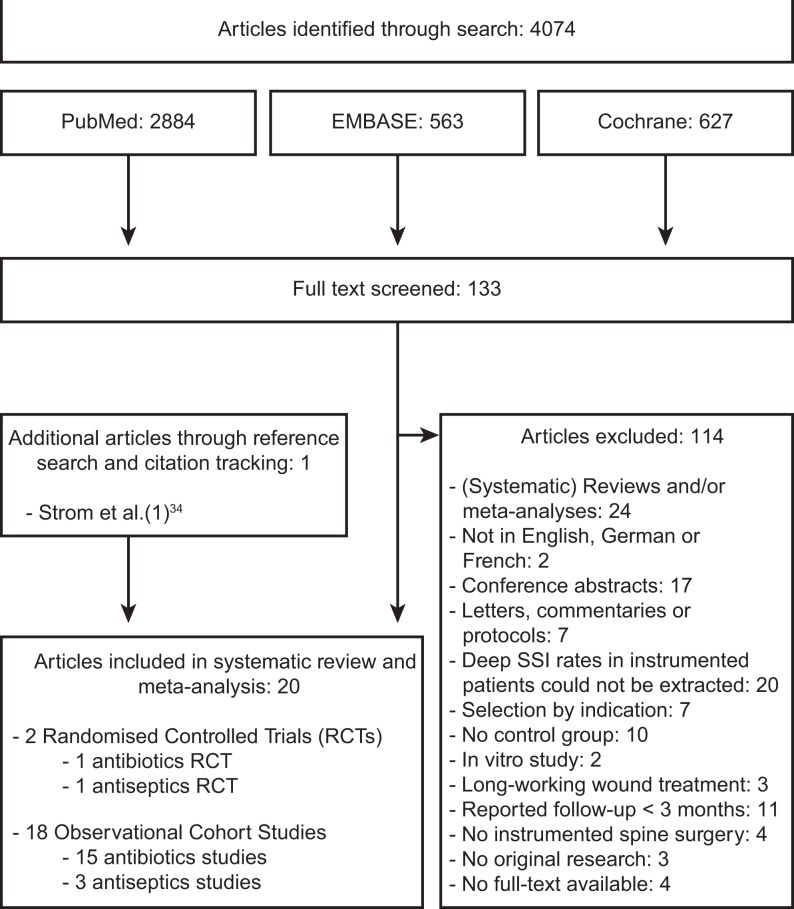
The search in the MEDLINE, EMBASE, and Cochrane libraries yielded a total of 4074 results. After removal of duplicates and title and abstract screening, 133 articles were eligible for full-text assessment. After review, 114 articles were excluded. Through reference screening and citation tracking, one additional article was found that matched the eligibility criteria. Finally, a total of 20 studies were included in the systematic review and meta-analysis. A PRISMA flowchart of this process can be found in Figure 1.
Figure 1.
PRISMA flow diagram.
RCT, randomized controlled trial; SSI, surgical site infection.
Baseline Characteristics
Of the 20 included studies, 2 were RCTs30,31 and 18 were observational cohort studies (Table 1).32–49 Eight studies investigated many different types of spinal surgery.30,38–41,43,46,47 Five studies investigated deformity surgery, in either adults,42 children,45,48,49 or both.32 One study investigated all types of spinal surgery in children.44 Three studies investigated cervical spinal surgery,34,36,37 and 3 studies investigated thoracolumbar or lumbar spinal surgery.31,33,35 Sixteen studies investigated the use of intrawound antibiotics (all studies investigated vancomycin),30,33–42,44–48 while 4 studies investigated the use of intrawound irrigation with antiseptics (all studies investigated povidone-iodine, one study also added hydrogen peroxide).31,32,43,49 Baseline equivalence regarding characteristics between control and intervention groups was present in 11 studies30–35,37–39,47,49 and unclear or not present in 9 studies.36,40–46,48 Characteristics of the intervention treatments and the use of perioperative antibiotic prophylaxis can be found in Table 2.
Table 1.
Study Demographics.
