Abstract
Study Objectives
Chronic sleep restriction in adolescents is widespread, yet we know little about how to apportion the limited amount of sleep obtained to minimize cognitive impairment: should sleep occur only nocturnally, or be split across separate nocturnal and daytime nap periods? This is particularly relevant to hippocampal-dependent cognitive functions that underpin several aspects of learning.
Method
We assessed hippocampal function in four groups by evaluating short-term topographical memory with the Four Mountains Test (4MT). All participants began with 9 hours nocturnal time-in-bed (TIB) for 2 days before following different sleep schedules over the next 3 days. Each day, one group had 5 hours nocturnal TIB (5.0h; n = 30), another, 6.5 hours nocturnal TIB (6.5h; n = 29), and a third had 6.5 hours split into 5 hours nocturnal TIB and a 1.5 hour TIB daytime nap (5.0 + 1.5h; n = 29). A control group maintained 9 hours nocturnal TIB (9.0h; n = 30). The 4MT was administered mid-afternoon (1.5 hours after awakening for those who napped).
Results
Performance of the 5.0h and 6.5h nocturnal TIB groups was significantly impaired relative to the 9.0h control group. Performance of participants on the split- sleep schedule (5.0 + 1.5h) did not significantly differ from controls.
Conclusions
These findings suggest that hippocampal function is sensitive to moderate multi-night sleep restriction, but deficits can be ameliorated by splitting sleep, at least for a period after waking from a daytime nap. While this split sleep schedule should not be considered a replacement for adequate nocturnal sleep, it appears to benefit the cognitive and neurophysiological functions that underpin learning in those who are chronically sleep deprived.
Keywords: sleep restriction, sleep deprivation, memory, spatial memory, topographical memory, split sleep, nap
Statement of Significance.
While many studies indicate that napping is beneficial to cognition, it remains unclear whether the nap itself leads to cognitive improvement, or if the same benefits are achievable by simply getting more nocturnal sleep instead. Here we show that splitting sleep between a nocturnal period and a daytime nap improves hippocampal-dependent cognitive function under conditions of chronic sleep restriction, even when total time available for sleep is controlled. In the absence of adequate nocturnal sleep, a split sleep schedule may optimize cognition.
Introduction
Chronic sleep restriction is associated with a wide range of physical and psychological deficits [1, 2] and has become increasingly prevalent in adolescents [3, 4]. In one study, less than 8% of high school students in the United States reported obtaining optimal sleep [5], while populations in East Asia consistently obtain below 6 hours on weekday nights [6–8], well below the 8–10 hours recommended by the National Sleep Foundation [9]. While the consequences of sleep deficits on development and academic achievement can be substantial, questions remain as to the amount of sleep restriction that leads to cognitive impairment, which cognitive faculties and underlying neurophysiology are worst affected, and the extent to which interventions such as daytime naps can alleviate these deficits.
Most experimental research into the consequences of sleep loss on cognition have examined performance after a night of total sleep deprivation, which negatively impacts a wide range of cognitive functions [10]. Cognition that relies on prefrontal cortical function, such as working memory (WM) and executive function, is particularly sensitive to total sleep deprivation [11–13]. However, a night of total sleep loss rarely occurs outside of a laboratory setting. A more common pattern of partially restricted sleep across several consecutive days reduces alertness and sustained attention [7, 8, 14], but more complex cognitive operations such as WM are less consistently affected [15]. Four nights of 5 hours time-in-bed (TIB) was found to impair WM and executive function (n-back task) in adolescents [7], but several other studies show resilience to similar schedules of chronic sleep restriction, for n-back [16] and verbal WM [17] in adolescents, and visual WM in adults [18, 19].
It is widely recognized that extending nocturnal sleep in adolescents toward the recommended 8–10 hours [9] is critical to their long-term well-being, which may be achieved via methods such as delaying school start times [20]. Habitual napping may be another low cost and scalable way to relieve cognitive impairment arising from chronic sleep restriction [21]. Although the potentially helpful practice of splitting sleep across a nocturnal period and a short afternoon nap is common in some countries [22, 23], only a handful of experimental studies have examined the cognitive benefits of this practice. Psychomotor vigilance and processing speed were shown to decline with total sleep obtained within a 24-hour period, but splitting sleep into either nocturnal and afternoon nap periods [24], or two equivalent periods across 24 hours [25, 26] in adults did not affect performance. These findings suggest that cognitive performance is determined by total time available for sleep, regardless of how that sleep is distributed.
Such findings seem at odds with observations that daytime naps enhance a number of cognitive operations, including attention [27–29], WM [8] and long-term memory [30–33]. These benefits are thought to result from active processes taking place primarily during non-rapid-eye-movement (NREM) sleep that refresh the capacity to process information [34] and reorganize memory networks [31]. Critically, napping in these studies constitutes an additional period of sleep to supplement a fixed amount of nocturnal sleep, rather than splitting the total sleep obtained across 24-hours into nocturnal and nap periods. It therefore remains an open question as to whether a split sleep schedule is beneficial to cognition, particularly in cognitive domains such as WM and long-term memory that are critical for effective learning. The present study aimed to explore this issue by assessing whether splitting sleep can alleviate cognitive impairments in chronically sleep-deprived adolescents, with a focus on hippocampal-dependent memory functions.
The Four Mountains Test (4MT) is a delayed match-to-sample task that assesses short-term topographical memory, and is critically dependent on the hippocampus for processing viewpoint invariant (allocentric) spatial information [35–39]. In healthy adults, hippocampal volume correlates with 4MT ability [40], while performance is impaired in patients with conditions linked to hippocampal atrophy [35–39]. Notably, 4MT performance in patients with fronto-temporal dementia is comparable to age-matched controls [39]. This suggests the task is less reliant on WM functions typically ascribed to prefrontal cortex [11, 12, 41].
The 4MT has not previously been used in adolescents or in the context of sleep research. Performance on this task is directly relevant to behaviors that rely on a “cognitive map,” such as spatial navigation, and it may provide a novel behavioral indication of hippocampal function under different schedules of chronic sleep restriction. Since the encoding of hippocampal-dependent episodic memories is sensitive to sleep deprivation [6, 42, 43] and benefits from daytime naps [32, 33], we reasoned that the 4MT may show a similar impairment after chronic sleep restriction and benefit from a split sleep schedule.
To explore these questions, we compared 4MT performance with a non-hippocampal dependent test of WM and executive function, the n-back task [44], in four groups of adolescents who underwent different schedules of chronic sleep restriction on 3 consecutive days. Groups with only 5 hours nocturnal TIB (5.0h), 6.5 hours nocturnal TIB (6.5h), or 5 hours nocturnal TIB with a 1.5-hour daytime nap opportunity (5.0 + 1.5h) were compared to a control group who had 9 hours nocturnal TIB (9.0h) (Figure 1). Consistent with our prior work, n-back performance was not expected to differ between groups after only three nights of restricted sleep. We predicted a decline in 4MT performance with sleep loss, while the split sleep schedule was expected to enhance performance relative to the other two chronically sleep deprived groups.
