Abstract
Study Objectives
Insomnia is a prominent complaint in patients with alcohol use disorders (AUD). However, despite the importance of sleep in the maintenance of sobriety, treatment options for sleep disturbance associated with a history of AUD are currently limited. Recent clinical trials have demonstrated that suvorexant, a dual Hct/OX receptor antagonist, normalizes sleep in patients with primary insomnia; yet, its potential for the treatment of sleep pathology associated with AUD has not been investigated in either preclinical or clinical studies.
Methods
This study employed a model whereby ethanol vapor exposure or control conditions were administered for 8 weeks to adult rats. Waking event-related oscillations (EROs) and EEG sleep were evaluated at baseline before exposure and again following 24 hr of withdrawal from the exposure. Subsequently, the ability of vehicle (VEH) and two doses (10, 30 mg/kg IP) of suvorexant to modify EROs, sleep, and the sleep EEG was investigated.
Results
After 24 hr following EtOH withdrawal, the ethanol-treated group had increases in waking ERO θ and β activity, more fragmented sleep (shorter duration and increased frequency of slow wave (SW) and rapid eye movement [REM] sleep episodes), and increased θ and β power in REM and SW sleep. Suvorexant induced a dose-dependent decrease in the latency to REM and SW sleep onsets but also produced REM and SW sleep fragmentation and increased β energy in waking EROs when compared with VEH.
Conclusions
Taken together, these studies suggest that suvorexant has overall sleep-promoting effects, but it may exacerbate some aspects of sleep and EEG pathology.
Keywords: alcohol, EEG, event-related oscillations, suvorexant, slow-wave sleep
Statement of Significance.
Insomnia is one of the problems that is associated with alcohol use disorder. However, the mechanisms underlying alcohol-associated sleep disturbances and potential targets for therapy remain under-investigated. Recent clinical trials have demonstrated that the dual Hypocretin/Orexin receptor antagonist suvorexant may have therapeutic value in the treatment of primary insomnia; yet, the use of this class of drugs in the treatment of alcohol-associated sleep disturbances has not been studied in an animal model. We examined the role of suvorexant on alcohol-associated insomnia in rats. We found that suvorexant promotes sleep, however, increased rapid eye movement, and slow-wave sleep fragmentation. Future studies are needed to explore the role of hypocretin/orexin-receptor 1 or receptor 2 on alcohol-associated sleep pathology.
Introduction
Insomnia is a prominent complaint of patients with alcohol use disorder (AUD). AUD-induced insomnia can also fail to resolve over the course of recovery, is a leading cause of patients’ relapse to drinking [1], and may have psychosocial and psychiatric consequences [2]. It has been reported that after acute withdrawal, chronic alcohol users may complain of light, fragmented sleep and demonstrate a deficit in slow-wave sleep (SWS) that can persist for months [3, 4]. Insomnia is one of the eight core criteria in the diagnosis of alcohol withdrawal syndrome [5] and refers to nonrestorative or poor-quality sleep as reported by the patient [6, 7] that occurs even in the absence of mood disorders [8]. There are a number of studies that have demonstrated that alcohol relapse is more likely among persons with persistent sleep disturbances who are recovering from an AUD [9]. A number of sleep measures have been employed in these studies, and decreased sleep efficiency, decreased total sleep time, increased rapid eye movement (REM) sleep, increased sleep pressure, and decreased SWS have all been found to be good predictors of alcohol relapse [1]. Foster and colleagues [10], using the Nottingham Health Profile Questionnaire (NHP), found that sleep latency was the most significant predictor of relapse. Additional studies that used spectral power analysis of the sleep electroencephalogram found enhancement in the β2 band (24–32 Hz) during REM sleep in those patients with AUDs who relapsed when compared with either alcohol abstainers or controls, suggesting that sleep may be lighter in AUD [11]. Other studies have shown that REM measures may be particularly good markers of relapse to AUD in nondepressed inpatients with primary alcoholism [12]. Furthermore, patients who reported using alcohol to help them fall asleep have also been found to be more vulnerable to relapse to AUD [13, 14]. Despite the importance of sleep in the maintenance of sobriety, treatment options for sleep disturbance in patients recovering from AUD are currently limited [6, 15]. This is especially problematic since some drugs, in particular hypnotics, may not be suitable for the treatment of sleep disturbance associated with AUD because of their addiction liability and potential interactions with alcohol [16].
