Abstract
Study Objectives
To assess potential effects of lemborexant on next-morning driving performance in adult and elderly healthy volunteers.
Methods
Randomized, double-blind, double-dummy, placebo and active-controlled, four period incomplete crossover study in 48 healthy volunteers (22 females), 23–78 years old. Participants were treated at bedtime for eight consecutive nights with two of three dose levels of lemborexant (2.5, 5, or 10 mg), zopiclone 7.5 mg (on the first and last night with placebo on intervening nights), or placebo. Driving performance was assessed in the morning on days 2 and 9 using a standardized highway driving test in normal traffic, measuring standard deviation of lateral position (SDLP). Drug–placebo differences in SDLP >2.4 cm were considered to reflect clinically meaningful driving impairment.
Results
Mean drug–placebo differences in SDLP following lemborexant 2.5, 5, and 10 mg on days 2 and 9 were 0.74 cm or less. The upper bound of the 95% confidence intervals (CIs) for lemborexant treatment groups were all below 2.4 cm and the 95% CIs included zero, indicating that the effects were neither clinically meaningful nor statistically significant. Symmetry analysis further supported the lack of clinically meaningful impairment with lemborexant.
Conclusions
When assessed starting ~9 h after lemborexant administration at bedtime the previous night, there was no statistically significant or clinically meaningful effect on driving performance in healthy adults and elderly, as assessed by either mean differences in SDLP relative to placebo or symmetry analysis. In this study, lemborexant at doses up to 10 mg was well-tolerated.
Clinical Trial Registration
clinicaltrials.gov, NCT02583451. https://clinicaltrials.gov/ct2/show/NCT02583451.
Keywords: hypnotics, lemborexant, zopiclone, orexin antagonist, driving, adults, elderly, plasma concentrations
Statement of Significance.
Lemborexant is an investigational compound for the treatment of insomnia and other sleep disorders. Lemborexant is thought to promote sleep by blocking receptors for the wake-promoting orexins in the brain. Results of the present study are the first to indicate that lemborexant in doses up to 10 mg taken at bedtime has no measurable effects on actual automobile driving performance the next morning in healthy adults and elderly.
Introduction
Lemborexant (E2006) is a novel competitive antagonist at orexin-1 and orexin-2 receptors, that is, a dual orexin receptor antagonist (DORA) that is being developed for the treatment of insomnia disorder and irregular sleep wake rhythm disorder [1–3]. The hypothalamic neuropeptides orexin-A and orexin-B have been implicated in the regulation of sleep/wake behavior, feeding, energy homeostasis, and reward seeking [4]. In sleep/wake control, orexin stabilizes wakefulness via direct projections to most wake-promoting centers, affecting neurotransmitters, such as acetylcholine, histamine, norepinephrine, and serotonin, and indirect inhibition of the sleep-promoting system in the ventrolateral preoptic nucleus [5, 6]. Phasic activity of orexin neurons during sleep increases the probability of awakening. Animal and clinical studies have shown that blockade of orexin receptors promotes sleep [7, 8]. Orexin levels have been found to peak at the end of the active phase, and fall to about half their maximum levels during sleep. Recent evidence has found that plasma orexin-A levels are significantly higher in patients with insomnia disorder compared with good sleepers [9]. Data such as these support the hypothesis that insomnia can be due to an inability of the brain to switch off wake-promoting systems such as the orexin system, as well as an inability to switch on sleep promoting circuits [10].
In clinical trials, lemborexant has been studied at doses of between 1 and 25 mg for insomnia disorder and irregular sleep wake rhythm disorder. Following oral administration, it is well absorbed, with an average tmax occurring between 1 and 2 h after evening dosing, an effective half-life of 17–24 h [11]. Plasma concentrations reach steady state after approximately eight nights of dosing.
A concern associated with use of sleep-promoting drugs is their potential to impair driving ability the morning after bedtime use, due to residual sedative effects [12–14]. Results from initial screening of lemborexant’s potential to impair next-day performance using subjective assessments of sleepiness and neurocognitive tests (e.g. simple and choice reaction time tests, digit symbol substitution tests) suggested that residual effects of lemborexant are minimal after bedtime doses up to 25 mg [15]. However, to support safety for driving of the lemborexant doses studied in phase 3 clinical trials, a dedicated study was needed; hence the present study was conducted. The protocol followed the Food and Drug Administration (FDA) guidance for industry [12] to include a positive control and placebo groups, elderly subjects, initial and steady state exposures for drugs with long half-lives, and a within-subjects crossover design.
The aim of the present study was to evaluate potential next-morning residual effects of lemborexant 2.5, 5, and 10 mg on driving, after single and repeated bedtime use in healthy adults (21–64 years) and elderly (≥65 years). The doses of lemborexant were selected to include the doses (5 and 10 mg) that are being studied in the clinical development program. Driving performance was assessed by the standard deviation of lateral position (SDLP in cm) in a standardized on-the-road driving test [16–18]. The effects of lemborexant were to be compared with those of placebo by analysis of mean drug–placebo changes, upper bounds of the 95% confidence intervals (CIs) around the mean drug–placebo changes, and by symmetry analysis of individual drug–placebo changes in SDLP. Based on results from previous studies with alcohol [19, 20], a mean drug–placebo difference in SDLP with an upper bound of the 95% CI <2.4 cm would not be considered clinically meaningful.
Symmetry analysis was used to evaluate driving performance at the individual subject level to gauge whether there was a statistically significant imbalance between the number of subjects with drug–placebo differences larger than the criterion of 2.4 cm (driving relatively impaired vs. after receiving placebo), compared with the number of subjects with drug–placebo differences less than −2.4 cm (driving relatively better vs. after receiving placebo). Although a cutoff point for individual performance changes in SDLP has not formally been validated, it is reasonable to set a threshold at the same criterion used for the mean difference in SDLP, that is, increases versus placebo exceeding 2.4 cm. It may be concluded that the drug has no clinically meaningful effect on driving if large changes in SDLP following drug treatment are balanced, in that the number of participants showing an increase in SDLP above 2.4 cm and below −2.4 cm are not different. If significantly more participants show an increase in SDLP larger than 2.4 cm than a decrease of the same size, the assertion may be made that the drug increases the risk of impairment [21–24].