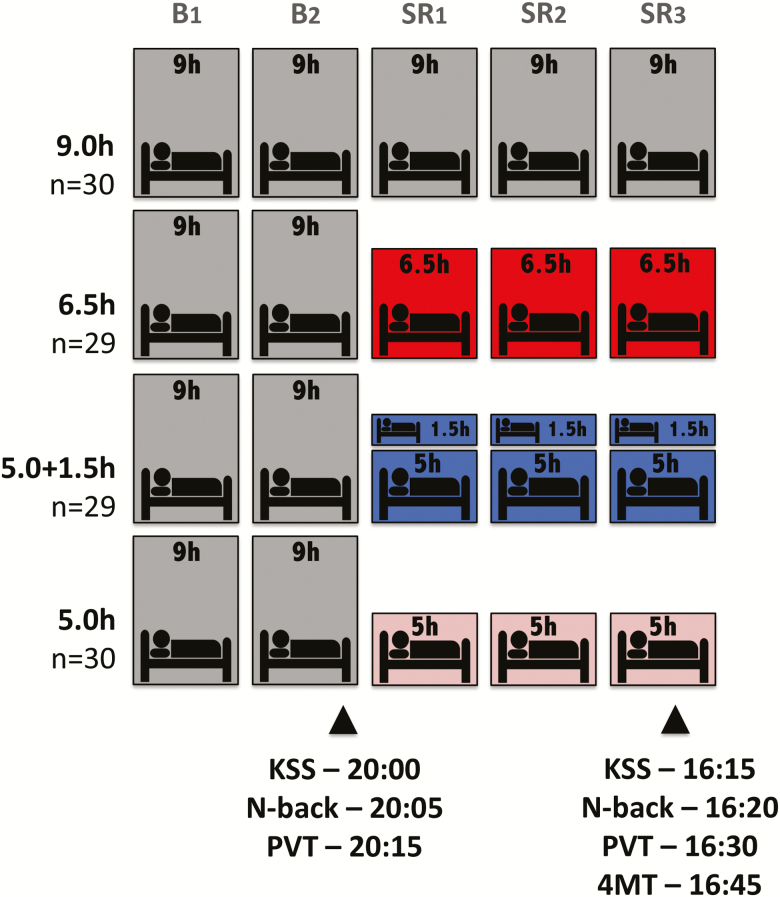
Figure 1.
Protocol. Each of the four groups received 9.0-hour nocturnal TIB for two nights prior to a 3-day manipulation period. These groups subsequently had: 9 hours nocturnal TIB (23:00–08:00), 6.5 hours nocturnal TIB (12:15–06:45), 5 hours nocturnal TIB (01:00–06:00) with a 1.5 hour TIB daytime nap opportunity (14:00–15:30), or 5 hours nocturnal TIB (01:00–06:00). On the second baseline day (B2), several measures from an evening test battery (KSS, N-back, and PVT) were analyzed to establish that groups did not differ prior to sleep manipulation. The Four Mountains Test took place at 16:45 on the third manipulation day (SR3), after the KSS, N-back, and PVT. Note that stated times on SR3 refer to the 6.5h and 5.0 + 1.5h groups (NFS4 study), while tests were consistently 30 minutes earlier for the 9.0h and 5.0h groups (NFS3 study). For the three manipulation nights, the 9.0h group could sleep from 23:00 to 08:00, the 6.5h group from 12:15 to 06:45, and the 5.0h group from 01:00 to 06:00. The 5.0 + 1.5h group were permitted the same nocturnal TIB as the 5.0h group (01:00–06:00), but had an additional 1.5 hour TIB during a mid-afternoon nap (14:00–15:30).
Methods
Participants
One hundred twenty adolescents between 15–19 years of age were selected from volunteers reporting no history of chronic or medical conditions, psychiatric illness or sleep disorders, were not habitual short sleep sleepers (>6 hours actigraphically assessed average TIB), had a body mass index (BMI) ≤ 30, consumed <5 caffeinated beverages a day and had not travelled across >2 time zones 1 month prior to the study. Participants and parents provided written informed consent, in compliance with a protocol approved by the National University of Singapore Institutional Review Board, and received monetary compensation after completion.
Participants were randomized into two pairs of groups as part of the Need for Sleep 3 (NFS3: 9.0h and 5.0h groups) [6] and Need for Sleep 4 studies (NFS4: 6.5h and 5.0 + 1.5h groups). Two participants withdrew due to personal reasons or illness, leaving a final sample comprised of 118 participants (58 females, 16.3 ± 0.8 years [mean ± SD]). Groups did not differ in gender, BMI, consumption of caffeinated beverages, or on tests of nonverbal intelligence, morning-eveningness preference, levels of daytime sleepiness, symptoms of chronic sleep reduction, self-reported sleep quality, self-reported and actigraphically assessed sleep habits, or levels of anxiety and depression (p > 0.05; Table 1). There was a significant group difference for age (one-way analysis of variance [ANOVA]: F(1,118) = 3.32, p = 0.023), where 9.0h and 5.0h groups in NFS3 were approximately 6 months younger than 6.5h and 5.0 + 1.5h groups in NFS4.
Table 1.
Screening characteristics
| 9.0h | 6.5h | 5.0 + 1.5h | 5.0h | F/χ2 | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| n | 30 | - | 29 | - | 29 | - | 30 | - | - | - |
| Age (y) | 16.1 | 0.6 | 16.6 | 1.1 | 16.6 | 0.7 | 16.1 | 0.6 | 3.32 | 0.023* |
| Gender (number of males) | 15 | - | 15 | - | 15 | - | 15 | - | 0.08 | 0.972 |
| Caffeinated drinks per day | 0.4 | 0.5 | 0.6 | 0.8 | 0.6 | 0.7 | 0.5 | 0.8 | 0.76 | 0.517 |
| Body mass index | 20.0 | 3.5 | 21.3 | 3.5 | 20.7 | 2.8 | 20.3 | 3.3 | 0.91 | 0.437 |
| Raven’s Advanced Progressive Matrices score | 8.8 | 1.9 | 8.8 | 1.9 | 9.2 | 1.6 | 8.8 | 1.5 | 0.29 | 0.834 |
| Beck Anxiety Inventory score | 8.0 | 5.5 | 9.3 | 6.7 | 10.4 | 6.3 | 9.4 | 6.9 | 0.60 | 0.618 |
| Beck Depression Inventory score | 8.6 | 5.8 | 11.0 | 5.3 | 9.2 | 5.5 | 9.3 | 6.3 | 0.79 | 0.501 |
| Morningness-Eveningness Questionnaire score | 53.3 | 6.4 | 49.0 | 7.5 | 50.7 | 7.1 | 52.1 | 8.4 | 1.71 | 0.169 |
| Epworth Sleepiness Scale score | 6.7 | 2.8 | 8.2 | 3.4 | 7.9 | 3.8 | 7.0 | 3.4 | 1.35 | 0.262 |
| Chronic Sleep Reduction Questionnaire | ||||||||||
| Total score | 35.2 | 5.3 | 35.2 | 6.0 | 36.1 | 4.7 | 35.0 | 5.1 | 0.23 | 0.879 |
| Shortness of sleep | 13.1 | 2.2 | 12.7 | 2.1 | 13.0 | 2.0 | 13.1 | 2.2 | 0.25 | 0.864 |
| Irritation | 6.4 | 1.7 | 6.4 | 1.5 | 6.8 | 1.9 | 6.9 | 2.3 | 0.58 | 0.631 |
| Loss of energy | 8.1 | 2.2 | 8.5 | 2.1 | 8.0 | 2.0 | 7.5 | 1.5 | 1.19 | 0.319 |
| Sleepiness | 7.6 | 1.6 | 7.7 | 2.3 | 8.3 | 1.5 | 7.5 | 1.8 | 1.08 | 0.362 |
| Pittsburgh Sleep Quality Index global score | 4.7 | 2.1 | 4.5 | 1.5 | 4.2 | 1.8 | 4.6 | 1.5 | 0.32 | 0.815 |
| Actigraphy | ||||||||||
| TIB on weekdays (h) | 6.6 | 1.0 | 7.0 | 0.8 | 6.8 | 1.1 | 6.7 | 0.8 | 0.74 | 0.533 |
| TIB on weekends (h) | 8.3 | 1.0 | 8.5 | 1.1 | 8.2 | 1.1 | 8.4 | 0.8 | 0.49 | 0.690 |
| TIB on average (h) | 7.2 | 0.8 | 7.4 | 0.6 | 7.2 | 0.9 | 7.4 | 0.7 | 0.86 | 0.462 |
| TST on weekdays (h) | 5.4 | 0.9 | 5.5 | 0.8 | 5.5 | 0.9 | 5.4 | 0.8 | 0.24 | 0.870 |
| TST on weekends (h) | 6.8 | 0.9 | 6.8 | 1.1 | 6.6 | 1.0 | 6.6 | 0.8 | 0.33 | 0.804 |
| TST on average (h) | 5.8 | 0.7 | 5.9 | 0.7 | 5.8 | 0.7 | 5.8 | 0.8 | 0.27 | 0.849 |
| Sleep efficiency (%) | 81.6 | 6.2 | 79.0 | 5.6 | 81.0 | 6.6 | 78.8 | 7.1 | 1.67 | 0.178 |
y = year; h = hour; SD = standard deviation; TIB = time-in-bed; TST = total sleep time; actigraphy threshold: medium.