| Author | Year | Study Type | Intervention | Contemporary Study Populations | Type of Surgery | MINORS Score | Follow-up Length | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Intrawound antibiotics | ||||||||
| Garg et al | 2018 | Retrospective cohort | Vancomycin | Historical controls | Pediatric posterior spinal fusion | 16 | Minimum: 3 months; intervention: median 17 months; control: median 26 months | None |
| Thompson et al | 2018 | Retrospective cohort | Vancomycin | Historical controls | Pediatric scoliosis growing rod surgery | 13 | Minimum: 3 months | None |
| Haller et al | 2017 | Retrospective cohort | Vancomycin | Historical controls | Rib-based distraction surgeries | 14 | Minimum: 6 months | None |
| Hey et al | 2017 | Retrospective cohort | Vancomycin | Contemporary groups | General instrumented spinal surgery | 15 | Minimum: 3 months | None |
| Liu et al | 2015 | Retrospective cohort | Vancomycin | Historical controls | Adult instrumented spinal surgery | 14 | Minimum: 3 months | None |
| Hill et al | 2014 | Retrospective cohort | Vancomycin | Contemporary groups | General spinal surgery | 14 | Intervention: mean 8.76 months; control: mean 10.03 months | None |
| Emohare et al | 2014 | Retrospective cohort | Vancomycin | Contemporary groups | General spinal surgery | 12 | Intervention: mean 20.7 months; control: mean 21.7 months | NR |
| Theologis et al | 2014 | Retrospective cohort | Vancomycin | Historical controls | Complex adult deformity reconstruction | 11 | Intervention: mean 18 months; control: mean 34 months | None |
| Tubaki et al | 2013 | RCT | Vancomycin | Contemporary groups | General spinal surgery | 16 | Minimum: 3 months | None |
| Strom et al (1)34 | 2013 | Retrospective cohort | Vancomycin | Historical controls | Instrumented posterior cervical fusion | 12 | Minimum: 1 year | None |
| Strom et al (2)35 | 2013 | Retrospective cohort | Vancomycin | Historical controls | Lumbar laminectomy and fusion | 12 | Intervention: mean 1.9 years; control: mean 4.5 years | None |
| Pahys et al | 2013 | Retrospective cohort | Vancomycin | Historical controls | Posterior cervical spinal surgery | 13 | Minimum: 3 months | None |
| Caroom et al | 2013 | Retrospective cohort | Vancomycin | Historical controls | Posterior cervical decompression and fusion | 16 | Intervention: minimum 6 months; control: mean 18 months | None |
| Heller et al | 2013 | Retrospective cohort | Vancomycin | Historical controls | General instrumented spinal surgery | 14 | Minimum: 3 months | None |
| Kim et al | 2013 | Retrospective cohort | Vancomycin | Contemporary groups | General instrumented spinal surgery | 12 | Minimum: 3 months; mean: 5.8 months | None |
| Sweet et al | 2011 | Retrospective cohort | Vancomycin | Historical controls | Instrumented thoracolumbar fusion | 17 | Intervention: mean 2 years; control: mean 3.4 years | None |
| Intrawound antiseptics | ||||||||
| De Luna et al | 2017 | Prospective cohort | Povidone-iodine | Contemporary groups | Adult and pediatric scoliosis surgery | 15 | Minimum: 2 years | NR |
| Herwijnen et al | 2016 | Retrospective cohort | Povidone-iodine | Historical controls | Pediatric idiopathic scoliosis surgery | 13 | Minimum: 8 months | None |
| Ulivieri et al | 2011 | Retrospective cohort | Povidone-iodine and H2O2 | Historical controls | General instrumented spinal surgery | 11 | NR | None |
| Chang et al | 2006 | RCT | Povidone-iodine | Contemporary groups | Instrumented lumbosacral posterolateral fusion | 17 | Intervention: mean 19.4 months; control: mean 19.1 months | None |
Abbreviations: MINORS, Methodological Index for Non-Randomized Studies; RCT, randomized controlled trial; NR, not reported; H2O2, hydrogen peroxide.
Table 2.
Treatment Characteristics
| Author | Year | Preoperative Prophylaxis | Intraoperative Intervention Treatment | Intraoperative Control Treatment | Postoperative Prophylaxis |
|---|---|---|---|---|---|
| Intrawound antibiotics | |||||
| Garg et al | 2018 | IV cefazolin or vancomycin (depending on MRSA risk) | 0.5-2 g of vancomycin combined with autograft and placed subfascially | NR | Standard perioperative antibiotics |
| Thompson et al | 2018 | IV cefazolin or vancomycin (depending on MRSA risk) | 0.5-1 g of vancomycin powder applied over implants and bone graft before closure | NR | Oral cephalexin or oral sulfamethoxazole/trimethoprim for 2 days |
| Haller et al | 2017 | 50 mg/kg IV cefuroxime | 0.5 g of vancomycin powder placed between fascia and subcutaneous tissue before closure | Saline irrigation | NR |
| Hey et al | 2017 | 1000 mg IV cefazolin | 1 g of vancomycin powder in subfascial space | NR | IV cefazolin for 2 days |
| Liu et al | 2015 | IV cefazolin or clindamycin | 0.5-2 g of vancomycin powder evenly spread over muscle, fascia, implants, and autograft before closure | 1-2 L saline irrigation before wound closure | IV cefazolin or clindamycin every 8 hours for 1 day |
| Hill et al | 2014 | 1000-2000 mg IV cefazolin | 1-2 g of vancomycin powder into the wound before closure | NR | IV cefazolin for 1 day |
| Emohare et al | 2014 | Perioperative IV cefazolin | 1 g of vancomycin powder into all wound layers prior to closure | NR | Perioperative IV cefazolin |
| Theologis et al | 2014 | Routine perioperative antibiotics | 2 g of vancomycin powder in subfascial space | NR | Routine perioperative antibiotics |
