Abstract
Study Design:
It consisted of evaluation of the pedicle screws for presence of residual nonmicrobial contaminants and tabulation of the minimum steps and time required for reprocessing implants as per guidelines and its comparison with actual practice.
Objective:
An evaluation of the nonmicrobial contaminants prevalent on the pedicle screws used for spine surgery and the underlying practice cause behind the source.
Methods:
The first component consisted of a random selection of 6 pedicle screws and its assessment using optical microscopy, scanning electron microscopy with energy dispersive spectroscopy, and Fourier transform infrared spectroscopy. The second component consisted of review of implant reprocessing guidelines and its applicability.
Results:
Three types of contaminants were identified: corrosion, saccharide of unknown origin, and soap residue mixed with and were mostly present at the interfaces with low permeability. In addition, manufacturer’s guideline recommends 19 hours of reprocessing, whereas the real-time observation revealed a turnaround time of 1 hour 17 minutes.
Conclusion:
Repeatedly reprocessed pedicle screws host corrosion, carbohydrate, fat, and soap, which could be a cause of surgical site infection and inflammatory responses postsurgery. The cause behind it is the impracticality of repeated cleaning and inspection of such devices.
Keywords: SSI, implant exposure, surgical site infection, terminally sterile devices, sterile processing department, SPD, preoperative implant handling
Introduction
Surgical site infection (SSI) is known to occur at the rate of 12.7% following spinal fusion.1 Pedicle screws are the key implants used in these procedures and have become synonymous with the term spinal fusion. The sterilization processing department (SPD) is at the core of conventional processing of these screws, with daily activities ranging from receiving contaminated surgical instruments and implants, to redistributing them sterilized to the various departments and surgical units in the facility (both on and off campus). The services performed includes decontamination, washing, reassembly in trays or as single items, wrapping, labeling, and sterilization.2 Efficiency in this process is paramount for the proper surgical management of the patients, consequently avoiding costly delays and SSIs.3 A caveat with this process is the low ratio of used to reprocessed implants (∼0.03-0.08), resulting in multiple reprocessing life cycle per individual implant before implantation. Thus, on the postoperatively returned implants with low cleanliness assurance in adjunct with highly contaminated instruments (harboring macroscopic human tissues and blood-borne pathogens) deems thorough reprocessing and visual inspection critical to avoid buildup of contaminations or formation of biofilm thereof.
However, the most common implant used in spinal fusion, pedicle screws, are multicomponent with lumens, interfaces, and crevices in the range of 0.2 to 1.5 mm. This raises a concern regarding the practicality of a repeated cleaning process, at the heart of which lies the manufacturer’s instruction for cleaning and sterilization. The purpose of this study was to evaluate the pedicle screws retrieved from reprocessed units stored and currently in circulation at surgical centers for presence of residual nonmicrobial contaminants and/or foreign material. In conjunction, multiple manufacturer’s instructions were reviewed to determine the basic steps and the minimum time recommended to clean and sterilize a tray of 164 pedicle screw implants and practicality of such a procedure.
Methods
The study design consisted of 2 components: (1) evaluation of the pedicle screws for presence of residual nonmicrobial contaminants and/or foreign material and (2) tabulation of the minimum steps and time required for reprocessing implants as per manufacturer’s guidelines and its comparison with actual practice.
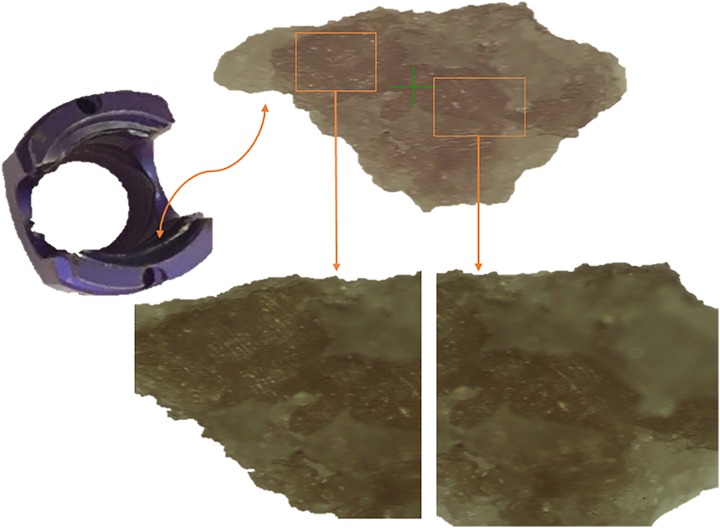
The first component consisted of a random selection of 6 pedicle screws from 4 different trays of cleaned, wrapped, and sterilized implants. The screws were retrieved using clean gloves, followed by its immediate placement inside of clean zip-lock polybags. Each pedicle screw was disassembled and sent for optical microscopy, scanning electron microscopy with energy dispersive spectroscopy, and Fourier transform infrared spectroscopy. The type and the size of contaminants were recorded.