*p < 0.05.
Design
Data are reported from the first half of NFS protocols that spanned 11 days (NFS3) and 15 days (NFS4). All groups were permitted two baseline nights (B1–B2) of the same 9 hour sleep opportunity, followed by a 3 day sleep restriction period (SR1–SR3) where groups diverged (Figure 1), prior to the 4MT. For the three manipulation nights, the 9.0h group could sleep from 23:00 to 08:00, the 6.5h group from 00:15 to 06:45, and the 5.0h group from 01:00 to 06:00. The 5.0 + 1.5h group were permitted the same nocturnal TIB as the 5.0h group (01:00–06:00), but had an additional 1.5 hour TIB during a mid-afternoon nap (14:00–15:30). Participants were constantly monitored and were not permitted to sleep outside of these specified times.
Materials
Four Mountains Test
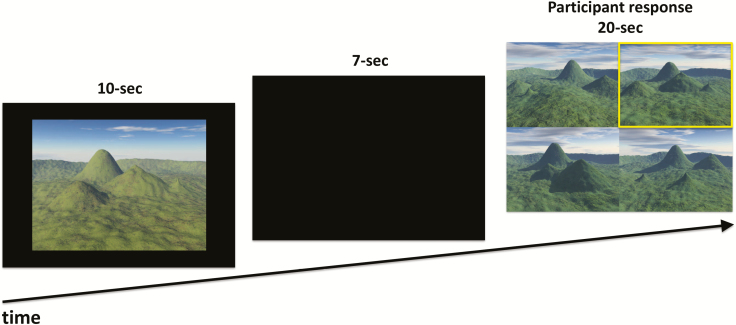
A 30-trial electronic version of the delayed match-to-sample task described previously [35], was programmed in E-Prime 2.0 (Psychology Software Tools, Inc, Sharpsburg, PA). Trials began with a 10-second presentation of a sample landscape containing four mountains of varying shape, size, and relative distance from each other, creating a unique topography (Figure 2). Each landscape was rendered from a virtual camera in one of seven predefined viewpoints. This was followed by a 7-second blank screen before the presentation of four alternative choice of landscapes arranged in a 2 × 2 grid. The target image displayed the same landscape as the sample but from a different virtual camera position. Non-topographical features of images were also varied between sample and test images to ensure that task performance was based solely on topography. These included sunlight direction, cloud cover, atmospheric conditions, and the color and texture of surfaces. The three foil images shared the same viewpoint and non-topographical features as the target, but the topography differed from the target in terms of shape, size, and relative location of mountains. On-screen position of targets and foils was randomized for each trial.
Figure 2.
The Four Mountains Test. Participants viewed a landscape containing four mountains. After a delay, they had to identify the same place from an alternative viewpoint (highlighted in yellow) from three distractor scenes.
Participants selected landscape images with a keyboard press (“Q,” “W,” “A,” or “S”). This highlighted the chosen image with a yellow box. Corrections before 20 seconds were permitted. The next trial began after 500 ms. Trials were presented in a randomized single block lasting approximately 16 minutes.
N-back task
Both 1-back and 3-back tasks [44] were performed to establish that groups were matched for WM and executive function at baseline, and during sleep restriction to contrast with 4MT performance. A letter appeared centrally for 1000 ms, followed by a 3000 ms blank screen inter-stimulus interval prior to presentation of the next letter. For the 1-back task, participants were required to respond with a button press to indicate whether the current stimulus matched (Y) or did not match (N) the letter in the previous trial. The three-back task required participants to respond as to whether the current stimulus matched the letter presented three trials previously. The match to mismatch ratio was 08:24. Two performance indicators were measured: A′ provided the participant’s ability to discriminate between matches and mismatches (range: 0–1; chance performance = 0.5), while B″ indicated their tendency towards liberal () or conservative () response bias.
Psychomotor vigilance and self-reported sleepiness
The psychomotor vigilance test (PVT) [45] provided an objective indication of sustained attention. Participants responded with the space bar when a counter appeared on screen, at random intervals between 2000 ms and 10 000 ms. A beep alerted participants via headphones if no response was detected within 10 000 ms. This was performed in a 10-minute continuous block. Lapses (responses slower than 500 ms) were measured. The Karolinska Sleepiness Scale (KSS) provided an indication of self-reported sleepiness.
Procedure
Participants’ habitual term-time sleep was actigraphically assessed (Actiwatch AW-2, Philips Respironics, Inc., Pittsburgh, PA) for a 1-week period 1–3 months prior to the study (Table 1). One week prior to the study participants adhered to a sleep schedule (23:00–08:00), confirmed with actigraphy. The protocol took place during a school holiday period inside a boarding school in Singapore. Participants slept in twin-share bedrooms, while all testing and free time were strictly monitored in specified classrooms and common rooms throughout the 11-day and 15-day protocols. Breakfast (07:15–09:30), lunch (12:00–13:00), dinner (17:30–18:30) and snacks between meals were provided each day. Breakfast was delayed until 11:00 on B2 and SR3 in the 6.5h and 5.0 + 1.5h groups (NFS4) because of a glucose monitoring test that required a period of fasting (data not reported here).