| Tubaki et al | 2013 | 750 mg IV cefuroxime | 1 g of vancomycin powder placed directly onto the tissues, taking care not to expose bone graft or dura | 1 L saline irrigation | 750 mg IV cefuroxime every 8 hours for 1 day or until drain removal depending on noninstrumented or instrumented surgery |
| Strom et al (1)34 | 2013 | IV cefazolin | 1 g of vancomycin powder placed onto all tissues, taking care not to expose bone graft or instrumentation | 3 L pulse lavage with bacitracin prior to bone graft placement | NR |
| Strom et al (2)35 | 2013 | IV cefazolin | 1 g of vancomycin powder placed onto all tissues, taking care not to expose bone graft or instrumentation | 3 L pulse lavage with bacitracin prior to bone graft placement | NR |
| Pahys et al | 2013 | Standard IV perioperative cephalosporins | Preoperative alcohol foam disinfectant, 0.5 g of vancomycin powder added to the wound at the end of the procedure + second drain placement | NR | Standard IV perioperative cephalosporins |
| Caroom et al | 2013 | IV antibiotics according to policy | 1 g of vancomycin powder applied subfascially along bone graft and instrumentation after saline irrigation | NR | IV antibiotics according to policy, continued until 24 hours after drain removal |
| Heller et al | 2013 | 20 mg/kg IV cefazolin | 0.5-2 g of vancomycin powder into the wound before closure | NR | 1000 mg IV cefazolin every 8 hours for 1 day |
| Kim et al | 2013 | 1000 mg IV cefazolin | 1 g of vancomycin powder placed directly onto the tissues, taking care not to expose bone graft or dura | NR | 1000 mg IV cefazolin every 8 hours for 1 day |
| Sweet et al | 2011 | 2000 mg IV cefazolin | 1 g of vancomycin powder sprinkled into the deep and superficial portion of the wound before closure, 1 g mixed with bone graft | NR | IV cefazolin for 1 day |
| Intrawound antiseptics | |||||
| De Luna et al | 2017 | 1000 mg IV cefazolin | 2 L 3% povidone-iodine for 5-10 minutes, followed by 1 L saline irrigation prior to bone graft placement | 2 L saline irrigation for 5-10 minutes prior to bone graft placement | 1000 mg IV cefazolin every 12 hours for 2 days |
| Herwijnen et al | 2015 | Weight dependent IV flucloxacillin and gentamicin | 3 L saline irrigation followed by 1 L 1% povidone-iodine for 3 minutes followed by 3 L saline irrigation | 6 L saline irrigation followed by 1 L saline irrigation with 80 mg dissolved gentamicin | IV flucloxacillin every 8 hours for 1 day |
| Ulivieri et al | 2011 | 2000 mg IV amoxicillin + 400 mg IV clavulanic acid | Irrigation with solution of 10 mL 10% povidone-iodine + 5 mL H2O + 1 mL H2O2 for 1 minute followed by copious saline irrigation | NR | 6 hours postoperative 2000 mg IV amoxicillin + 400 mg IV clavulanic acid; 1000 mg amoxicillin + 200 mg clavulanic acid for 7 days if hardware was implanted |
| Chang et al | 2006 | 1000 mg IV cefazolin and 60 mg IV gentamicin | 0.35% povidone-iodine irrigation for 3 minutes followed by 2 L saline irrigation | 2 L saline irrigation | 1000 mg IV cefazolin every 6 hours and 60 mg IV gentamicin every 12 hours for 2 days; after that, oral cefazolin for 3 days |
Abbreviations: IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; NR, not reported; H2O2, hydrogen peroxide.
Fourteen studies provided a clear definition of SSI. In 5 studies,32,44–46,48 this was the (deep) SSI definition used by the Centers for Disease Control and Prevention.50 Three studies defined SSI as a combination of clinical symptoms, elevated serum inflammation markers (erythrocyte sedimentation rate, C-reactive protein, white blood cell count), and bacterial culture results.31,33,43 One study solely relied on the results of culture and/or radiographic findings.39 Five studies used the need for reoperation or nonresponse to antibiotics,36,38,42,47,49 and 6 studies did not provide a clear SSI definition.30,34,35,37,40,41
Study Quality and Heterogeneity
The median MINORS quality score for all studies was 14 (range 11-17) out of a maximum score of 24. The 2 RCTs included in this review yielded a higher median score of 16.5 (range 16-17), while the observational studies had a median score of 13.5 (range 11-17). Some statistical heterogeneity was observed when looking at the pooled result with a Tau2 of 0.43. The I 2 index for heterogeneity remained <50% (I 2 = 38.6%) and may represent moderate heterogeneity.
Meta-Analysis
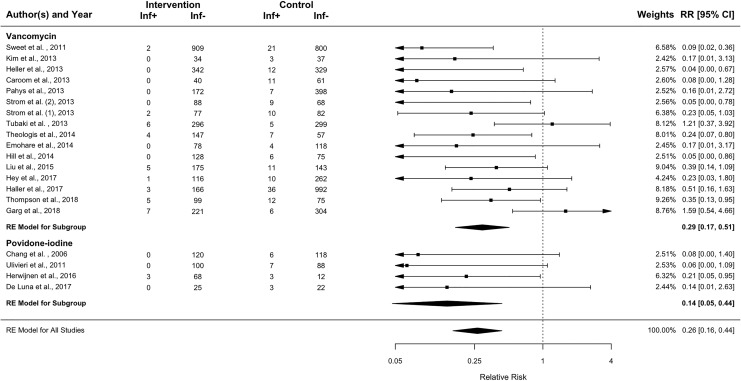
Deep SSIs were reported in 38 of the 3439 patients that received intrawound treatments (1.1%), compared with 189 deep SSIs in the 4529 control patients (4.2%). Table 3 contains the deep SSI rates for all studies. With this data, a meta-analysis was performed (Figure 2). When the results of the antibiotic interventions and the antiseptic irrigation interventions were pooled, the relative risk for deep SSI was 0.26 (95% CI 0.16-0.44, P < .0001). For the patients treated with local antibiotics, the pooled relative risk for deep SSI was 0.29 (95% CI 0.17-0.51, P < .0001) when compared with the control group. Patients that were irrigated with antiseptics had a pooled relative risk of 0.14 (95% CI 0.05-0.44, P = .0006). If heterogeneity would be ignored, the Mantel-Haenszel method that uses the fixed-effects model yields even lower relative risks with a relative risk of deep SSI for antibiotics and antiseptics combined of 0.23 (95% CI 0.16-0.33), a relative risk for antibiotics of 0.26 (95% CI 0.18-0.37), and a relative risk for antiseptics of 0.05 (95% CI 0.01-0.31).
Table 3.