FDA-approved pharmacological treatments for primary insomnia include benzodiazepines and nonbenzodiazepine hypnotics, tricyclic antidepressants, off-label use of drugs such as other antidepressants, antihistamines, herbal preparations, and antipsychotics and more recently therapeutic drugs that target orexin/hypocretin receptors [17–19]. Although evidence of sleep improvement is presented for individual drugs for insomnia [18, 20, 21], no evidence-based clinical practice guidelines have been published to date by the American Academy of Sleep Medicine (AASM) to draw conclusions regarding the overall efficacy of pharmacotherapy in the insomnia population [18]. It has been suggested that insomnia disorder and AUD might be best thought of as comorbid disorders, each of which requires its own treatment rather than categorizing insomnia as a symptom of a primary illness [5, 6, 15]. The development of animal models of alcohol-induced insomnia [22–24] allows for the experimental control necessary to study the effects of ethanol, independent of many factors that confound human studies, such as psychiatric comorbidity and other substance use.
Recent literature supports a prominent role for the hypothalamic peptide hypocretin/orexin (Hct/OX) system [25, 26] as a master regulator of the sleep–wake cycle [27–29]. Mice lacking Hct/OX peptides [30, 31] or both Hct/OX-R1 and –R2 receptors (Hct/OX-R1, Hct/OX-R2) display a “narcoleptic-like” phenotype with cataplexy [32, 33]. Additionally, disrupted Hct/OX-R2 signaling has been demonstrated to be a cause of familial canine narcolepsy [34]. These findings have spurned efforts to discover Hct/OX receptor agonists to treat narcolepsy and, alternatively, Hct/OX receptor antagonists to treat insomnia disorders [35]. Antagonism of Hct/OX receptors is hypothesized to facilitate sleep by transiently blocking orexinergic activity in the lateral hypothalamus that is postulated to mediate the transition between arousal and sleep [36–38].
A recent body of literature also supports a role for the Hct/OX system in a number of behaviors relevant to AUD including reward/motivated behavior [39, 40], addiction [41–43], feeding, and energy metabolism (for review see [44]). There are also data to suggest that there is specificity with regard to Hct/Ox receptor signaling and these target behaviors. Although a stronger overall role for OX-R1 signaling has been found for addictive behaviors [45–51], both Hct/OX-R1 and Hct/OX-R2 signaling have been shown to modify alcohol drinking [42]. Most data suggest that OX-R1 receptor signaling may play a greater role in behaviors directed towards highly salient reinforcers, such as cocaine self-administration, consumption of a high fat diet [45, 46, 52, 53], the motivational properties of opiates and cue induced reinstatement of heroin seeking [54], higher alcohol preference and intake [49], and increased alcohol drinking in dependent mice [55].
The Hct/OX neuropeptide system may be an attractive target for treatment of insomnia associated with AUD, since it may theoretically target both the insomnia associated with AUD and the motivational properties underlying the drive to use alcohol. Additionally, drugs targeting Hct/OX may have less addiction liability than traditional hypnotics and perhaps less potential for daytime sleepiness. The drug suvorexant is the first approved selective dual Hct/OX receptor antagonist (DORA) that has shown therapeutic value in normalizing sleep in primary insomnia [19, 56, 57]. Although suvorexant has been demonstrated to improve sleep in rats [56, 58, 59] and its potential for the treatment of chronic ethanol-induced sleep pathology has been proposed [60], it has not been experimentally investigated in either preclinical or clinical studies of AUD sleep pathology.
Studies from our laboratory, and others, in rats, have demonstrated that chronic intermittent ethanol vapor exposure in adults results in increases in the latency to sleep onset, increases in the number of SWS episodes, and reductions in the amount of time spent in each SWS episode [22, 61–63]. Despite the development of these animal models, their use in identifying the mechanisms underlying alcohol-induced insomnia, and in the investigation of potential therapeutic targets, has been limited.