Testing took place in a classroom with participants using individual laptops. Participants sat approximately 1 meter apart across six perpendicular rows and were instructed not to look at other visible screens during task performance. Participants performed the n-back and PVT three times daily as part of a test battery on each day of the experiment. Timings varied by 30 minutes between NFS3 and NFS4 studies. Analysis focused on the final baseline test battery (20:00) when participants had familiarized themselves with the tests, and on manipulation day SR3 when the 4MT test took place. On day SR3, the 9.0h and 5.0h groups from NFS3 performed the n-back task at 15:50, the PVT at 16:00, and the 4MT at 16:15. The 6.5h and 5.0 + 1.5h groups in NFS4 performed the same tasks 30 minutes later: the n-back at 16:20, the PVT at 16:30, and the 4MT at 16:45. Participants were briefed altogether in each of the studies. They were shown four examples of the test stimuli and received feedback on the correct answers, as well as an explanation of why the foils were incorrect. Participants were instructed that each target image would be on screen for 10 seconds, and that they should study the shape and arrangement of mountains carefully. They were instructed to select the image which showed the same place as the target within 20 seconds and could change their answer within that period.
Statistical analysis
A one-way ANOVA and follow-up independent samples t-tests compared the four experimental groups, or Kruskal–Wallis H-test and Mann–Whitney U-tests where Shapiro-Wilk indicated a non-normal distribution. Spearman’s Rho correlations explored the relationship between 4MT performance and sleep features.
Polysomnography
Sleep was recorded using SOMNOtouch RESP portable devices (SOMNOmedics, GmbH, Germany) only for the NFS4 study (6.5h and 5.0 + 1.5h groups). Recordings were performed on three nights (B2, SR1, and SR3) and also the naps that followed on SR1 and SR3 in the 5.0 + 1.5h group. Electrodes were applied by trained technicians. Electroencephalography (EEG) was recorded from two main channels (C3 and C4 according to the 10–20 system) referenced to the contralateral mastoids. The common ground and reference electrode were placed at Fpz and Cz. Left and right electromyogram and electrooculogram were also attached. Impedance <10 K Ohms was verified at each electrode. The sampling rate was 256 Hz. Data was scored utilizing the Z3Score automated EEG system [46] and verified by a trained researcher. Prior research has linked post-nap cognitive performance with spindles [32] and slow-wave sleep (SWS) [33, 47]; therefore, spindles and slow-wave activity (SWA) were analyzed at C3 referenced to A2. Slow (12–13.5 Hz) and fast (13.5–15 Hz) spindle density (spindles per minute) was assessed using an adapted automated algorithm [48]. Spectral analysis was performed on artifact-free nonoverlapping 5-second epochs, focusing on SWA (0.6–4 Hz) using a fast Fourier transform routine (Hamming window; 0.2 Hz bin resolution). Total SWA was summed across all SWS epochs and expressed as a percentage of total SWA in the baseline night (B2). As an exploratory analysis, total SWA in the first hour of nocturnal sleep was also computed as a marker of sleep pressure.
Results
Four Mountains Test
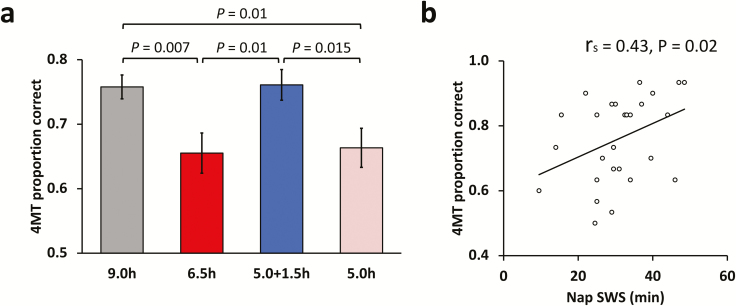
See Table 2 for a summary of all cognitive tests. A one-way ANOVA showed a significant main effect of group (F(3,117) = 4.768, p = 0.004). Follow-up t-tests revealed that relative to the 9.0h control group, the 5.0h group (t(58) = 2.67, p = 0.01; Cohen’s d = 0.69), and the 6.5h group (t(57) = 2.832, p = 0.007; Cohen’s d = 0.74) performed worse (Figure 3). In contrast, performance of the 5.0 + 1.5h group was not significantly different from the 9.0h control group (t(57) = 0.055, p = 0.956; Cohen’s d = 0.02). Moreover, the 5.0 + 1.5h group performed significantly better than the 5.0h (t(57) = 2.5, p = 0.015; Cohen’s d = 0.65) and 6.5h groups (t(56) = 2.67, p = 0.01; Cohen’s d = 0.7). There was no significant difference between 5.0h and 6.5h groups (t(57) = 0.205, p = 0.839; Cohen’s d = 0.053).
Table 2.
Performance for all cognitive tests
| 9.0h | 6.5h | 5.0 + 1.5h | 5.0h | F/χ2 | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Baseline—B2 | ||||||||||
| PVT lapses | 2.60 | 3.15 | 3.21 | 4.97 | 2.24 | 2.63 | 4.63 | 5.51 | 3.19 | 0.363 |
| KSS | 4.47 | 1.48 | 4.67 | 1.41 | 4.79 | 1.46 | 4.86 | 1.38 | 1.56 | 0.669 |
| 1-back A′ | 0.97 | 0.03 | 0.96 | 0.06 | 0.98 | 0.02 | 0.97 | 0.03 | 2.85 | 0.415 |
| 1-back B″ | 0.12 | 0.63 | 0.33 | 0.63 | 0.02 | 0.71 | 0.32 | 0.70 | 4.30 | 0.231 |
| 3-back A′ | 0.93 | 0.05 | 0.90 | 0.08 | 0.92 | 0.06 | 0.91 | 0.08 | 2.46 | 0.483 |
| 3-back B″ | 0.27 | 0.68 | 0.33 | 0.69 | 0.18 | 0.75 | 0.34 | 0.74 | 1.50 | 0.681 |
| Sleep restriction—SR3 | ||||||||||
| PVT lapses | 2.93 | 4.62 | 11.45 | 14.02 | 2.90 | 3.57 | 8.48 | 9.98 | 20.80 | 0.000** |
| KSS | 4.53 | 1.80 | 5.28 | 1.75 | 4.50 | 1.32 | 5.87 | 1.59 | 15.07 | 0.002** |
| 1-back A′ | 0.95 | 0.04 | 0.96 | 0.06 | 0.96 | 0.06 | 0.96 | 0.05 | 3.77 | 0.288 |
| 1-back B″ | 0.34 | 0.76 | 0.15 | 0.73 | 0.11 | 0.74 | 0.41 | 0.70 | 4.44 | 0.218 |
| 3-back A′ | 0.90 | 0.18 | 0.91 | 0.10 | 0.95 | 0.05 | 0.89 | 0.94 | 7.22 | 0.065 |
| 3-back B″ | 0.15 | 0.72 | 0.17 | 0.71 | -0.20 | 0.83 | 0.41 | 0.71 | 8.80 | 0.032* |
| 4MT (proportion correct) | 0.76 | 0.10 | 0.66 | 0.16 | 0.76 | 0.13 | 0.66 | 0.16 | 4.77 | 0.004** |
h = hour.
*p < 0.05; **p < 0.01.
Figure 3.
Behavioral results and sleep correlation. (a) There was significantly lower performance in the 5.0h and 6.5h groups relative to the 9.0h control group. By contrast, there was no impairment to performance when 6.5h TIB was split across nocturnal sleep and a daytime nap (5.0 + 1.5h group). (b) Duration of SWS during the nap prior to the task was significantly correlated with 4MT performance. Error bars represent standard error of the mean (SEM).