The second component consisted of review of 4 major manufacturer’s instructions for reprocessing pedicle screws. Each instruction manual was then converted into a tabulation with the various steps and the minimum time required for each step as per the written guidelines. The common steps were recorded along with the minimum time required in each step. This was then compared with the real-time observations from state of art SPDs on the exact procedure of reprocessing a pedicle screw set.
Results
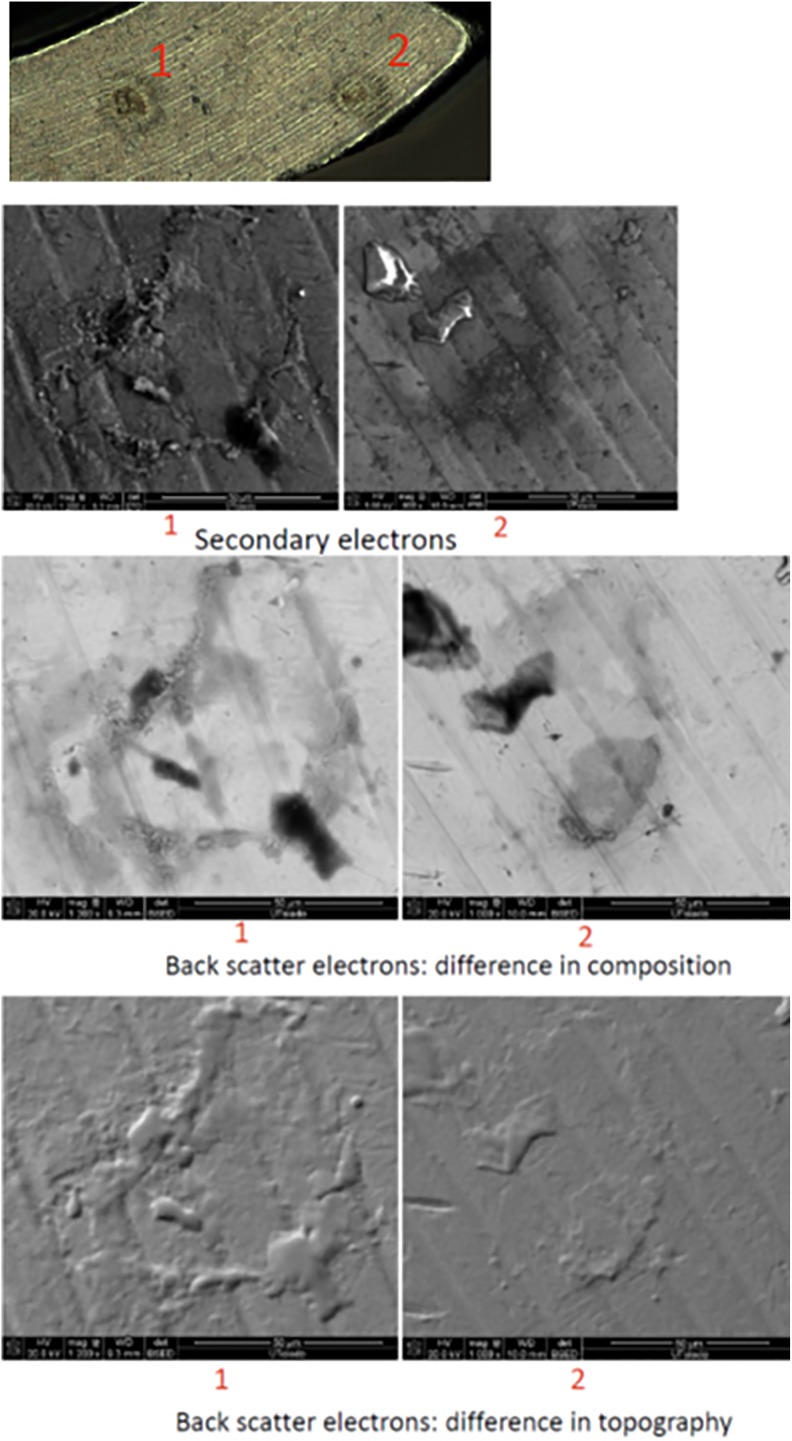
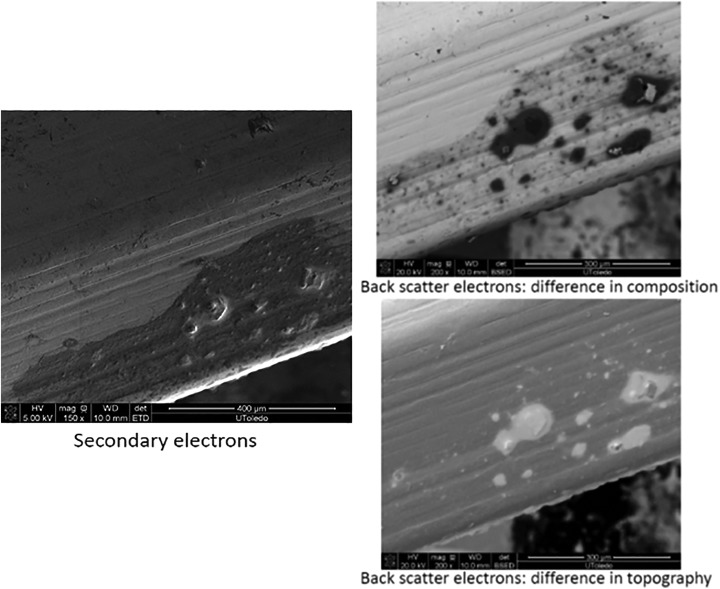
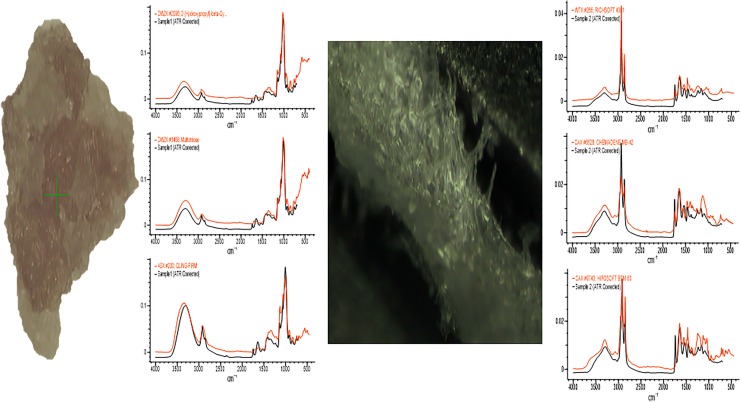
Three types of contaminants were identified: corrosion, saccharide of unknown origin (biofilm, endotoxins, fatty tissue), and soap residue mixed with fat, each occupying isolated diametrical areas of 1.4 mm, 1.5 mm, and 3.4 mm, respectively (Figures 1 –5 and Table 1). In addition, salt residues were also found at interfaces between the tulip head and shaft (Table 2). The corrosion stains were present on the outer surfaces of the implants, whereas an active corrosion with material erosion was seen at the inner rim of the pedicle screw head (tulip) and some parts of the washer. The saccharides and soap were present in the interfaces with low permeability (interior region of the multipiece assembled device).
Figure 1.
Corrosion on the washer.
Figure 2.
Corrosion on the tulip interface.
Figure 3.
Saccharide of unknown origin.
Figure 4.
Soap residue mixed with fat.
Figure 5.
FTR conforming the results.
Table 1.
Summary of the Results in a Tabulated Form.
| Sample | Company | Size | Results | Equipment Used for Analysis |
|---|---|---|---|---|
| 1 | Company A | 7.5 × 55 mm |
|
|
| 2 | Company A | 7.5 × 45 mm |
|
|
| 3 | Company B | 6.5 × 45 mm |
|
|
| 4 | Company C | 7.5 × 45 mm |
|
|
| 5 | Company D | 5.5 × 45 mm |
|
|
| 6 | Company D | 7.5 × 55 mm |
|
Table 2.