Instrumented Deep Surgical Site Infection Rates.
| Author | Year | Instrumented Intervention Patients | Instrumented Control Patients | Deep SSI rate in Instrumented Intervention Patients | Deep SSI Rate in Instrumented Control Patients |
|---|---|---|---|---|---|
| Intrawound antibiotics | |||||
| Garg et al | 2018 | 228 | 310 | 3.1% (7/228) | 1.9% (6/310) |
| Thompson et al | 2018 | 104 | 87 | 4.8% (5/104) | 13.8% (12/87) |
| Haller et al | 2017 | 169 | 1028 | 1.8% (3/169) | 3.5% (36/1028) |
| Hey et al | 2017 | 117 | 272 | 0.9% (1/117) | 3.7% (10/272) |
| Liu et al | 2015 | 180 | 154 | 2.8% (5/180) | 7.1% (11/154) |
| Hill et al | 2014 | 128 | 81 | 0% (0/128) | 7.4% (6/81) |
| Emohare et al | 2014 | 78 | 122 | 0% (0/78) | 3.3% (4/122) |
| Theologis et al | 2014 | 151 | 64 | 2.7% (4/151) | 10.9% (7/64) |
| Tubaki et al | 2013 | 302 | 304 | 2.0% (6/302) | 1.6% (5/304) |
| Strom et al (1)34 | 2013 | 79 | 92 | 2.5% (2/79) | 10.9% (10/92) |
| Strom et al (2)35 | 2013 | 88 | 77 | 0% (0/88) | 11.7% (9/77) |
| Pahys et al | 2013 | 172 | 405 | 0% (0/172) | 1.7% (7/405) |
| Caroom et al | 2013 | 40 | 72 | 0% (0/40) | 15.3% (11/72) |
| Heller et al | 2013 | 342 | 341 | 0% (0/342) | 3.5% (12/341) |
| Kim et al | 2013 | 34 | 40 | 0% (0/34) | 7.5% (3/40) |
| Sweet et al | 2011 | 911 | 821 | 0.2% (2/911) | 2.6% (21/821) |
| Intrawound antiseptics | |||||
| De Luna et al | 2017 | 25 | 25 | 0% (0/25) | 12.0% (3/25) |
| Herwijnen et al | 2016 | 71 | 15 | 4.2% (3/71) | 20.0% (3/15) |
| Ulivieri et al | 2011 | 100 | 95 | 0% (0/100) | 7.4% (7/95) |
| Chang et al | 2006 | 120 | 124 | 0% (0/120) | 4.8% (6/124) |
Abbreviation: SSI, surgical site infection.
Figure 2.
Forest plot of random effects model showing the relative risks and 95% confidence intervals of intrawound treatment compared to controls. A relative risk below 1 favors intervention treatment over control treatment.
RE, random effects; Inf+, number of patients with deep surgical site infection; Inf−, number of patients without deep surgical site infection; RR, relative risk; CI, confidence interval.
Pooling the high-quality studies (5 studies) resulted in a relative risk of 0.33 (95% CI 0.08-1.42). Pooling the medium (9 studies) and lower (6 studies) quality studies resulted in a relative risk of 0.29 (95% CI 0.18-0.49) and 0.18 (95% CI 0.08-0.40), respectively, indicating that the lower quality studies may overestimate an effect.
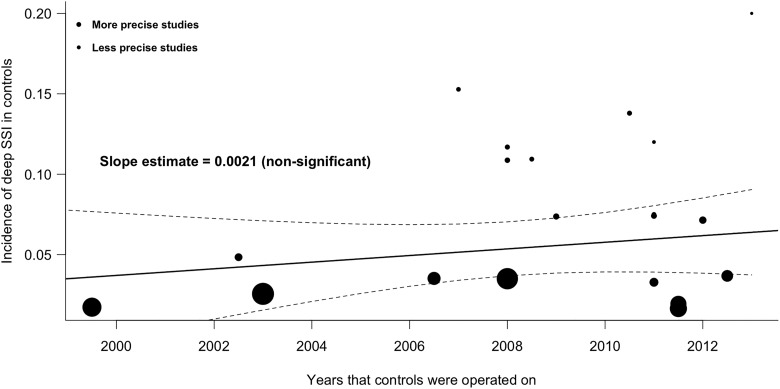
The regression analysis of the incidence of SSIs over time shows that the risk of deep SSI in the control groups did not decrease but rather showed a nonsignificant, inclining slope (Figure 3). From this, we can conclude that in a period of about 12 years, the incidence of deep SSI has not significantly decreased in the study populations.
Figure 3.
Weighted regression analysis of the incidence of SSI in control groups over time. Area between dashed lines is 95% confidence interval.
SSI, surgical site infection.
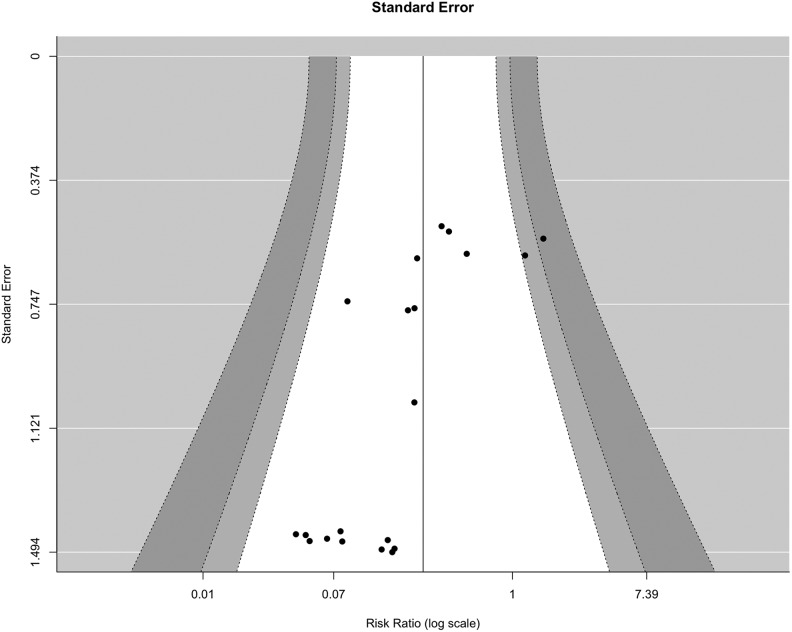
To analyze publication bias, a funnel plot was made that indicated asymmetry (Figure 4). This may be explained by the difficulty to publish studies without an effect (publication bias). However, since the standard error (the y-axis) of the relative risks is mathematically linked to the relative risk itself, studies with few events automatically have a high standard error, which causes the clustering in the lower left corner.