Thus, there was a similar performance deficit when obtaining 5 or 6.5 hours nocturnal TIB for three consecutive nights. However, if 6.5 hours TIB was split into 5 hours nocturnal TIB and a 1.5 hour TIB daytime nap (5.0 + 1.5h group), performance was comparable to the 9.0h controls.
We also examined the number of trials where no response was made (misses) as an indirect measure of attention. These were very low across the whole sample (M = 1.5%, SD = 2.8%) and Kruskal–Wallis H-test showed no significant group differences (χ2(3) = 1.042, p = 0.791).
N-back task
Prior to the sleep manipulation (B2), there were no 1-back or 3-back group differences in A′ (p > 0.414) and (p > 0.230). On SR3, there were no significant group effects for 1-back A′ (χ2(3) = 3.77, p = 0.288), and a trend for 3-back A′ (χ2(3) = 7.22, p = 0.065). For response bias, groups did not differ for 1-back (χ2(3) = 4.44, p = 0.218), but there was a significant group effect for 3-back (χ2(3) = 8.80, p = 0.032). This appeared to be driven by the 5.0 + 1.5h group who had a significantly more liberal response bias than the 5.0h group (U = 258, p = 0.006), while no other group comparisons yielded a significant difference (p > 0.063). This liberal bias of the 5.0 + 1.5h group may account for the trend of a group difference in 3-back A′, where this group performed numerically better than the others (Table 2).
Next, we correlated n-back performance on SR3 with the 4MT within each group separately (16 comparisons). Only 3-back A′ for the 5.0h group was significantly correlated with 4MT performance (Rs = 0.398, p = 0.029), although this did not survive false discovery rate (FDR) correction for multiple comparisons.
In sum, the split sleep schedule was associated with a shift in response bias for the more cognitively demanding 3-back task, but there were no significant group differences for accuracy and little indication of a relationship between n-back and 4MT performance. This suggests that unlike the 4MT, the 3 days of restricted sleep had relatively little impact on n-back performance. Together, these findings indicate that the sleep-related deficit observed in short-term topographical memory (4MT) was unlikely to reflect a more general impairment to WM and executive function (n-back).
Psychomotor vigilance and self-reported sleepiness
At baseline (B2), there were no significant group differences for PVT lapses, (χ2(3) = 3.19, p = 0.363) or self-reported sleepiness (χ2(3) = 1.56, p = 0.669; Table 2). After the sleep manipulation, however (SR3), there was a significant main effect of group for lapses (χ2(3) = 20.8, p < 0.001) and self-reported sleepiness (χ2(3) = 15.07, p = 0.002). Follow-up Mann–Whitney U-tests showed a similar pattern to the 4MT, whereby the 5.0 + 1.5h group had significantly fewer lapses (p < 0.004) and less self-reported sleepiness (p < .033) than 6.5h and 5.0h groups. The 9.0h group also had fewer lapses than 6.5h and 5.0h groups (p < 0.002), while self-reported sleepiness was significantly lower than the 5.0h group (p = 0.003) and trending to be lower than the 6.5h group (p = 0.063). There were no significant differences for these measures between 9.0h and 5.0 + 1.5h groups (p > 0.481), or between 6.5h and 5.0h groups (p > 0.181).
Despite the similar pattern of results between PVT and 4MT at the group level, there were no significant correlations between PVT lapses or self-reported sleepiness and 4MT performance within any group (p > 0.081), indicating a dissociation within each participant between the effects of sleep restriction on attention, alertness and short-term topographical memory.
Actigraphy
In the screening period prior to inclusion in the study, participants showed a sleep pattern typical for Singaporean adolescents—shortened sleep on weekdays (TIB = 6.83 ± 0.94 hours, total sleep time [TST] = 5.44 ± 0.84 [mean ± SD]) and sleep extension on weekends (TIB = 8.31 ± 1.00 hours, TST = 6.69 ± 0.96; Table 1). In the week prior to commencement of the study, participants adhered to a sleep schedule (23:00–08:00) confirmed with actigraphy (TIB = 8.9 ± 0.37, TST = 7.45 ± 0.53). Actigraphy during the study confirmed that our manipulation was effective at reducing TST in each group (Table 3). Note that actigraphy using the manufacturer’s default sensitivity settings underestimates adolescent TST by an average of ~30 minutes [49]; therefore, absolute values for TST should be interpreted with caution.
Table 3.
TST across baseline and manipulation nights (assessed with actigraphy)
| 9.0h | 6.5h | 5.0 + 1.5h | 5.0h | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Baseline nocturnal (B1–B2) | ||||||||
| TST | 453.6 | 32.6 | 453.8 | 42.0 | 455.1 | 33.2 | 456.8 | 30.6 |
| Manipulation nocturnal (SR1–SR3) | ||||||||
| TST | 446.7 | 30.0 | 326.4 | 29.0 | 246.5 | 20.9 | 259.9 | 17.1 |
| Manipulation nap (SR1–SR3) | ||||||||
| TST | - | - | - | - | 72.9 | 8.7 | - | - |
h = hour; TST = total sleep time (minutes); actigraphy threshold: medium.
Polysomnography
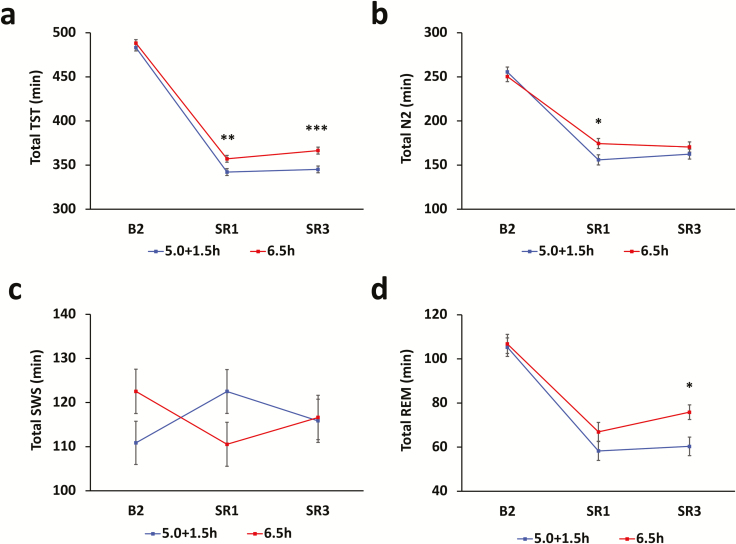
Data was obtained for three of the four nights prior to the experimental day (B2, SR1, and SR3), for the 6.5h and 5.0 + 1.5h groups (Figure 4). Table 4 details nocturnal sleep, nap sleep and total sleep across each 24-hour period (i.e. nocturnal sleep and the following nap combined for the 5.0 + 1.5h group). There were no significant group differences in sleep macro-architecture during the baseline night (B2) for any measure (TST, Stage 1 sleep [N1], Stage 2 sleep [N2], SWS, rapid-eye movement sleep [REM], p > 0.05; Figure 4). The 5.0 + 1.5h group obtained significantly less TST than the 6.5h group on SR1 (p = 0.007) and SR3 (p < 0.001). This was due to increased sleep latency on both days (p < 0.001), because participants were required to fall asleep on two separate occasions, but wake after sleep onset (WASO) did not differ between groups (p > 0.05). This resulted in a reduction in total N2 on SR1 (p = 0.024) and REM on SR3 (p = 0.011), while SWS did not differ between groups at any point (p > 0.05).