In the present study, we hypothesized that chronic alcohol exposure and withdrawal would enhance activity in the Hct/OX system causing an increased excitatory drive within wake-promoting neurons and resulting in insomnia. We then postulated that blocking Hct/OX-R1/R2, with the dual orexin receptor antagonist, suvorexant, would reduce the excitatory drive caused by the chronic alcohol exposure and withdrawal thus promoting sleep. If our hypothesis is true, then it would represent a new therapeutic strategy for alleviating protracted alcohol withdrawal-induced insomnia. We used an animal model of chronic alcohol exposure to study the effects of two doses of suvorexant on waking and sleep physiology. For the evaluation of suvorexant on waking electrophysiology, we used measures of event-related oscillations (EROs). EROs are oscillatory changes in EEG rhythms that are synchronized or enhanced by a time-locked cognitive and/or sensory stimulus (see [64–67]). EROs have shown to be good measures of normal [68, 69] and abnormal cognitive functioning, as well as specific endophenotypes for AUDs [70–72]. We also evaluated the effects of suvorexant on sleep parameters and the spectral content of the sleep EEG during REM and SWS episodes in rats who were exposed to chronic alcohol vapor and withdrawal or control conditions.
Methods
Animal subjects
Forty-four male Wistar rats were obtained from Charles River (USA) and arrived on postnatal day (PD) 60. Rats were pair-housed in standard plastic cages in a temperature-controlled room with a 12 hr light/dark cycle. All testing was conducted at the onset of the light cycle, which occurred at 08:00 am. Food and water were available ad libitum. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996) and was reviewed and approved by The Scripps Research Institute’s Animal Care and Use Committee.
Surgical procedure
The general methods used for surgical and electrophysiological recording procedures in our studies have been described previously [22, 62]. In this study, rats (PD 146–155) were surgically implanted with screw electrodes in the skull overlying the frontal (FCTX, AP: 1.5 mm, ML: ± 3.0 mm, FR1) and parietal cortex (PCTX, AP: −4.5 mm, ML: ± 4.5 mm) with a corresponding reference electrode placed posterior to lambda overlying the cerebellum. Surgical coordinates were obtained from the Paxinos and Watson [73] atlas. An electromyography (EMG) wire electrode was inserted into the rats’ neck muscle. EEG and EMG electrodes were connected to a multipin PlasticsOne connector and the assembly was anchored to the skull with dental acrylic and anchor screws. Rats were given at least 2 weeks of recovery before EEG recording.
Ethanol vapor exposure
Ethanol vapor exposure has been shown to reliably allow for the titration of blood ethanol concentrations (BECs) that are sufficient for inducing physical dependence. The ethanol vapor inhalation procedures and the chambers used in this study have been described previously [74]. In this study, the ethanol vapor chambers were calibrated to produce high to moderate BECs between 175 and 225 mg/dL. In brief, rats (n = 44) were divided into two groups when they arrived in the vivarium, and then the two groups were balanced to ensure that the ethanol and control groups were not significantly different based on body weight (Ethanol group [vapor exposure], n = 24; Control [air-exposed] group, n = 20). After the baseline recordings, ethanol rats were housed in sealed chambers, infused with vaporized 95% ethanol from 08:00 pm to 10:00 am. For the remaining 10 hr of the day, ethanol vapor was not infused into the chambers. Rats were exposed to this vapor cycle for 8 weeks (PD 177–236). Blood samples were collected from the tip of the tail every 3–4 days during the 8 week exposure period to assess BECs (average for 8 weeks, 182.3 ± 6.9 mg/dL). Control (air-exposed) animals were handled identically as described for the ethanol rats except that they were maintained in standard cages throughout the experiment. BECs were determined using the Analox micro-statAM1 (Analox Instr. Ltd., Lunenberg, MA). Following the 8 week exposure, ethanol animals were transferred to standard polycarbonate cages for the duration of the experiment.