Figure 4.
Funnel plot to assess publication bias. White area is within 95% pseudo–confidence interval limits.
Adverse Events
None of the included articles reported any adverse events such as renal toxicity, hypotension, or prolonged wound leakage. Two articles studied the potential effects of vancomycin on compromised bone healing in terms of nonunion rate.33,34 Strom et al34 found a nonunion rate of 5.1% for the treated group versus 5.4% for the control group (P = 1.000). Sweet et al33 found no significant difference between the intervention and control groups either (0.33% for the treated group vs 0.49% for the control group). For the application of antiseptics, only Chang et al31 investigated the nonunion rates and found no significant difference between treated patients and controls when using a 3-minute 0.35% povidone-iodine irrigation (10.8% vs 12.1%, P = .28).
Discussion
This systematic review and meta-analysis indicates a positive effect of perioperative intrawound prophylaxis to reduce the risk of SSI, with a relative risk of 0.26 (95% CI 0.16-0.44) compared with no intrawound treatment. When viewed separately, both antibiotics and antiseptics were significantly effective with relative risks of 0.29 (∼3 times lower risk) and 0.14 (∼7 times lower risk), respectively.
In the present review, we deliberately decided to include both RCTs and observational studies. The reason for this is that the RCT is no longer regarded as the only optimal design for surgical (intervention) studies, mainly due to inherent disadvantages.22–24,51 For example, double blinding is difficult or impossible.52 Furthermore, surgical RCTs often have very low recruitment rates, which make them less representative of usual practice.22,53 Due to the limited financial resources, sample size is often small and the follow-up period is short, which makes these studies less useful for complication research.22,24,51,54,55 Observational comparative studies are by design more subjected to confounders and bias. However, a large part of confounding bias in observational comparative studies can be mitigated by sound methodological practices. In fact, meta-epidemiological studies have shown that both designs provide a comparable level of evidence for surgical research questions.21,23,55–57 To limit bias by selection on indication, we specifically addressed this item in the study selection process.
Interestingly, the only RCT investigating intrawound antibiotics found no effect of treatment.30 However, this study investigated treatment in both instrumented and uninstrumented spine surgeries and therefore yielded a relatively low total SSI rate of 1.65%. The RCT investigating intrawound antiseptic prophylaxis in instrumented spine surgery found a reduction of deep SSIs, with a rate of 0% in the intervention group versus 4.84% in the control group.31
To our knowledge, this is the first systematic review and meta-analysis in spinal surgery patients to pool data from both antibiotic and antiseptic intrawound treatments into a single meta-analysis. By focusing on deep SSIs (as opposed to superficial SSIs) and instrumented patients only, we also investigated the most clinically relevant complication in a vulnerable patient group, as deep SSIs in instrumented spine surgery patients often have disastrous consequences. We are also the first to analyze SSI rates against time in a meta-regression analysis. In a recent systematic review and meta-analysis of the prophylactic use of vancomycin powder in spine surgery, Evaniew et al17 found results similar to our study (OR 0.19, 95% CI 0.08-0.47), but they included only 8 studies, included all types of patients (implanted and non-implanted) and both deep and superficial SSIs. Also, Bakhsheshian et al58 found an effect of vancomycin powder in the prevention of deep SSIs in their meta-analysis of 12 studies with an odds ratio of 0.23 (95% CI 0.11-0.50). With respect to intrawound povidone-iodine treatment, the meta-analysis by Mueller et al18 that included many different types of surgery and both contaminated and infected wounds also indicated a protective effect (OR 0.70, 95% CI 0.51-0.97).
Complications and Adverse Events
Based on the literature search that we performed, few adverse events have been reported of any intrawound prophylaxis. Vancomycin is most often used as intrawound antibiotic prophylaxis because of its potency to treat infections with gram-positive skin commensals such as Staphylococcus aureus and Staphylococcus epidermidis. Side effects mentioned in the literature are sudden hypotension, renal toxicity, ototoxicity, and the Red Man syndrome,59 which, however, have only been reported in cases when vancomycin was administered intravenously.59 The literature on adverse events when using intrawound vancomycin mostly consists of case reports, which mention one anaphylactic reaction with circulatory collapse 30 minutes after administration,60 one patient with unexplained renal failure and 2 patients with transient hearing loss.61 A recent systematic review of DeFrancesco et al found only one case of adverse drug reaction (transient rash) in almost 1400 children undergoing posterior spinal surgery for early onset scoliosis, a rate of only 0.072%.62 In addition, patients in this study that had previously shown adverse drug reactions to intravenous vancomycin did not react to intrawound vancomycin powder. Nonunion of bone is another potential complication of local antibiotics at high concentrations. Edin et al63 reported cytotoxicity occurring at vancomycin levels ≥10 000 µg/mL; Rathbone et al64 found that concentrations ≥5000 µg/mL impaired the number of osteoblasts and their function; and a recent study by Eder et al65 reported similar dose-dependent effects at concentrations of only 3000 µg/mL. The included clinical studies did not report increased nonunion rates. This is likely because the vancomycin levels in the drain fluid never exceeded 1500 µg/mL33,65,66 and resorption into the blood was negligible, with mean serum levels not exceeding 2.5 µg/mL, far below the toxic serum concentrations.33,66 It is important to note that vancomycin seems to be the least cytotoxic of studied antibiotics. Other antibiotics (eg, gentamicin) can be more harmful to osteoblasts and especially to cartilage when applied intra-articularly.64 A serious disadvantage of intrawound antibiotics is its effect on antimicrobial resistance. Studying this phenomenon following intrawound use is difficult, but some studies that investigated culture findings after vancomycin use exist. One such study found no increase in the number of SSIs with vancomycin-resistant strains in patients treated with intrawound vancomycin.67 It did, however, find significantly more infections with gram-negative bacteria. In contrast to this, however, another study found no differences in culture profiles when comparing the period before and after intrawound vancomycin.68 Although these 2 studies have not yet shown the onset of vancomycin-resistant infections, the theoretical effects are definitely a cause for concern and therefore a preference for irrigation with antiseptics to antibiotics could be argued.