Figure 4.
Sleep parameters prior to the 4MT. Graphs represent combined sleep characteristics over each 24-hour period. (a) The 5.0 + 1.5h group obtained significantly less total sleep time than the 6.5h group during the sleep restriction period (SR1–SR3). (b) Underlying this difference was significantly less stage 2 sleep on SR1 and (d) less REM sleep on SR3. (c) Slow-wave sleep did not differ between groups at any point. Error bars represent SEM.
Table 4.
Sleep architecture of the 6.5h and 5.0 + 1.5h groups during baseline and manipulation nights, measured with polysomnography
| 5.0 + 1.5h | 6.5h | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | P | ||
| B2 | Nocturnal | ||||||
| N1 | 11.5 | 7. 8 | 8.6 | 6.1 | 1.54 | 0.130 | |
| N2 | 255.5 | 31.1 | 251.3 | 37.6 | 0.46 | 0.650 | |
| SWS | 110.9 | 22.2 | 121.2 | 31.6 | −1.43 | 0.158 | |
| REM | 105.3 | 20.3 | 106.6 | 22.0 | −0.224 | 0.824 | |
| TST | 483.2 | 27.1 | 487.8 | 26.6 | −0.634 | 0.529 | |
| N2 latency | 36.9 | 18.5 | 30.6 | 12.3 | 1.494 | 0.140 | |
| WASO | 20.4 | 19.5 | 22.2 | 20.1 | −0.341 | 0.735 | |
| Sleep efficiency | 89.5 | 5.0 | 90.3 | 4.9 | −0.589 | 0.559 | |
| SR1 | Nocturnal | ||||||
| N1 | 3.6 | 3.0 | 5.3 | 3.5 | −1.88 | 0.065 | |
| N2 | 124.5 | 21.0 | 174.4 | 32.6 | −6.72 | 0.000* | |
| SWS | 94.7 | 17.1 | 110.5 | 33.0 | −2.25 | 0.030* | |
| REM | 48.5 | 18.7 | 66.9 | 18.5 | −3.68 | 0.001* | |
| TST | 271.3 | 16.4 | 357.1 | 14.3 | −20.67 | 0.000* | |
| N2 latency | 22.9 | 14.4 | 23.1 | 10.3 | −0.075 | 0.941 | |
| WASO | 6.4 | 10.8 | 10.3 | 9.9 | −1.38 | 0.172 | |
| Sleep efficiency | 90.4 | 5.6 | 91.6 | 3.7 | −0.938 | 0.352 | |
| Nap | |||||||
| N1 | 1.8 | 1.8 | - | - | - | - | |
| N2 | 31.2 | 12.0 | - | - | - | - | |
| SWS | 27.8 | 13.0 | - | - | - | - | |
| REM | 10.1 | 10.2 | - | - | - | - | |
| TST | 70.9 | 15.7 | - | - | - | - | |
| N2 latency | 13.9 | 8.2 | - | - | - | - | |
| WASO | 5.7 | 12.1 | - | - | - | - | |
| Sleep efficiency | 78.7 | 17.4 | - | - | - | - | |
| Total | |||||||
| N1 | 5.5 | 3.8 | 5.3 | 3.5 | 0.25 | 0.802 | |
| N2 | 156.8 | 25.3 | 174.4 | 32.6 | −2.23 | 0.030* | |
| SWS | 122.2 | 23.1 | 110.5 | 33.0 | 1.51 | 0.136 | |
| REM | 58.1 | 25.2 | 66.9 | 18.5 | −1.49 | 0.144 | |
| TST | 342.6 | 28.2 | 357.1 | 14.3 | −2.39 | 0.022* | |
| N2 latency | 36.8 | 19.3 | 23.1 | 10.3 | 3.26 | 0.002* | |
| WASO | 11.7 | 17.2 | 10.3 | 9.9 | .381 | 0.705 | |
| Sleep efficiency | 87.8 | 7.3 | 91.6 | 3.7 | −2.42 | 0.019* | |
| SR3 | Nocturnal | ||||||
| N1 | 4.5 | 6.6 | 3.7 | 3.2 | .58 | 0.563 | |
| N2 | 125.8 | 22.2 | 170.2 | 26.7 | −6.79 | 0.000* | |
| SWS | 84.8 | 16.4 | 116.2 | 23.3 | −5.87 | 0.000* | |
| REM | 50.6 | 14.6 | 76.5 | 21.1 | −5.38 | 0.000* | |
| TST | 265.7 | 17.1 | 366.6 | 9.2 | −27.75 | 0.000* | |
| N2 latency | 29.6 | 16.1 | 17.1 | 8.9 | 3.55 | 0.000* | |
| WASO | 5.3 | 7.7 | 6.8 | 6.2 | −0.778 | 0.440 | |
| Sleep efficiency | 88.5 | 5.7 | 94.0 | 2.4 | −4.59 | 0.000* | |
| Nap | |||||||
| N1 | 2.0 | 1.4 | - | - | - | - | |
| N2 | 36.7 | 12.3 | - | - | - | - | |
| SWS | 31.0 | 9.4 | - | - | - | - | |
| REM | 9.8 | 8.4 | - | - | - | - | |
| TST | 79.5 | 3.9 | - | - | - | - | |
| N2 latency | 9.7 | 3.9 | - | - | - | - | |
| WASO | 1.4 | 1.4 | - | - | - | - | |
| Sleep efficiency | 88.2 | 4.4 | - | - | - | - | |
| Total | |||||||
| N1 | 6.5 | 7.1 | 3.7 | 3.2 | 1.86 | 0.068 | |
| N2 | 162.5 | 26.1 | 170.2 | 26.7 | −1.10 | 0.276 | |
| SWS | 115.9 | 21.2 | 116.2 | 23.3 | −0.061 | 0.952 | |
| REM | 60.3 | 17.7 | 76.5 | 21.1 | −3.11 | 0.003* | |
| TST | 345.1 | 18.6 | 366.6 | 9.2 | −5.53 | 0.000* | |
| N2 Latency | 39.3 | 18.1 | 17.1 | 8.9 | 5.74 | 0.000* | |
| WASO | 6.7 | 8.0 | 6.8 | 6.2 | −0.037 | 0.971 | |
| Sleep efficiency | 88.5 | 4.8 | 94.0 | 2.4 | −5.41 | 0.000* |
N1 = stage 1 sleep (minutes); N2 = stage 2 sleep (minutes); SWS = slow-wave sleep (minutes); REM = rapid-eye movement sleep (minutes); TST = total sleep time (minutes); N2 latency = time to first epoch of stage 2 sleep (minutes); WASO = wake after sleep onset (minutes); SE = sleep efficiency (% TIB).
*p < 0.05.
Assessment of sleep characteristics in the SR3 nap prior to the 4MT showed that performance was positively correlated with SWS duration (Rs = 0.42, p = 0.023) and total SWA (0.6–4 Hz) (r = 0.4, p = 0.03). Other sleep stages and TST during the nap did not significantly correlate with 4MT performance (p > 0.05).
As a final exploratory analysis, we examined SWA in the first hour of nocturnal sleep on SR3 as an indicator of the amount of accumulated sleep pressure. The 5.0 + 1.5h group (M = 81.85%, SD = 32.97) had significantly lower SWA than the 6.5h group (M = 119.23%, SD = 53.12), t(52) = 3.131, p = 0.003, which suggests alleviation of sleep pressure by the nap under the split sleep schedule.