In addition, the associations between morning plasma concentrations of lemborexant and SDLP were evaluated. Finally, “lapses” in driving performance [25] were analyzed as a secondary measure of driving impairment. Zopiclone 7.5 mg was selected as an active control, to demonstrate assay sensitivity versus placebo, as has been the case in other studies [22–24, 26].
Methods
Participants
Participants were recruited via advertisements placed in local newspapers. Adult (21 to <65 years old) and elderly (≥65 years old) healthy volunteers were eligible to enroll if they possessed a valid driver’s license, had driving experience of ≥3,000 km/year on average within the last 3 years, body mass index (BMI) between 18 and 30 kg/m2 (inclusive), and normal vision (corrected or uncorrected). Participants were required to be in good health, as confirmed by their medical history, physical examination, vital sign measurement, electrocardiogram, and laboratory safety tests (blood chemistry and hematology).
Participants who met any of the following criteria were excluded from the study: history or present evidence of any clinically significant physical, neurological, psychiatric, or sleep disorders, alcoholism or drug abuse; use of medication known to affect driving performance or hepatic drug metabolism; systolic blood pressure (BP) >140 mmHg (adults) or >150 mmHg (elderly) or diastolic BP >90 mmHg (all ages); resting heart rate <50 or ≥100 beats per minute; major surgery, blood donation or participation in any other clinical trial within 4 weeks before screening; smoking >6 cigarettes per week; alcohol consumption >14 (females) or >21 (males) drinks per week; caffeine consumption >3 cups per day. All participants were tested for drug use (amphetamines, benzodiazepines, barbiturates, cannabis, cocaine, methadone, tricyclic antidepressant, and opiates), at prestudy screening and at the start of each test session. Eligible volunteers had to have a regular sleep pattern, defined as time spent in bed between 7.0 and 8.5 h, with bedtime between 22:00 and 1:00 h, and wake time between 5:00 and 9:00 h, as confirmed by a 1-week sleep diary [27] before randomization. A total of 85 volunteers were screened for this study, of which 37 were excluded as screen failures, most (n = 33) because their blood pressure exceeded the criteria.
During the study, participants were required to abstain from prescription and over-the-counter medication. They also had to refrain from smoking and/or consuming caffeine and alcohol from the time of arrival at the site on treatment days until the completion of all tests the next day. In addition, alcoholic drinks and caffeine were not permitted from 12 and 5 h before arrival, respectively. Furthermore, participants were required not to drive their own vehicles from intake of the first dose until 24 h after the last dose of each treatment period.
Ethical approval was obtained from the Medical Ethics Committee of Maastricht University, and all volunteers provided written informed consent prior to enrollment. The study was carried out in compliance with the current revision of the Declaration of Helsinki, and the International Conference on Harmonization guidelines for Good Clinical Practice.
Design
The study was conducted from October 2015 to January 2017, according to a single center, randomized, double-blind, double-dummy, placebo- and active drug-controlled, four-period, incomplete crossover design. Each treatment period lasted for 8 days, and residual effects were assessed in the mornings of days 2 and 9. Treatments were bedtime doses of lemborexant (2.5, 5, or 10 mg), and placebo for eight consecutive days, and zopiclone 7.5 mg as an active control on days 1 and 8 only, with placebo given for the 6 days in between (days 2–7). While all participants were to receive zopiclone 7.5 mg and placebo, each participate was assigned to receive only two of the three dose levels of lemborexant. Randomization to 1 of 12 treatment sequences was stratified by age (adult vs. elderly) in a 1:1 ratio, and was balanced for sex such that there were no fewer than 10 males or 10 females per age group. Washout intervals between treatment periods were at least 14 days. The study was registered at clinicaltrials.gov (NCT02583451).
Assessments
Highway driving test
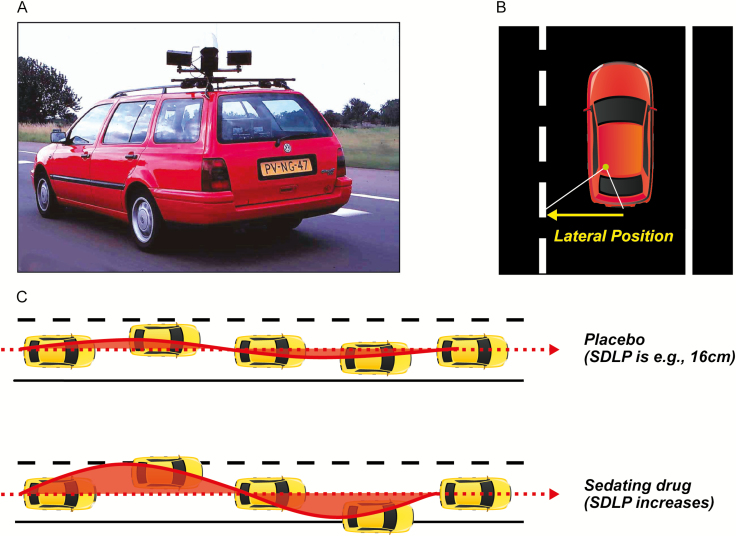
Residual effects were assessed using a standardized on-the-road driving test, which assesses SDLP as a measure of driver vehicle control (Figure 1) [16–18].
Figure 1.