Most antiseptics are cytotoxic well before they achieve the minimal bactericidal concentration.19 Povidone-iodine is an exception to this by achieving bactericidal concentrations before cytotoxicity occurs at the relatively low concentration of 1.3 g/L.19 Although the included studies used substantially higher concentrations, no adverse events associated with the use of povidone-iodine were reported. Also, nonunion rates between treated patients and controls did not differ.31
Limitations
Our study had several limitations. First, deep SSI rates in many different types of instrumented spinal surgery were studied. This makes general applicability of the observed results difficult. Second, a publication bias based on the included studies cannot be excluded. Third, many different patient demographics and highly divergent follow-up times were present in the included studies, which caused study heterogeneity. Fourth, the SSI definitions were not similar in the included studies and were not always clearly defined, making it easier for the investigators to be biased when defining whether someone developed an SSI based on the desired outcome (expectancy bias). Finally, the amount, concentration, and method of application varied across studies, as did the type, amount, and length of the perioperative antibiotics that were used.
Conclusion
Based on data from 20 studies, we found a 3 to 7 times reduction in deep SSIs in instrumented spinal surgery when antibiotic intrawound prophylaxis (relative risk 0.29, 95% CI 0.17-0.51, P < .0001) or antiseptic intrawound prophylaxis (relative risk 0.14, 95% CI 0.05-0.44, P = .0006) was used. No adverse events were reported. Although the nonstandardized methods and the large heterogeneity of the currently investigated interventions preclude recommendation for a specific treatment regime, the application of intrawound treatments in general should be considered for instrumented spinal surgery patients.
Appendix
Search Strategy
Date of search: April 16, 2018.
| Database | Search Syntax | Results |
|---|---|---|
| PubMed library | (“Surgical Wound Infection”[Mesh] OR surgical wound infection*[tiab] OR surgical site infection* [tiab] OR SSI[tiab] OR joint infection[tiab] OR deep infection[tiab] OR postoperative wound infect*[tiab]) AND (local administration[tiab] OR local application[tiab] OR intrawound[tiab] OR intra-wound[tiab] OR intrasite[tiab] OR intra-site[tiab] OR powder[tiab] OR vancomycin[tiab] OR gentamicin[tiab] OR gentamycin[tiab] OR dermacyn[tiab] OR iodine[tiab] OR povidone-iodine[tiab] OR PVP-I[tiab] OR betadine[tiab] OR chlorhexidin*[tiab] OR bacitracin[tiab] OR benzalkonium[tiab] OR castile soap[tiab] OR anti-infect*[tiab] OR antiseptic*[tiab] OR surfactant*[tiab] OR microbicides[tiab]) | 2884 |
| EMBASE library | (‘surgical wound infection’:ab,ti OR ‘surgical site infection’:ab,ti OR SSI:ab,ti OR ‘joint infection’:ab,ti OR ‘deep infection’:ab,ti OR ‘postoperative wound infection’:ab,ti) AND (‘local administration’:ab,ti OR ‘local application’:ab,ti OR intrawound:ab,ti OR ‘intra wound’:ab,ti OR intrasite:ab,ti OR ‘intra site’:ab,ti OR powder:ab,ti OR vancomycin:ab,ti OR gentamicin:ab,ti OR gentamycin:ab,ti OR dermacyn:ab,ti OR iodine:ab,ti OR ‘povidone iodine’:ab,ti OR ‘PVP I’:ab,ti OR betadine:ab,ti OR chlorhexidin*:ab,ti OR bacitracin:ab,ti OR benzalkonium:ab,ti OR ‘castile soap’:ab,ti OR ‘anti infectant’:ab,ti OR antiseptic*:ab,ti OR surfactant*:ab,ti OR microbicides:ab,ti) AND [embase]/lim NOT [medline]/lim | 563 |
| Cochrane library | (“surgical wound infection”:ab,ti,kw OR “surgical site infection”:ab,ti,kw OR SSI:ab,ti OR “joint infection”:ab,ti OR “deep infection”:ab,ti OR “postoperative wound infection”:ab,ti) AND (“local administration”:ab,ti OR “local application”:ab,ti OR intrawound:ab,ti OR intra-wound:ab,ti OR intrasite:ab,ti OR intra-site:ab,ti OR powder:ab,ti OR vancomycin:ab,ti OR gentamicin:ab,ti OR gentamycin:ab,ti OR dermacyn:ab,ti OR iodine:ab,ti OR povidone-iodine:ab,ti OR PVP-I:ab,ti OR betadine:ab,ti OR chlorhexidin*:ab,ti OR bacitracin:ab,ti OR benzalkonium:ab,ti OR “castile soap”:ab,ti OR anti-infect*:ab,ti OR antiseptic*:ab,ti OR surfactant*:ab,ti OR microbicides:ab,ti) | 627 |
Footnotes
Authors’ Note: No ethical committee approval was deemed necessary for this systematic review and meta-analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. [DOI] [PubMed] [Google Scholar]
- 2. Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009;34:1422–1428. [DOI] [PubMed] [Google Scholar]
- 3. Blam OG, Vaccaro AR, Vanichkachorn JS, et al. Risk factors for surgical site infection in the patient with spinal injury. Spine (Phila Pa 1976). 2003;28:1475–1480. [DOI] [PubMed] [Google Scholar]
- 4. Cahill PJ, Warnick DE, Lee MJ, et al. Infection after spinal fusion for pediatric spinal deformity: thirty years of experience at a single institution. Spine (Phila Pa 1976). 2010;35:1211–1217. [DOI] [PubMed] [Google Scholar]