Energy Dispersive X-Ray Spectroscopy Showing Presence of Salt Elements.
| Element | Weight (%) | Atomic (%) | |
|---|---|---|---|
| C K | 14.08 | 32.64 | |
| O K | 13.29 | 23.13 | |
| Al K | 6.06 | 6.25 | |
| Si K | 0.14 | 0.14 | |
| Cl K | 3.92 | 3.08 | Salt element |
| K K | 8.58 | 6.11 | Salt element |
| Ti K | 41.15 | 23.92 | |
| V K | 1.24 | 0.68 | |
| Fe K | 0.14 | 0.07 | |
| Br K | 11.38 | 3.97 | Salt element |
| Total | 100.00 |
The tabulated form of manufacturer’s guideline consisted of at least 19 disjoint steps and a minimum of 19 man-hours required for reprocessing an implant tray with 164 pedicle screws (Table 3). In comparison, the real-time observation revealed a substantial lower turnaround time for each set, as it only included the processes of mechanical washing (in the same chamber and along with all the dirty instruments), thermal disinfection, packaging for sterilization, steam sterilization, and drying (Table 3).
Table 3.
Quantification of a Typical Manufacturer’s Reprocessing Guideline.
| Process | Step | Minimum Time (Minutes) | Type | Allocation | For 164 Devices and 1 Person (Guideline) | Real-Time Observation |
|---|---|---|---|---|---|---|
| Inspection | 1 | 0.15 | Visual | Per device | 24.6 | N/A |
| Precleaning | 2 | 1 | Running water | Per device | 164 | N/A |
| 3 | 2 | Cleaner and brush | Per device | 328 | N/A | |
| 4 | 0.1 | Water | All | 0.1 | N/A | |
| 5 | 1 | Water jet | Per device | 164 | N/A | |
| 6 | 15 | Ultrasonic cleaner | All | 15 | N/A | |
| 7 | 2 | DI water jet | Per device | 328 | N/A | |
| Inspection | 8 | 0.15 | Visual | Per device | 24.6 | N/A |
| Mechanical washer | 9 | 2 | Prewash | All | 2 | 4 |
| 10 | 2 | Wash I | All | 2 | 12 | |
| 11 | 5 | Wash II | All | 5 | ||
| 12 | 2 | Rinse | All | 2 | 5 | |
| 13 | 40 | Dry | All | 40 | 20 | |
| Thermal disinfection | 14 | 2.5 | At 93°C | All | 2.5 | 8 |
| Inspection | 15 | 0.15 | Visual | Per device | 24.6 | N/A |
| Packaging for sterilization | 16 | N/A | Wraps | All | 3 | 3 |
| Sterilization | 17 | 4 | Steam | All | 4 | 4 |
| Drying | 18 | 20 | Up to 60 minutes | All | 20 | 20 |
| Total steps | 18 | Total time | 19 hours | 1 hour 16 minutes | ||
Discussion
Reprocessing and sterilization of orthopedic implants is a labor-intensive process and requires great precision and technical know-how. Ineffective execution can compromise patient’s health along with wasting hundreds of thousands of dollars. Furthermore, the medical device turnaround time has drastically reduced, along with a streak of sophisticated devices being released every year.4 Alfa et al showed through their study that the screws in the sterilization racks have limited access to the cleaning fluids resulting in insufficient cleaning and rinsing in an automated washer.5,6 Additionally, their study demonstrated an increase in endotoxin levels post reprocessing. Complementing it was the study from Litrico et al, who reported results on presterile single packed screws and compared it with an older series, which used reprocessed implants, and was performed by the same team.7 They found that the infection rate was lower with presterile single packed screws compared with the reprocessed implants (2% vs 6%).
SSI adds an enormous burden to individuals and society in terms of medications, reoperations, extended stays at the hospital, lost productivity and wages, and emotional and physical trauma afflicted on patients and their families.8 Our results indicate that implantable pedicle screws, which are reprocessed, harbor contaminants. This could be one of the direct cause of SSI, resulting in additional burden and morbidity to patients involved, which could have been avoided. While the exact source of each contaminant is unknown, presence of foreign material residues on the inner surfaces and at the interfaces of a pedicle screw is unacceptable for an immune-compromised elderly patient. The sources of these contaminants could range from mucous-like deposits from bacteria, biofilms, fatty tissue residues left over from reprocessing the implants with other contaminated instruments, and insufficient rinsing after cleansing with detergent. Previous studies on endoscopes and general orthopedic also indicate that the execution of reprocessing in the health facilities are impractical considering the workload and the intricacies of such devices. Some countries (eg, Japan and Scotland) have banned reprocessing of implants used for spine surgery. In Scotland, for example, the deadline for conversion of all orthopedic units to prepackaged, sterile, single-use implants was December 31, 2007.9 It was pointed out by the Scottish Health Department that repeatedly reprocessing implants in the hospital is a suboptimal clinical practice.