Highway driving test. (A) Subjects drive a specially instrumented vehicle for about 1 h over a 100 km primary highway circuit, accompanied by a licensed driving instructor having access to dual controls. The subjects’ task is to drive with a steady lateral position between the delineated boundaries of the slower (right) traffic lane, while maintaining a constant speed of 95 km/h. (B) A camera on top of the car continuously registers the lateral position of the car on the road with respect to the left lane delineation. (C) The standard deviation of lateral position (SDLP in cm) is an index of road tracking error or “weaving.” SDLP scores increase compared with placebo after the use of many sedating drugs including low doses of alcohol. SDLP, standard deviation of lateral position.
In this test, participants operate a specially instrumented vehicle for about 1 h over a 100-km (61-mile) primary highway circuit (road E25) between the Dutch cities of Maastricht and Kelpen-Oler, accompanied by a licensed driving instructor having access to dual controls (brakes and accelerator). The participants’ task is to drive with a steady lateral position between the delineated boundaries of the slower (right) traffic lane, while maintaining a constant speed of 95 km/h (58 mph). Participants may deviate from those instructions only to pass a slower vehicle, and to leave and re-enter the highway at the turnaround point. During the drive, the vehicle’s speed and lateral distance to the left lane-line are continuously recorded via a camera mounted on the top of the vehicle. These signals are captured at a rate of 4 Hz and stored on an onboard computer disk file for later preprocessing and analysis. Preprocessing consists of off-line visual inspection of all data by trained processors to mark data segments that reveal signal loss or disturbances, such as passing maneuvers and the turn-around point. The preprocessed dataset is then used to calculate means and variances of lateral position and speed of clean (unmarked) data. The primary outcome variable is standard deviation of lateral position (SDLP in cm), which is a measure of “weaving” or road tracking error. In addition, the number of lapses of attention were analyzed, as defined [25], that is, moving laterally from the chosen position in the lane by at least 100 cm for a minimum of 8 s. A computer algorithm was developed for automated detection and scoring of lapses of attention according to the criterion given above. Performance as measured by mean SDLP in this test has repeatedly been found sensitive to residual effects of zopiclone 7.5 mg [22–24, 26, 28].
Pharmacokinetics
Blood samples (4 mL) for lemborexant and zopiclone determinations were obtained predose on the first day of each treatment period, starting with the second treatment period, to measure any residual concentrations of previous treatment, and following each driving test, at approximately 10.5 h post bedtime dosing. Plasma samples were stored frozen at −20°C and later analyzed. The determination of lemborexant and S-zopiclone were based on a validated liquid chromatography with tandem mass spectrometry (LC-MS/MS) assay methods. Lower limit of quantification (LLOQ) for lemborexant and metabolites was 0.0500 ng/mL, and for S-zopiclone (the active isomer of zopiclone) was 0.500 ng/mL. Samples were assayed by Medpace Bioanalytical Laboratories (Cincinnati, OH).
Procedures
Within 2 weeks before the first treatment period, participants slept one night in the same facilities as during treatment conditions, to overcome possible sleep disturbances associated with sleeping in an unfamiliar environment. In the morning following their habituation night, participants were individually trained to perform the driving test.
On days 1 and 8 of each treatment period participants arrived at the test facility at approximately 21:00, upon which their eligibility and compliance with study restrictions was verified by questioning, urine screens for drugs of abuse and pregnancy, and breath testing for alcohol. A maximum of four participants were treated on the same night and tested the following day, with 3-min difference between their activities. At 23:30, the first subject was administered drug or placebo with 240 mL water in the presence of an investigator, and retired to bed. At 07:30 the first participant was awakened. Following toilet and dress, participants were provided a standardized light breakfast without caffeine and transported to the highway. The driving test of the first participant started at approximately 08:30, that is, 9 h after bedtime dosing. After completion of the driving test, participants were transported to the test facility. Upon arrival, a blood sample was taken at approximately 10.5 h after dosing. Hereafter, participants were transported home by study personnel.
During days 2 to 7 of all treatment periods, trial medication was taken by the participants at their homes. On day 5 of each treatment period, participants were contacted by telephone to check treatment compliance and possible adverse events.
Approximately 14 days after the last treatment period, participants’ health and well-being were confirmed by questioning them about adverse events, and by physical examination, including electrocardiograms and laboratory tests (blood chemistry and hematology).
Statistical analyses
The primary endpoint was mean SDLP. Secondary endpoints were symmetry analysis of individual changes from placebo in SDLP (see below) and mean number of lapses. Sample size was determined based on power calculations to rule out a clinically relevant mean difference in SDLP between lemborexant and placebo. In this study, the criteria for a clinically relevant mean difference in SDLP was met when the upper bound of a two-sided 95% CI for the mean difference fell above the threshold for impairment, that is, 2.4 cm, and the lower bound of the 95% CI fell above zero. A mean increase in SDLP of 2.4 cm as compared with placebo, corresponds to the effects previously found for alcohol while participants drove with average blood alcohol concentrations of 0.5 g/L [19, 20]. A sample size of 48 participants provided a power of at least 80% to detect a >1.0 cm difference in mean SDLP between lemborexant and placebo, assuming a within-subject variance in SDLP of 3.55 cm [2, 29] and each treatment comparison of lemborexant versus placebo occurring in 32 of 48 participants [30].
SDLP was analyzed using repeated-measures analyses of variance, for the second and ninth day of each treatment period. The model included fixed effects for age group, sequence, period, time, treatment and the interaction of treatment and time, and a repeated effect for time, with subject within period. In addition, age by treatment and sex by treatment interactions terms were included in the model post hoc. As a secondary analysis, SDLP was also analyzed using symmetry analysis of individual changes from placebo in SDLP. To perform this symmetry analysis, the McNemar test used for each treatment condition and treatment day separately to test whether the number of participants with an increase in SDLP >2.4 cm (reflecting impairment) differed significantly (p < 0.05) from the number of participants with a decrease in SDLP < −2.4 cm (reflecting improvement).