- 5. Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008;17:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fry DE. The economic costs of surgical site infection. Surg Infect (Larchmt). 2002;3(suppl 1):S37–S43. [DOI] [PubMed] [Google Scholar]
- 7. Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183–189. [DOI] [PubMed] [Google Scholar]
- 8. Tanner J, Swarbrook S, Stuart J. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst Rev. 2008;(1):CD004288. [DOI] [PubMed] [Google Scholar]
- 9. Widmer AF, Rotter M, Voss A, et al. Surgical hand preparation: state-of-the-art. J Hosp Infect. 2010;74:112–122. [DOI] [PubMed] [Google Scholar]
- 10. AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90:915–919. [DOI] [PubMed] [Google Scholar]
- 11. Bratzler DW, Houck PM, Richards C, et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;140:174–182. [DOI] [PubMed] [Google Scholar]
- 12. Coates T, Bax R, Coates A. Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects. J Antimicrob Chemother. 2009;64:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2015;(2):CD004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salassa TE, Swiontkowski MF. Surgical attire and the operating room: role in infection prevention. J Bone Joint Surg Am. 2014;96:1485–1492. [DOI] [PubMed] [Google Scholar]
- 15. Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014;14:397–407. [DOI] [PubMed] [Google Scholar]
- 16. Kang DG, Holekamp TF, Wagner SC, Lehman RA., Jr Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015;15:762–770. [DOI] [PubMed] [Google Scholar]
- 17. Evaniew N, Khan M, Drew B, Peterson D, Bhandari M, Ghert M. Intrawound vancomycin to prevent infections after spine surgery: a systematic review and meta-analysis. Eur Spine J. 2015;24:533–542. [DOI] [PubMed] [Google Scholar]
- 18. Mueller TC, Loos M, Haller B, et al. Intra-operative wound irrigation to reduce surgical site infections after abdominal surgery: a systematic review and meta-analysis. Langenbeck’s Arch Surg. 2015;400:167–181. [DOI] [PubMed] [Google Scholar]
- 19. van Meurs SJ, Gawlitta D, Heemstra KA, Poolman RW, Vogely HC, Kruyt MC. Selection of an optimal antiseptic solution for intraoperative irrigation: an in vitro study. J Bone Joint Surg Am. 2014;96:285–291. [DOI] [PubMed] [Google Scholar]
- 20. Muller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281–1287. [DOI] [PubMed] [Google Scholar]
- 21. Shrier I, Boivin JF, Steele RJ, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 2007;166:1203–1209. [DOI] [PubMed] [Google Scholar]
- 22. Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. 2010;63:238–245. [DOI] [PubMed] [Google Scholar]
- 24. Jacobs WCH, Kruyt MC, Verbout AJ, Oner FC. Spine surgery research: on and beyond current strategies. Spine J. 2012;12:706–713. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 London, England: Cochrane Collaboration; 2008. [Google Scholar]
- 27. Agency for Healthcare Research and Quality Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 28. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. A N Z J Surg. 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- 29. Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976). 2013;38:2149–2155. [DOI] [PubMed] [Google Scholar]
- 31. Chang FY, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J. 2006;15:1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Luna V, Mancini F, De Maio F, Bernardi G, Ippolito E, Caterini R. Intraoperative disinfection by pulse irrigation with povidone-iodine solution in spine surgery. Adv Orthop. 2017;2017:7218918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011;36:2084–2088. [DOI] [PubMed] [Google Scholar]
- 34. Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976). 2013;38:991–994. [DOI] [PubMed] [Google Scholar]
- 35. Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Lumbar laminectomy and fusion with routine local application of vancomycin powder: decreased infection rate in instrumented and non-instrumented cases. Clin Neurol Neurosurg. 2013;115:1766–1769. [DOI] [PubMed] [Google Scholar]
- 36. Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95:549–554. [DOI] [PubMed] [Google Scholar]
- 37. Caroom C, Tullar JM, Benton EG, Jr, Jones JR, Chaput CD. Intrawound vancomycin powder reduces surgical site infections in posterior cervical fusion. Spine (Phila Pa 1976). 2013;38:1183–1187. [DOI] [PubMed] [Google Scholar]
- 38. Heller A, McIff TE, Lai SM, Burton DC. Intrawound vancomycin powder decreases staphylococcal surgical site infections following posterior instrumented spinal arthrodesis. J Spinal Disord Tech. 2015;28:E584–E589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim HS, Lee SG, Kim WK, Park CW, Son S. Prophylactic intrawound application of vancomycin powder in instrumented spinal fusion surgery. Korean J Spine. 2013;10:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hill BW, Emohare O, Song B, Davis R, Kang MM. The use of vancomycin powder reduces surgical reoperation in posterior instrumented and noninstrumented spinal surgery. Acta Neurochir (Wien). 2014;156:749–754. [DOI] [PubMed] [Google Scholar]