Discussion
We investigated how different sleep schedules affect a hippocampal-dependent test of short-term topographical memory. Performance was impaired after three nights of relatively mild nocturnal sleep restriction of 6.5 hours TIB, and was comparable to a more extreme schedule of sleep restriction (5 hours nocturnal TIB). In contrast, when sleep was split into 5 hours nocturnal sleep and a 1.5 hour daytime nap, performance was similar to a control group obtaining the recommended amount of sleep for adolescents [9] (9 hours nocturnal TIB).
The improved performance we observed after splitting sleep contrasts with prior research in adults, where overall performance was suggested to be determined by total sleep obtained within a 24-hour period, irrespective of whether sleep was split or not [24–26]. There is a wealth of research showing that a nap benefits cognition when it supplements a fixed amount of nocturnal sleep [32, 33], but persons who napped in these studies obtained more sleep in the 24-hour period prior to testing. This makes it difficult to determine if the cognitive benefits stem from the additional sleep time or the distribution of sleep. The present split sleep design allows us to definitively attribute the benefit on memory to sleep distribution as total TIB was controlled.
The superior performance of students under the split sleep schedule (5.0 + 1.5h group) is interesting given that TST was less relative to the 6.5h nocturnal sleep group. This appears to simply be due to the fact that participants under the split sleep schedule were required to fall asleep twice. This results in a numerically longer sleep latency total that reduces TST given the fixed total sleep opportunity. Stage 2 and REM sleep duration were less under the split sleep schedule while SWS duration was unaffected. It is likely that the splitting of sleep afforded an additional opportunity to dissipate sleep pressure that built up by the mid-afternoon following hours of prior wakefulness. Evidence for such dissipation of sleep pressure comes from the finding of lower SWA in the first hour of nocturnal sleep in the split compared to 6.5h nocturnal sleep group. However, the mechanistic basis for why split sleep yields superior cognition under conditions of multi-night sleep restriction remains to be investigated in future studies.
Notwithstanding, participants who obtained more SWS and had greater SWA in the nap prior to the 4MT performed better at the task. A relationship between hippocampal-dependent long-term memory operations and SWS has been consistently observed [33, 50], and here we show a similar relationship for a hippocampal-dependent short-term memory task. These findings are consistent with the idea that SWS benefits cognitive function, perhaps through the downscaling of synapses potentiated during extended wakefulness. This could renew the capacity of networks to encode new information [34] and may account for the enhanced ability of the split sleep group to encode and manipulate scenes in the 4MT.
The impairment to 4MT performance after only three nights of 6.5 hours TIB contrasts with the lack of a significant effect on n-back performance. The latter finding agrees with prior studies where several nights of sleep restriction did not affect WM and executive function [16–18]. In adolescents, n-back performance decrements only emerged after four nights of 5 hours TIB [7]. One study utilized a visuospatial WM task that required the maintenance and manipulation of a visual image [19], but did not depend on the allocentric spatial processing that is critical to performance of the 4MT [35]. They found that 3 weeks of 6.5 hours TIB per 28-hour period led to deficits in speed but not accuracy of this task [19]. Taken together, these findings suggest that allocentric spatial processing may be more vulnerable to sleep restriction than WM.
Deficits to WM and executive function after a night of sleep deprivation are strongly linked to impaired prefrontal function [11–13]. While the 4MT tests a form of WM, it is thought to provide a sensitive index of hippocampal-dependent spatial processing [35–40], and neuropsychological evidence suggests it is less affected by prefrontal damage [39]. Speculatively, the high sensitivity of the 4MT to sleep restriction suggests that hippocampal function is particularly sensitive to chronic sleep loss. The current study did not measure brain activity during performance and to our knowledge, the only imaging studies of WM have examined total sleep deprivation rather than partial sleep restriction [11, 13]. Therefore, further work is necessary to uncover the neurophysiological correlates of these impairments.
The sensitivity of the 4MT to multiple nights of sleep restriction is congruent with observations of impaired episodic memory encoding after sleep loss and associated hippocampal dysfunction. A single night of sleep deprivation [42, 43] or five consecutive nights of only 5 hours TIB [6] significantly reduced the capacity to encode new information, possibly as a result of reduced hippocampal activity during encoding [43] as well as reduced capacity for long-term potentiation in the hippocampus [51, 52]. We show that a less severe and relatively common form of chronic sleep restriction (6.5 hours on three consecutive nights) can also impair hippocampal-dependent cognition.
The pattern of results for psychomotor vigilance and self-reported sleepiness were similar to the 4MT, but no significant correlations between the tasks were observed. This indicates that within individual participants, capability in one cognitive domain was not associated with their ability in another. Similar dissociations between cognitive measures have been noted several times in prior work: between self-reported and objective measures of sleep loss [53], as well as vigilance and memory [6, 43]. The long trials of the 4MT (10 seconds to encode, 20 seconds to make a response) make it unlikely that lapses in concentration associated with the PVT would impact on performance, supported by the low number of missed trials on the 4MT. Moreover, the rapid presentation of the n-back task makes it arguably more vulnerable to attentional lapses, and yet, it was not affected after three nights of sleep restriction. To summarize, while decline in vigilance after sleep deprivation is a robust observation [6–8, 10], it is unlikely to account for the short-term topographical memory effects we observed.
Several limitations to the present study should be kept in mind. While the split sleep group appeared to perform as well as controls, the sleep they obtained was far below the recommended 9 hours for this age group [9]. Therefore, we do not advocate for students to keep this type of chronically restricted sleep schedule. Not only are there myriad other negative health consequences associated with insufficient sleep [2], but our observations may also be conditional on the time at which participants were tested. The 4MT took place at 16:45, 1.5 hours after the nap, and it is unclear if performance would differ at other times of day and other times relative to the time at which the nap took place. Sleepiness and sustained attention have been shown to be enhanced by a nap for a limited window only [29] and this may also be the case for the 4MT. Our prior study also suggests that morning 4MT performance would be likely reduced: a 1-hour nap enhanced afternoon PVT performance in adolescents under a 5-hour TIB nocturnal sleep schedule, but morning performance was similarly impaired to a no-nap condition [8]. The 4MT was only tested once in the current study in order to limit the effects of training and memory consolidation between sessions. Therefore, other times of day could be tested in future studies to provide a complete picture of performance under a split sleep schedule. It may also be useful to examine the change in performance between baseline and sleep restriction tests in order to assess the impact of different sleep schedules at the individual level.
In sum, hippocampal-dependent topographical memory appears to be negatively affected by even three nights of relatively mild sleep restriction, but this deficit is recovered when sleep is split across a nocturnal period and a daytime nap. This suggests that under conditions of chronic sleep restriction, a split sleep schedule may optimize the cognitive and neurophysiological functions that underpin some aspects of learning.
Acknowledgments
Thank you to Lee Xuan Kai, Cher Wei Shan, Lydia Teo Manling, Chong Shin Wee, Nicholas Chee, Shirley Koh, Teo Teck Boon, June Lo, Jesisca Tandi, Karen Sasmita, Ksenia Vinogradova, Azrin Bin Jamaluddin, Alyssa Ng, Andrew Dicom, James Teng, Kian Wong, Ruth Leong, and Vaisakh Puthusseryppady who provided assistance in data collection and sleep scoring. The staff of Nanyang Girls Boarding School provided a conducive environment for the conduct of this research.