All statistical analyses were done by using the SAS statistical program version 9.3 (SAS Institute, Inc., Cary, NC). No adjustments were made for multiple comparisons.
The relationship between observed plasma concentrations of lemborexant following the driving tests and SDLP were initially analyzed graphically, and emergent relationships were followed by PK/PD modelling using NONMEM 7.3. Exposure–response relationships were described using direct linear Emax models. The exposure parameter was the observed postdriving lemborexant concentration after each driving test.
Results
Demographic data
Forty-eight volunteers (26 male, 22 females) were randomized; all completed the study. Their mean (±SD) age was 58.5 (±13.3) years, and their mean height, weight, and BMI were 174 (±10.7) cm, 76.7 (±13.4) kg, and 25.2 (±2.6) kg/m2, respectively. Median age of the adults (12 males, 12 females) was 49 years (range 23–64 years), and median age of the elderly (14 males, 10 females) was 67 years (range 65–78 years). Twenty-one of the adult participants were Caucasian, one was African American; one was Japanese, and one was Asian. All elderly participants were Caucasian. Scores from the 1-week sleep diaries before randomization confirmed that all volunteers were good sleepers, with participants self-reporting an estimated mean (SD) sleep onset latency of 10.0 (±5.5) min, mean total sleep time of 432 (±49) min, and a mean sleep efficiency score of 90.6% (±7.9%).
Prematurely terminated tests
Three driving tests were terminated before scheduled completion, all after use of zopiclone (3 of total 96 post-zopiclone drives [3.125%]). No tests were stopped after use of lemborexant or placebo. One adult female reported sleepiness and requested to stop both driving tests in the zopiclone condition (after 70% and 87% completion, respectively). One test of an elderly male was terminated by the driving instructor on day 9 in the zopiclone condition (after 62% completion), because the instructor judged the participant too drowsy to continue safely. The SDLP scores for these tests were calculated from the data collected until termination of driving.
SDLP
Table 1 shows least squares (LS) mean estimates of SDLP and treatment effects versus placebo with 95% CI, at both test days in each treatment condition, overall and split by subgroups for age and sex.
Table 1.
LS-mean (SE) of standard deviation of lateral position (SDLP in cm), and mean drug–placebo changes ΔSDLP [95% CI] at both test days in each treatment condition, for all participants, and for each age group and sex separately
| Group and treatment condition | N | LS mean (SE)SDLPDay 2 | LS mean (SE)SDLPDay 9 | ΔSDLPDay 2 | 95% CI | P for maineffect oftreatment | ΔSDLPDay 9 | 95% CI | P for maineffect oftreatment |
|---|---|---|---|---|---|---|---|---|---|
| All | |||||||||
| PBO | 48 | 17.84 (0.46) | 17.82 (0.44) | — | — | — | — | — | — |
| LEM2.5 | 32 | 17.85 (0.58) | 18.30 (0.57) | 0.02 | [−1.44 to 1.48] | 0.983 | 0.48 | [−0.94 to 1.90] | 0.506 |
| LEM5 | 32 | 18.06 (0.58) | 18.18 (0.57) | 0.23 | [−1.23 to 1.69] | 0.760 | 0.36 | [−1.06 to 1.78] | 0.652 |
| LEM10 | 32 | 18.57 (0.58) | 18.56 (0.57) | 0.73 | [−0.73 to 2.19] | 0.324 | 0.74 | [−0.68 to 2.16] | 0.306 |
| ZOP | 48 | 19.88 (0.46) | 19.70 (0.44) | 2.04 | [0.77 to 3.32]† | 0.002* | 1.88 | [0.64 to 3.12]† | 0.003* |
| Adults | |||||||||
| PBO | 24 | 17.20 (0.53) | 17.27 (0.51) | — | — | — | — | — | — |
| LEM2.5 | 16 | 17.04 (0.68) | 17.35 (0.66) | −0.16 | [−1.88 to 1.55] | 0.849 | 0.08 | [−1.58 to 1.75] | 0.921 |
| LEM5 | 16 | 17.74 (0.68) | 17.35 (0.66) | 0.54 | [−1.18 to 2.26] | 0.534 | 0.08 | [−1.58 to 1.74] | 0.924 |
| LEM10 | 16 | 17.85 (0.68) | 18.10 (0.66) | 0.65 | [−1.07 to 2.36] | 0.456 | 0.83 | [−0.83 to 2.49]† | 0.321 |
| ZOP | 24 | 18.72 (0.53) | 19.05 (0.51) | 1.51 | [0.01 to 3.01]† | 0.048* | 1.79 | [0.34 to 3.24]† | 0.016* |
| Elderly | |||||||||
| PBO | 24 | 18.47 (0.60) | 18.37 (0.59) | — | — | — | — | — | — |
| LEM2.5 | 16 | 18.67 (0.76) | 19.25 (0.75) | 0.20 | [−1.73 to 2.13] | 0.837 | 0.88 | [−1.02 to 2.78]† | 0.359 |
| LEM5 | 16 | 18.38 (0.76) | 19.01 (0.75) | −0.09 | [−2.02 to 1.84] | 0.926 | 0.64 | [−1.26 to 2.54]† | 0.505 |
| LEM10 | 16 | 19.29 (0.76) | 19.02 (0.75) | 0.82 | [−1.11 to 2.75]† | 0.399 | 0.65 | [−1.25 to 2.55]† | 0.496 |