Previous studies have also observed that during reprocessing (mostly instruments) 79% of visual inspections are not performed correctly, 57% of the washer-disinfectors are obsolete or not suitable for performing a validated process, 64% of the reprocessing facility are in need of renovation, and 100% demonstrates a lack of a validated reprocessing method.10 When categorized by the date of facility establishment, an older facility had a higher number of deficiencies over a newer one. This indicates an existence of resistance in change of standard operation, and hence quality, with regard to changes in technology and accessibility. Nevertheless, the current study observed and collected implant samples from state-of-the art facilities following a strict standard of operation as directed by the hospital administration. In addition, our study did not record or access instrument reprocessing. Therefore, the failure mode here is not the lack of compliance by SPD but the underlying impracticality of repeated cleaning and sterilization of hundreds of small implants with multiple components, each with interface clearances of less than a fraction of millimeter.
The medical device turnaround time has drastically reduced, with streak of sophisticated devices being released every year.4 There already exists evidence that the amount of microscopic carbohydrate residue and endotoxins on any device increases after reprocessing.5 The increase in more complex medical devices being released to the market undoubtedly necessitates new requirements for handling such devices, and therefore instead of prescribing impractical reprocessing guidelines for SPD, focus should be on providing in a presterile package. The current study demonstrates the risks associated with repeated reprocessing of pedicle screws and the overall inapplicability of manufacturer’s guidelines in a clinical scenario. This information would be crucial for hospitals to reduce liability toward the patients, surgeons, and the administration, by taking appropriate steps to mitigate repeated reprocessing of pedicle screws.
Conclusion
Despite improvements in health care, the practice of reprocessing implants has stayed unaltered. In our evaluation, we discovered corrosion, carbohydrate, fat, and soap on reprocessed pedicle screw implants obtained from reprocessed implant sets in clinical circulation. These results indicate that the reprocessed devices have the potential to not be thoroughly cleaned during reprocessing and prior to sterilization. In par with this is the impracticality of cleaning and inspection methodology prescribed by the manufacturers for cleaning these single-use devices.
Footnotes
Authors’ Note: The device(s)/drug(s) is/are Food and Drug Administration–approved or approved by corresponding national agency for this indication.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Coauthors are employed or have stock options in Spinal Balance Inc, a company providing sterile orthopedic implants, in addition to their respective academic affiliations.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. McClelland S, 3rd, Takemoto RC, Lonner BS, et al. Analysis of postoperative thoracolumbar spine infections in a prospective randomized controlled trial using the Centers for Disease Control surgical site infection criteria. Int J Spine Surg. 2016;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell MA. Hospital uses team approach to improve processes, reduce costs. AORN J. 1998;68:68–72. [DOI] [PubMed] [Google Scholar]
- 3. Chobin N, Trattler B. Perspectives from sterile processing and perioperative services. Mater Manag Health Care. 2003;12:22–25. [PubMed] [Google Scholar]
- 4. Swanson SC. Shifting the sterile processing department paradigm: a mandate for change. AORN J. 2008;88:241–247. [DOI] [PubMed] [Google Scholar]
- 5. Alfa MJ. The “Pandora’s box” dilemma: reprocessing of implantable screws and plates in orthopedic tray sets. Biomed Instrum Technol. 2012;(suppl):55–59. [DOI] [PubMed] [Google Scholar]
- 6. Alfa MJ, Olson N, Al-Fadhaly A. Cleaning efficacy of medical device washers in North American healthcare facilities. J Hosp Infect. 2010;74:168–177. [DOI] [PubMed] [Google Scholar]
- 7. Litrico S, Recanati G, Gennari A, Maillot C, Saffarini M, Le Huec JC. Single-use instrumentation in posterior lumbar fusion could decrease incidence of surgical site infection: a prospective bi-centric study. Eur J Orthop Surg Traumatol. 2016;26:21–26. [DOI] [PubMed] [Google Scholar]
- 8. Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183–189. [DOI] [PubMed] [Google Scholar]
- 9. Burns H. Migration to single-use pre-sterilised individually wrapped small orthopaedic implants in NHS Scotland (Letter). Edinburgh, Scotland: Scottish Executive Heath Department; 2006. [Google Scholar]
- 10. Thiede B, Kramer A. Evaluation of reprocessing medical devices in 14 German regional hospitals and at 27 medical practitioners’ offices within the European context—consequences for European harmonization. GMS Hyg Infect Control. 2013;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]