- 41. Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW, Jr, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 2014;14:2710–2715. [DOI] [PubMed] [Google Scholar]
- 42. Theologis AA, Demirkiran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: a cost analysis. Spine (Phila Pa 1976). 2014;39:1875–1880. [DOI] [PubMed] [Google Scholar]
- 43. Ulivieri S, Toninelli S, Petrini C, Giorgio A, Oliveri G. Prevention of post-operative infections in spine surgery by wound irrigation with a solution of povidone-iodine and hydrogen peroxide. Arch Orthop Trauma Surg. 2011;131:1203–1206. [DOI] [PubMed] [Google Scholar]
- 44. Garg S, Bloch N, Potter M, et al. Topical vancomycin in pediatric spine surgery does not reduce surgical site infection: a retrospective cohort study. Paper presented at: 52nd Annual Meeting & Course; September 6-9, 2017; Philadelphia, PA. [DOI] [PubMed] [Google Scholar]
- 45. Haller JM, Heflin JA, Hulet DA, Ding Q, Presson AP, Smith JT. Intrawound vancomycin powder associated with reduced surgical site infection in rib-based distraction surgery [published online August 2, 2017]. J Pediatr Orthop. doi:10.1097/BPO.0000000000001042. [DOI] [PubMed] [Google Scholar]
- 46. Hey HW, Thiam DW, Koh ZS, et al. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine (Phila Pa 1976). 2017;42:267–274. [DOI] [PubMed] [Google Scholar]
- 47. Liu N, Wood KB, Schwab JH, et al. Comparison of intrawound vancomycin utility in posterior instrumented spine surgeries between patients with tumor and nontumor patients. Spine (Phila Pa 1976). 2015;40:1586–1592. [DOI] [PubMed] [Google Scholar]
- 48. Thompson GH, Poe-Kochert C, Hardesty CK, Son-Hing J, Mistovich RJ. Does vancomycin powder decrease surgical site infections in growing spine surgery? A preliminary study. J Bone Joint Surg Am. 2018;100:466–471. [DOI] [PubMed] [Google Scholar]
- 49. van Herwijnen B, Evans NR, Dare CJ, Davies EM. An intraoperative irrigation regimen to reduce the surgical site infection rate following adolescent idiopathic scoliosis surgery. Ann R Coll Surg Engl. 2016;98:320–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection. 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. [PubMed] [Google Scholar]
- 51. Lassen K, Hφye A, Myrmel T. Randomised trials in surgery: the burden of evidence. Rev Recent Clin Trials. 2012;7:244–248. [DOI] [PubMed] [Google Scholar]
- 52. Boutron I, Tubach F, Giraudeau B, Ravaud P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J Clin Epidemiol. 2004;57:543–550. [DOI] [PubMed] [Google Scholar]
- 53. Abraham NS, Young JM, Solomon MJ. A systematic review of reasons for nonentry of eligible patients into surgical randomized controlled trials. Surgery. 2006;139:469–483. [DOI] [PubMed] [Google Scholar]
- 54. Vandenbroucke JP. Why do the results of randomised and observational studies differ? BMJ. 2011;343:d7020. [DOI] [PubMed] [Google Scholar]
- 55. Jacobs WCH, Kruyt MC, Verbout AJ, Oner FC. Effect of methodological quality measures in spinal surgery research: a meta-epidemiological study. Spine J. 2012;12:339–348. [DOI] [PubMed] [Google Scholar]
- 56. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. [DOI] [PubMed] [Google Scholar]
- 57. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015;83:816–823. [DOI] [PubMed] [Google Scholar]
- 59. Bruniera FR, Ferreira FM, Saviolli LR, et al. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19:694–700. [PubMed] [Google Scholar]
- 60. Mariappan R, Manninen P, Massicotte EM, Bhatia A. Circulatory collapse after topical application of vancomycin powder during spine surgery. J Neurosurg Spine. 2013;19:381–383. [DOI] [PubMed] [Google Scholar]
- 61. Molinari RW, Khera OA, Molinari WJ., 3rd Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21(suppl 4):S476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. DeFrancesco CJ, Flynn JM, Smith JT, et al. ; Children’s Spine Study Group. Clinically apparent adverse reactions to intra-wound vancomycin powder in early onset scoliosis are rare. J Child Orthop. 2017;11:414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996;(333):245–251. [PubMed] [Google Scholar]
- 64. Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29:1070–1074. [DOI] [PubMed] [Google Scholar]
- 65. Eder C, Schenk S, Trifinopoulos J, Schildboeck S, Kienzl M, Ogon M. Does intrawound application of vancomycin influence bone regeneration in spinal fusion? Eur Spine J. 2014;14:S2–S3. [DOI] [PubMed] [Google Scholar]
- 66. Armaghani SJ, Menge TJ, Lovejoy SA, Mencio GA, Martus JE. Safety of topical vancomycin for pediatric spinal deformity: nontoxic serum levels with supratherapeutic drain levels. Spine (Phila Pa 1976). 2014;39:1683–1687. [DOI] [PubMed] [Google Scholar]
- 67. Ghobrial GM, Thakkar V, Andrews E, et al. Intraoperative vancomycin use in spinal surgery: single institution experience and microbial trends. Spine (Phila Pa 1976). 2014;39:550–555. [DOI] [PubMed] [Google Scholar]
- 68. Carreon LY, Gum JL, Crawford CH, et al. Culture profile of surgical site infections after topical vancomycin powder use in instrumented spine fusions. Spine J. 2013;13:42S. [Google Scholar]