| ZOP | 24 | 21.04 (0.60) | 20.35 (0.59) | 2.57 | [0.89 to 4.25]† | 0.003* | 1.98 | [0.33 to 3.63]† | 0.020* |
| Males | |||||||||
| PBO | 26 | 17.11 (0.55) | 17.13 (0.49) | — | — | — | — | — | — |
| LEM2.5 | 18 | 17.21 (0.68) | 17.33 (0.60) | 0.09 | [−1.62 to 1.80] | 0.913 | 0.19 | [−1.32 to 1.71] | 0.801 |
| LEM5 | 17 | 17.41 (0.69) | 18.01 (0.62) | 0.30 | [−1.44 to 2.03] | 0.735 | 0.87 | [−0.66 to 2.41]† | 0.261 |
| LEM10 | 17 | 17.82 (0.70) | 17.79 (0.62) | 0.71 | [−1.04 to 2.46]† | 0.422 | 0.65 | [−0.90 to 2.20] | 0.406 |
| ZOP | 26 | 19.21 (0.55) | 19.09 (0.49) | 2.10 | [0.59 to 3.61]† | 0.007* | 1.96 | [0.62 to 3.29]† | 0.005* |
| Females | |||||||||
| PBO | 22 | 17.83 (0.58) | 17.77 (0.63) | — | — | — | — | — | — |
| LEM2.5 | 14 | 17.78 (0.74) | 18.65 (0.80) | −0.05 | [−1.91 to 1.81] | 0.958 | 0.88 | [−1.15 to 2.91]† | 0.390 |
| LEM5 | 15 | 17.84 (0.72) | 17.41 (0.78) | 0.01 | [−1.79 to 1.80] | 0.993 | −0.36 | [−2.32 to 1.61] | 0.718 |
| LEM10 | 15 | 18.45 (0.72) | 18.48 (0.78) | 0.62 | [−1.20 to 2.44]† | 0.498 | 0.71 | [−1.28 to 2.69]† | 0.481 |
| ZOP | 22 | 19.86 (0.58) | 19.63 (0.63) | 2.04 | [0.45 to 3.62]† | 0.013* | 1.86 | [0.12 to 3.60]† | 0.036* |
CI, confidence interval; LS mean, least-squares mean; PBO, placebo; LEM2.5, lemborexant 2.5 mg; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; SDLP, standard deviation of lateral position; SE, standard error; ZOP, zopiclone 7.5 mg.
*Statistically significant (p < 0.05) drug–placebo difference.
†Upper limit of the 95% CI exceeding the threshold for impairment (>2.4 cm).
Repeated measures analysis of variance showed that there was a significant main effect of treatment condition (F4,172 = 3.13, p = 0.0162). Overall, LS-mean changes from placebo in SDLP scores after lemborexant 2.5, 5, and 10 mg were very small (<0.75 cm) on both test days. The 95% CIs of these changes all included zero, and their upper limits were all below the threshold of 2.4 cm (Table 1), indicating that none of these changes was statistically significant, or clinically meaningful.
Assay sensitivity was clearly demonstrated, as shown by the significant impairing effects of zopiclone. Following zopiclone, mean SDLP was increased compared to placebo by 2.04 (95% CI: 0.77–3.32) cm on day 2, and by 1.88 (95% CI: 0.64–3.12) cm on day 9. These results show that the effects of zopiclone 7.5 mg on driving were statistically significant and clinically relevant on days 2 and 9.
There was a significant main effect of age group on SDLP (F1,172 = 10.74, p = 0.0013). As shown in Table 1, LS-mean SDLP for elderly subjects was higher than that for adult subjects, regardless of treatment. However, the interaction of age group by treatment was not significant (F4,163 = 0.36, p = 0.8377) and no significant sex by treatment interaction (F5,163 = 0.30, p = 0.9128), indicating that the SDLP treatment difference from placebo was not significantly different between age groups or between sexes. Mean SDLP, and the SDLP treatment difference from placebo, were similar in males and females (Table 1, all p-values > 0.05). There were no significant main effects of day/time (F1,187 = 0.30, p = 0.5873), period (F3,172 = 0.16. p = 0.9237) and sequence (F11,172 = 1.25, p = 0.2605) based on the primary model.
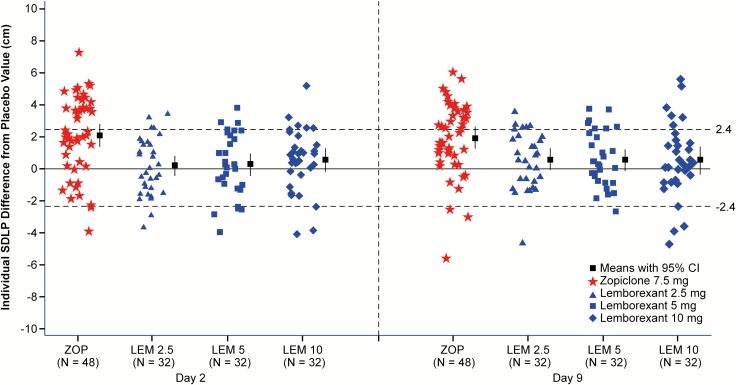
Individual subject differences from placebo in SDLP are shown in Figure 2. Symmetry analysis showed that following zopiclone, significantly more participants showed an increase in SDLP >2.4 cm than a decrease of the same magnitude on both days 2 and 9 (p-values < 0.0001). The symmetry analyses were not significant for any lemborexant dose on either days 2 or 9 (Table 2).
Figure 2.
Individual standard deviation of lateral position (SDLP, in cm) differences from placebo, mean and 95% confidence interval by treatment and day, following bedtime administration of lemborexant 2.5, 5, and 10 mg single dose (day 2, n = 32) and repeated doses (day 9, n = 32), and single doses of zopiclone 7.5 mg (days 2 and 9, n = 48). N = 28 on day 2, N = 27 on day 9. Horizontal dashed lines indicate thresholds for impairment (>2.4 cm) and improvement (<−2.4 cm). LEM2.5, lemborexant 2.5 mg; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; SDLP, standard deviation of lateral position; ZOP, zopiclone 7.5 mg.
Table 2.