Electrophysiological recordings
After recovery from surgery, rats were habituated to the EEG testing chambers prior to the electrophysiological testing. For waking EROs, animals were placed in individual polycarbonate chambers within a sound and electrically shielded recording chamber. Rats were unrestrained but were spatially restricted. Rats were first presented with auditory stimuli through a small speaker centered approximately 70 cm above the rat’s head. Auditory stimuli and EROs were elicited using an oddball plus “noise” paradigm described previously [75, 76]. Each auditory ERO session consisted of 312 trials that lasted approximately 10 min and each trial presented one of the three randomly generated tones: standard tone (84% probability, 75 dB, 1000 Hz), rare tone (10% probability, 85 dB, 2000 Hz), or noise tone (6% probability, 100 dB, white noise). Individual trials were 1000 ms in duration (200 ms prestimulus + 800 ms poststimulus) and the interval between tones varied from 750 to 1500 ms. A 5 hr EEG sleep recording occurred about 5 min after the conclusion of the ERO session. All rats were determined to be awake at the start of each EEG session.
Rats were given a baseline electrophysiology session (EROs, EEG sleep) prior to any ethanol or control exposure (PD 164–175). A second EEG, ERO, and 5 hr sleep recording session was conducted 24 hr after withdrawal from the vapor/air chambers (PD 234–242). Approximately 1 month after acute alcohol/control withdrawal, all rats had three additional recording sessions (ERO, 5 hr sleep): following the administration of vehicle (5% DMSO in saline), low (10mg/kg) and high (30 mg/kg) doses of suvorexant (PD 264–291) (described below) 1 hr prior to the recordings. For all sessions, the EEG recordings began at “lights on” at 08:00 and all rats were awake at the start of the recording. The EEG was recorded from two monopolar leads referenced to cerebellum ground (i.e. frontal cortex and parietal cortex) on a Sensorium preamplifier/amplifier unit (Shelburne, VT). Signals were transferred to a PC and digitized at a rate of 256 Hz. The EEG amplifier input range corresponding to the full range of the 12-bit analog-to-digital converter was about +/− 250 μV. Periodic calibration results were used to scale the digitized EEG to microvolts.
Acute suvorexant and vehicle administration
Sessions were conducted with a randomized order of administration for two doses of suvorexant (Astatech Inc., Bristol PA): low dose (10 mg/kg), high dose (30 mg/kg), and vehicle. Following the injection, rats were returned to their home cage for approximately 1 hr before being placed in the electrophysiological recording chamber. At least a week was given between doses to avoid carryover effects from the drug. The suvorexant concentrations were prepared fresh for each day of testing for groups containing three to six rats each, and the vehicle doses were given in equal frequency and at equivalent volumes to the suvorexant doses.
Waking EEG analyses of ERO energy
Data from single trials generated by the ERP stimuli were entered into a time frequency analyses algorithm, S-transform (ST), a generalization of the Gabor transform [77]. The S-transform mathematically resembles the continuous wavelet transform but it uses Gaussian windows which do not meet a requirement of wavelet analysis, and it includes a “phase correction” that is not part of wavelet analysis. The actual use of the S-transform was simplified by performing first a forward Fourier transform of the time series. Then, for each frequency of the Fourier transform, the results of multiplication were summed by a set of Fourier transforms of Gaussian windows of varying width. Finally, for each of these sums, the inverse of the Fourier transform was taken. The S-transform resulted in a time–frequency representation of the data. The exact code we used is a C language, S-transform subroutine available from the NIMH MEG Core Facility website (http://kurage.nimh.nih.gov/meglab/). This code is specifically for use with real time series, so it sets the input imaginary values, required by the S-transform, to zero, and it always uses the Hilbert transform so that each of the complex output time series is an analytic signal.
To reduce anomalies in the S-transform output at the beginning and the end of the output time series, we used a Hanning window over the initial and final 100 ms of the input time series. The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time–frequency energy distributions. To quantify S-transform magnitudes, a region of interest (ROI) is defined by specifying the band frequencies and time interval dimensions of the rectangular ROI. The time–frequency points saved from each S transformation are from 200 ms before to 800 ms after the onset of the stimulus, and from 1 through 50 Hz at intervals of 0.5 Hz. Energy is the square of the magnitude of the S-transform output in a time–frequency ROI. The S-transform output for a time/frequency ROI, for a specific EEG lead, is proportional to the input voltage of the lead over the time/frequency interval. The S-transform magnitude squared for a time/frequency interval is therefore proportional to volts squared. These analyses are similar to what has been described previously [78].