Funding
This work was supported by National Medical Research Council (STaR/0015/2013), the National Research Foundation Singapore (NRF2016-SOL002-001) and The Far East Organization.
Conflict of interest statement. None declared.
References
- 1. O’Brien LMO. The neurocognitive effects of sleep disruption in children and adolescents. Sleep. 2011;6(1):109–116. [Google Scholar]
- 2. Itani O, et al. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. [DOI] [PubMed] [Google Scholar]
- 3. Matricciani L, et al. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012;16(3):203–211. [DOI] [PubMed] [Google Scholar]
- 4. Keyes KM, et al. The great sleep recession: changes in sleep duration among US adolescents, 1991-2012. Pediatrics. 2015;135(3):460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eaton DK, et al. Prevalence of insufficient, borderline, and optimal hours of sleep among high school students - United States, 2007. J Adolescent Health. 2010;46(4):399–401. [DOI] [PubMed] [Google Scholar]
- 6. Cousins JN, et al. Memory encoding is impaired after multiple nights of partial sleep restriction. J Sleep Res. 2018;27(1):138–145. [DOI] [PubMed] [Google Scholar]
- 7. Lo JC, et al. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2015;39(3):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lo JC, et al. Neurobehavioral impact of successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2016;40(2). doi: 10.1093/sleep/zsw042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirshkowitz M, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 10. Lim J, et al. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krause AJ, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18(7):404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chee MW, et al. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24(19):4560–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frenda SJ, et al. Sleep less. think worse: the effect of sleep deprivation on working memory. J Appl Res Mem Cogn. 2016;5(4):463–469. [Google Scholar]
- 14. Wolfson AR, et al. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;1:875–887. [PubMed] [Google Scholar]
- 15. de Bruin EJ, et al. Effects of sleep manipulation on cognitive functioning of adolescents: a systematic review. Sleep Med Rev. 2017;32:45–57. [DOI] [PubMed] [Google Scholar]
- 16. Beebe DW, et al. Behavioral and brain functions preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav Brain Funct. 2009;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang F, et al. Effect of chronic sleep restriction on sleepiness and working memory in adolescents and young adults. J Clin Exp Neuropsychol. 2011;33(8):892–900. [DOI] [PubMed] [Google Scholar]
- 18. Drummond SP, et al. The effects of two types of sleep deprivation on visual working memory capacity and filtering efficiency. PLoS One. 2012;7(4):e35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pomplun M, et al. The effects of circadian phase, time awake, and imposed sleep restriction on performing complex visual tasks : evidence from comparative visual search. J Vision. 2017;12(7):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watson NF, et al. ; American Academy of Sleep Medicine Board of Directors. Delaying middle school and high school start times promotes student health and performance: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13(4):623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ribeiro S, et al. Sleep and school education. Trends Neurosci Educ. 2014; 3(1):18–23. [Google Scholar]
- 22. Stampi B. Why we nap. Evolution, Chronobiology, and Functions of Polyphasic and Ultrashort Sleep. Boston, MA: Birkhauser; 1992. [Google Scholar]
- 23. Ji X, et al. The relationship between midday napping and neurocognitive function in early adolescents. Behav Sleep Med. 2018; Feb 2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mollicone DJ, et al. Response surface mapping of neurobehavioral performance: testing the feasibility of split sleep schedules for space operations. Acta Astronaut. 2008;63(7-10):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kosmadopoulos A, et al. The effects of a split sleep-wake schedule on neurobehavioural performance and predictions of performance under conditions of forced desynchrony. Chronobiol Int. 2014;31(10):1209–1217. [DOI] [PubMed] [Google Scholar]
- 26. Jackson ML, et al. Investigation of the effectiveness of a split sleep schedule in sustaining sleep and maintaining performance. Chronobiol Int. 2014;31(10):1218–1230. [DOI] [PubMed] [Google Scholar]
- 27. Taub JM, et al. Effects of afternoon naps on physiological variables performance and self-reported activation. Biol Psychol. 1977;5(3):191–210. [DOI] [PubMed] [Google Scholar]
- 28. Tietzel AJ, et al. The recuperative value of brief and ultra-brief naps on alertness and cognitive performance. J Sleep Res. 2002;11(3):213–218. [DOI] [PubMed] [Google Scholar]
- 29. Lovato N, et al. The effects of napping on cognitive functioning. Prog Brain Res. 2010;185:155–166. [DOI] [PubMed] [Google Scholar]
- 30. Lau H, et al. Daytime napping: effects on human direct associative and relational memory. Neurobiol Learn Mem. 2010;93(4):554–560. [DOI] [PubMed] [Google Scholar]
- 31. Rasch B, et al. About sleep’s role in memory. Physiol Rev. 2014;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mander BA, et al. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21(5):R183–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antonenko D, et al. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37(7):1142–1151. [DOI] [PubMed] [Google Scholar]
- 34. Tononi G, et al. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hartley T, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17(1):34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bird CM, et al. Topographical short-term memory differentiates Alzheimer’s disease from frontotemporal lobar degeneration. Hippocampus. 2010;20(10):1154–1169. [DOI] [PubMed] [Google Scholar]
- 37. Chan D, et al. The 4 mountains test : a short test of spatial memory with high sensitivity for the diagnosis of pre-dementia Alzheimer’s disease. JOVE-J Vis Exp. 2016; 116:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moodley K, et al. Diagnostic differentiation of mild cognitive impairment due to Alzheimer’s disease using a hippocampus-dependent test of spatial memory. Hippocampus. 2015;25(8):939–951. [DOI] [PubMed] [Google Scholar]
- 39. Pengas G, et al. Lost and found: bespoke memory testing for Alzheimer’s disease and semantic dementia. J Alzheimers Dis. 2010;21(4):1347–1365. [DOI] [PubMed] [Google Scholar]
- 40. Hartley T, et al. An association between human hippocampal volume and topographical memory in healthy young adults. Front Hum Neurosci. 2012;6:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braver TS, et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. [DOI] [PubMed] [Google Scholar]
- 42. Poh JH, et al. Degradation of cortical representations during encoding following sleep deprivation. Neuroimage. 2017;153:131–138. [DOI] [PubMed] [Google Scholar]
- 43. Yoo SS, et al. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10(3):385–392. [DOI] [PubMed] [Google Scholar]
- 44. Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352–358. [DOI] [PubMed] [Google Scholar]
- 45. Dinges DF, et al. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Ins C. 1985;17(6):652–655. [Google Scholar]
- 46. Patanaik A, et al. An end-to-end framework for real-time automatic sleep stage classification. Sleep. 2018;41(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Der Werf YD, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12(2):122–123. [DOI] [PubMed] [Google Scholar]
- 48. Ferrarelli F, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–492. [DOI] [PubMed] [Google Scholar]
- 49. Pesonen AK, et al. The validity of a new consumer-targeted wrist device in sleep measurement: an overnight comparison against polysomnography in children and adolescents. J Clin Sleep Med. 2018;14(4):585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 51. Campbell IG, et al. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88(2):1073–1076. [DOI] [PubMed] [Google Scholar]
- 52. Vyazovskiy VV, et al. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200–208. [DOI] [PubMed] [Google Scholar]
- 53. Van Dongen HP, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2): 117–126. [DOI] [PubMed] [Google Scholar]