Symmetry analysis of numbers of participants whose SDLP increased more than 2.4 cm (indicating impairment) and numbers of participants whose SDLP decreased more than −2.4 cm (indicating improvement), n = 48
| Treatment condition | Day | ∆SDLP > 2.4 n (proportion) | ∆SDLP < −2.4 n (proportion) | McNemar test P-value | Reject H0 |
|---|---|---|---|---|---|
| LEM2.5 (n = 32) | 2 | 4 (12.5) | 2 (6.3) | 0.688 | No |
| 9 | 6 (18.8) | 1 (3.1) | 0.125 | No | |
| LEM5 (n = 32) | 2 | 4 (12.5) | 5 (15.6) | 1.000 | No |
| 9 | 7 (21.9) | 1 (3.1) | 0.070 | No | |
| LEM10 (n = 32) | 2 | 6 (18.8) | 3 (9.4) | 0.508 | No |
| 9 | 6 (18.8) | 3 (9.4) | 0.508 | No | |
| ZOP (n = 48) | 2 | 20 (41.7) | 1 (2.1) | <0.0001 | Yes |
| 9 | 24 (50.0) | 3 (6.3) | <0.0001 | Yes |
LEM2.5, lemborexant 2.5 mg; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; SDLP, standard deviation of lateral position; ZOP, zopiclone 7.5 mg.
Lapses of attention
Lapses of attention, defined as a deviation of at least 100 cm for at least 8 s [25], were not detected during any of the on-the-road tests.
Pharmacokinetic analysis
A total of 180 lemborexant plasma concentrations collected after driving (median 10.4 h) postdose were available (n = 91 on day 2, and n = 89 on day 9). Lemborexant concentrations measured after driving were similar to those observed at the corresponding time postdose in a previous study [15]. On day 2, mean (range) plasma concentrations of lemborexant were 1.66 (0.8–2.9), 2.94 (1.4–5.2), and 5.88 (03.22–30) ng/mL for 2.5, 5, and 10 mg doses, respectively. On day 9, mean (range) concentrations of lemborexant were 4.40 (2.0–9.1), 7.63 (3.81–12.3), and 15.28 (6.7–37.4) ng/mL, for 2.5, 5, and 10 mg doses, respectively. Mean concentrations of lemborexant were approximately 40% higher in elderly, as compared with adults. No differences between males and females were observed. There were no residual concentrations of lemborexant that were greater than 5% of Cmax predose on day 1 of periods 2, 3, and 4. Mean (range) plasma concentrations of S-zopiclone (the active isomer of zopiclone) were 12.97 (0.0–27.6) ng/mL on day 2, and 12.79 (7.2–22.2) ng/mL on day 9.
The relationship between placebo-corrected SDLP on days 2 and 9 and lemborexant concentration measured after the completion of the driving assessments (median 10.4 h postdose) is best described by a linear model without intercept.
PK/PD analyses showed no relationship between lemborexant concentrations and placebo-corrected SDLP following single dosing on day 2. Following multiple dosing of lemborexant 2.5, 5, and 10 mg for 8 days, a shallow, statistically significant linear relationship was detected. This relationship appeared to be similar in adult and elderly subjects, and in males and females, based on graphical evaluation of the large overlap of responses when split by age group and by sex. At median levels of exposure at approximately 10.4 h postdose, the PK/PD model predicted small increases in placebo-corrected SDLP of 0.19, 0.32, and 0.64 cm following lemborexant 2.5, 5, and 10 mg, respectively. Based on the large interindividual variability (60%–70%) in response (SDLP), and noting that the predicted increases in SDLP at the highest lemborexant concentrations are below the clinically meaningful threshold of 2.4 cm, the effect of observed lemborexant concentrations on SDLP is considered not clinically relevant.
Safety
Table 3 presents a summary of treatment-emergent adverse events (AEs) reported by ≥5% of the participants after any treatment.
Table 3.
Summary of adverse events reported by at least 5% of the participants after any treatment, n = 48
| Adverse event | PBO(n = 48)n (%) | LEM2.5(n = 32)n (%) | LEM5(n = 32)n (%) | LEM10(n = 32)n (%) | ZOP(n = 48)n (%) |
|---|---|---|---|---|---|
| Somnolence | 3 (6.3) | 3 (9.4) | 3 (9.4) | 10 (31.3) | 8 (16.7) |
| Headache | 8 (16.7) | 4 (12.5) | 3 (9.4) | 2 (6.3) | 1 (2.1) |
| Dizziness | 1 (2.1) | 2 (6.3) | 0 (0.0) | 0 | 3 (6.3) |
| Dysgeusia | 1 (2.1) | 0 (0.0) | 0 (0.0) | 0 | 7 (14.6) |
| Dry mouth | 1 (2.1) | 3 (9.4) | 1 (3.1) | 3 (9.4) | 2 (4.2) |
| Nausea | 0 | 0 | 0 | 2 (6.3) | 0 |
| Fatigue | 1 (2.1) | 0 | 2 (6.3) | 2 (6.3) | 0 |
| Influenza | 0 | 2 (6.3) | 1 (3.1) | 2 (6.3) | 0 |
| Back pain | 1 (2.1) | 0 | 0 | 2 (6.3) | 0 |
PBO, placebo; LEM2.5, lemborexant 2.5 mg; LEM5, lemborexant 5 mg; LEM10, lemborexant 10 mg; ZOP, zopiclone 7.5 mg.
After lemborexant, the most common AEs were somnolence, headache, and dry mouth. After zopiclone, somnolence, dysgeusia, and dizziness were the most common AEs. Overall, females reported AEs more frequently than males (37 vs. 28), and adults reported AEs more frequently than elderly (36 vs. 29). All AEs were of mild to moderate severity. There were no events of cataplexy or cataplexy-like events. There were no relevant treatment-related changes in laboratory, vital signs, or ECG safety parameters.
Discussion
The primary objective of the study was to assess the residual effects of lemborexant on automobile driving in a group of adults and elderly, after single and repeated bedtime dosing. Results showed that when lemborexant was taken in doses of 2.5, 5, and 10 mg 9 h before driving, mean drug–placebo changes were small (<0.74 cm), and the 95% CIs were well below the 2.4 cm threshold for impairment, and included zero. This indicates that, overall, there were no statistically significant or clinically relevant effects of lemborexant on SDLP, the primary measure of driving performance. In line with this, there were no statistically significant differences in proportions of drivers showing increases or decreases in individual SDLP scores of a magnitude larger than the criterion for impairment.
Driving impairment observed after zopiclone 7.5 mg demonstrated assay sensitivity. It significantly increased mean SDLP as compared with placebo by 2.04 and 1.88 cm on days 2 and 9, respectively, and impaired driving in almost 50% of participants, as defined by a change in SDLP of more than 2.4 cm. In addition, three driving tests were terminated prematurely after zopiclone 7.5 mg (3.1% of 96 tests), compared with zero after use of lemborexant or placebo. It should be noted however, that the number of driving tests stopped before completion is a poor predictor of a drug’s effect on driving performance, as shown by a review of 50 driving studies [31]. The impairing effects of zopiclone 7.5 mg on driving performance are consistent with previous studies that assessed the next day effects of zopiclone using the same standardized highway driving test [22–24].
Plasma concentrations of lemborexant after driving (approximately 10.4 h postdose) were approximately dose-proportional across the dose range from 2.5 to 10 mg, following both single and repeated administrations. The concentrations observed were in line with those predicted for 9 h post bedtime dose based on previous studies [11]. There was no relationship between plasma concentrations and drug–placebo changes in driving performance following a single dose. After repeated dosing, a weak but statistically significant relationship was observed, such that higher concentrations of lemborexant were associated with small increases in SDLP that were not clinically relevant. These results are in line with previous studies [23, 24, 32] showing that individual differences in blood concentrations of hypnotic drugs and changes in SDLP correlate poorly.
On average, the effects of lemborexant did not differ between adults and elderly, or between males and females; estimated mean drug–placebo changes in SDLP were comparable in adults and elderly, and in males and females. Occasionally, however, the upper limit of the 95% CI of lemborexant-placebo changes within these subgroups exceeded the threshold for impairment. This can be explained by larger variability in smaller sample sizes. Moreover, none of these changes differed significantly from placebo. It could be argued that interindividual variability in responses to lemborexant indicates that residual effects on driving cannot be ruled out completely for all patients, and all doses.
Contrary to expectation, lapses of attention defined as moving laterally from the chosen position in the lane by at least 100 cm for a minimum of 8 s, were not detected during the study. The failure to find lapses is not in line with conclusions of Verster et al. [25]. These authors retrospectively examined driving data from two published studies to evaluate the utility of lapses as an alternative outcome measure of driving impairment. They report that 23 out of 60 healthy volunteers showed lapses following placebo, and that bedtime use of zopiclone 7.5 mg was associated with an average of 2.5 lapses per driving test. One explanation for why lapses were not detected in the present study could be that the driving instructors would not have permitted participants to lapse this far (100 cm) for this duration (8 s) before taking control of the car. This is not likely, however, because when the instructor has to take control of the car, it is considered a reason to terminate the test. Alternatively, the discrepancy in detection of lapses may be due to methodological issues, including differences in vehicles, and roadways used, and differences in scoring routines of lapses. In the present study, lapses were detected using an automated computer algorithm, whereas lapses were previously identified by visual inspection of the data. Mean SDLP scores after placebo and zopiclone were comparable between studies, however.
In this study, lemborexant was well-tolerated. No subject discontinued from the study; all subjects completed all treatments.
As discussed for previous studies [22–24], the inclusion of healthy volunteers instead of patients could be considered a limitation of the study. However, healthy volunteers have been found to be more sensitive to residual effects of hypnotics than patients with insomnia [33]. Thus, studying drug effects on driving performance in healthy volunteers minimizes the risk of failing to detect clinically relevant impairment associated with the use of a drug. Future studies in patients may help to determine the interaction of these effects with the diagnosis of insomnia and other comorbid disorders, or concomitant medication. The present study aimed to determine the impairing potential of lemborexant per se, as compared with that of a drug with well-defined impairing properties, that is, zopiclone 7.5 mg.
In conclusion, results of the present study show no clinically meaningful residual effects of single and repeated doses of lemborexant 2.5, 5, and 10 mg on next-morning driving performance (9 h after bedtime dosing) in healthy adults and elderly.
Funding
This study was financially supported by Eisai, Inc., Woodcliff Lake, NJ. Eisai, Inc. is the owner and manufacturer of lemborexant. Maastricht University received financial support from Eisai to conduct this study.
Conflict of interest statement: Drs Vermeeren, Vuurman, Jongen, Van Leeuwen, Ramaekers, and Ms. Van Oers are employees of Maastricht University. The remaining authors are employees or former employee of Eisai. Editorial support was provided by John Bilbruck, PhD, CMPP of ProScribe—Envision Pharma Group, and was funded by Eisai, Inc. and Purdue Pharma LP.
Acknowledgments
The authors thank Lizzy Vuurman, MD, for assistance during medical screening of the volunteers; Henk Brauers, Hans Sleebe, Reggy Augustin, Roger Widdershoven, and John Vissers for ensuring safety of the participants during driving; Irma Brauers, Lizzy Vuurman, Lianne van Laarschot, Tim Calon, Eric Fonseca for assistance during data collection, Jagadeesh Aluri for support with pharmacokinetic analysis, and Ziad Hussein for the pharmacokinetic/pharmacodynamics modeling.
References
- 1. Murphy PJ, et al. Concentration-response modeling of ECG data from early-phase clinical studies as an alternative clinical and regulatory approach to assessing qt risk—experience from the development program of lemborexant. J Clin Pharmacol. 2017;57(1):96–104. [DOI] [PubMed] [Google Scholar]
- 2. Beuckmann CT, et al. In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J Pharmacol Exp Ther. 2017;362(2):287–295. [DOI] [PubMed] [Google Scholar]
- 3. Yoshida Y, et al. Discovery of (1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (E2006): a potent and efficacious oral orexin receptor antagonist. J Med Chem. 2015;58(11):4648–4664. [DOI] [PubMed] [Google Scholar]
- 4. Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15(11):719–731. [DOI] [PubMed] [Google Scholar]
- 5. de Lecea L, et al. Hypocretin (orexin) regulation of sleep-to-wake transitions. Front Pharmacol. 2014;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fulcher BD, et al. A physiologically based model of orexinergic stabilization of sleep and wake. PLoS One. 2014;9(3):e91982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scammell TE, et al. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michelson D, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13(5):461–471. [DOI] [PubMed] [Google Scholar]
- 9. Tang S, et al. Increased plasma orexin-A levels in patients with insomnia disorder are not associated with prepro-orexin or orexin receptor gene polymorphisms. Peptides. 2017;88:55–61. [DOI] [PubMed] [Google Scholar]
- 10. Nofzinger EA, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11): 2126–2128. [DOI] [PubMed] [Google Scholar]
- 11. Pastino G, et al. Pharmacokintetics of lemborexant: relationships to efficacy and safety. In: Poster presented at: SLEEP 2015, the 29th Annual Meeting of the Associated Professional Sleep Societies, June 5–10, 2015; Seattle. [Google Scholar]
- 12. Food and Drug Administration. Evaluating Drug Effects on the Ability to Operate a Motor Vehicle, Guidance for Industry 2017. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM430374.pdfAccessed October 2, 2017.
- 13. Dassanayake T, et al. Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf. 2011;34(2):125–156. [DOI] [PubMed] [Google Scholar]
- 14. Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18(5):297–328. [DOI] [PubMed] [Google Scholar]
- 15. Murphy P, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br J Clin Pharmacol. 1984;18(Suppl 1):121S–129S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramaekers JG. Drugs and driving research in medicinal drug development. Trends Pharmacol Sci. 2017;38(4): 319–321. [DOI] [PubMed] [Google Scholar]
- 18. Verster JC, et al. Standard operation procedures for conducting the on-the-road driving test, and measurement of the standard deviation of lateral position (SDLP). Int J Gen Med. 2011;4:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louwerens JW, et al. The relationship between drivers’ blood alcohol concentration (BAC) and actual driving performance during high speed travel. In: International Congress on Alcohol, Drugs and Traffic Safety Amsterdam: Exerpta Medica; 1987:183–186. [Google Scholar]
- 20. Jongen S, et al. A pooled analysis of on-the-road highway driving studies in actual traffic measuring standard deviation of lateral position (i.e., “weaving”) while driving at a blood alcohol concentration of 0.5 g/L. Psychopharmacology (Berl). 2017;234(5):837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laska E, et al. A maximally selected test of symmetry about zero. Stat Med. 2012;31(26):3178–3191. [DOI] [PubMed] [Google Scholar]
- 22. Vermeeren A, et al. Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use. Sleep. 2014;37(3):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vermeeren A, et al. On-the-road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non-elderly healthy volunteers. Sleep. 2015;38(11):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vermeeren A, et al. On-the-road driving performance the morning after bedtime use of suvorexant 15 and 30 mg in healthy elderly. Psychopharmacology (Berl). 2016;233(18):3341–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verster JC, et al. Lapses of attention as outcome measure of the on-the-road driving test. Psychopharmacology (Berl). 2014;231(1):283–292. [DOI] [PubMed] [Google Scholar]
- 26. Verster JC, et al. Zopiclone as positive control in studies examining the residual effects of hypnotic drugs on driving ability. Curr Drug Saf. 2011;6(4):209–218. [DOI] [PubMed] [Google Scholar]
- 27. Maich KHG, et al. Psychometric properties of the consensus sleep diary in those with insomnia disorder. Behav Sleep Med. 2018;16(2):117–134. [DOI] [PubMed] [Google Scholar]
- 28. Leufkens TR, et al. Zopiclone’s residual effects on actual driving performance in a standardized test: a pooled analysis of age and sex effects in 4 placebo-controlled studies. Clin Ther. 2014;36(1):141–150. [DOI] [PubMed] [Google Scholar]
- 29. Ramaekers JG, et al. Residual effects of esmirtazapine on actual driving performance: overall findings and an exploratory analysis into the role of CYP2D6 phenotype. Psychopharmacology (Berl). 2011;215(2):321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Senn S, et al. An incomplete blocks cross-over in asthma: a case study in collaboration. In: Vollmar J, Hothorn LJ, eds. Biometrics in the Pharmaceutical Industry 7: Cross-Over Clinical Trials. Stuttgart: Gustav Fischer Verlag; 1997: 3–26. [Google Scholar]
- 31. Verster JC, et al. The prevalence and nature of stopped on-the-road driving tests and the relationship with objective performance impairment. Accid Anal Prev. 2012;45:498–506. [DOI] [PubMed] [Google Scholar]
- 32. Verster JC, et al. Blood drug concentrations of benzodiazepines correlate poorly with actual driving impairment. Sleep Med Rev. 2013;17(2):153–159. [DOI] [PubMed] [Google Scholar]
- 33. Leufkens TR, et al. Residual effects of zopiclone 7.5 mg on highway driving performance in insomnia patients and healthy controls: a placebo controlled crossover study. Psychopharmacology (Berl). 2014;231(14):2785–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]