Rectangular ROIs were defined within the time–frequency analysis plane by specifying, for each ROI, a band of frequencies and a time interval relative to the stimulus onset time. Time 0 in these definitions is the onset of the stimulus. The 3 ROIs were δ band, 1–4 Hz, 200–500 ms; θ band, 4–7 Hz, 10–400 ms; and β band, 13–30 Hz, 0–300 ms. These regions were chosen a priori to coincide with the major EEG frequencies present in the rat and the latency windows of the N1, P3a, and P3b event-related potential components in the rat reported previously [75]. Using mean values over trials, the maximum energy values were calculated for each ROI, at each electrode location.
Rat sleep and EEG analyses
SWS was visually identified as synchronized slow-wave activity (1–4 Hz) during the 5 hr EEG recording session that included baseline, 24 hr withdrawal after ethanol vapor or control exposure, vehicle injection control, suvorexant 10 mg/kg, and suvorexant 30 mg/kg. Increases in EEG power of at least twice the amplitude of waking baseline EEG power lasting longer than 8 s were counted as episodes of SWS. REM was visually identified as synchronized θ activity (4–8 Hz) in the absence of muscle activity and preceded by an episode of SWS. Sleep patterns identified and analyzed for SWS and REM states: (1) onset latency of the first episode, (2) mean duration of all episodes, (3) total number of instances of each state, and (4) fragmentation ratio (total episodes/mean duration). The onset of the first SWS episode was identified from the raw EEG as the first transition from low-amplitude high-frequency EEG to SWS (high-amplitude low-frequency EEG) and lasting at least 8 s.
The 5 hr EEG recording was also analyzed for spectral characteristics. Raw EEG signals were amplified (50% gain), band-pass filtered (0.53–70 Hz), digitized at a rate of 256 Hz, and then transferred to an IBM-compatible PC. A Fourier transform of 4 s epochs was used to generate the power spectrum. Mean power density was quantified in μV2/octave and was assessed for three frequency bands: δ (1–4 Hz), θ (4–8 Hz), and β (16–32 Hz). EEG spectra were identified as containing artifact when average cortical power was > 2000 μV2/octave and were excluded only after visual analysis of the raw EEG and spectral distributions. Mean spectral power within each band was calculated for all SWS and REM epochs over the entire 5 hr recording session. These analysis procedures have been described previously [79].
Statistical analyses
Data analyses were based on the three aims of the study which were to test the effects of alcohol withdrawal, vehicle (VEH) administration, and two doses of suvorexant (10, 30 mg/kg) on: (1) waking EROs, (2) REM and SW sleep parameters, and (3) sleep EEG spectral characteristics, in the ethanol and control groups. For the ERO analyses, energy in the three time–frequency regions of interest (δ, θ, β) was compared in response to the infrequent tone, in the two leads (frontal cortex [FCTX] and parietal cortex [PCTX]) in the alcohol vapor and control animals for the 5 conditions using a group (ethanol and control) × 5 conditions (baseline, withdrawal, vehicle, suvorexant 10, and suvorexant 30) ANOVA. Similarly, alcohol vapor and control animals were compared on the REM and SW sleep measures (latency to onset, mean duration of episodes, number of episodes, and sleep fragmentation) between the two exposure groups also using a 2 group (ethanol and control) × 5 conditions (baseline, withdrawal, vehicle, suvorexant 10, and suvorexant 30) ANOVA. Power in the EEG in the three frequency bands (δ, θ, β) over all the SW and REM sleep episodes in leads FCTX and PCTX was also evaluated for the 5 conditions using a 2 group (ethanol and control) × 5 conditions (baseline, withdrawal, vehicle, suvorexant 10, and suvorexant 30) ANOVA. Post hoc analyses for group and/or condition were used when significant main effects were found. Significance was set at p < 0.05. Statistical analyses were performed using SPSS (IBM Corp, Armonk, NY).
Results
Effects of alcohol withdrawal and two doses of suvorexant on waking EROs
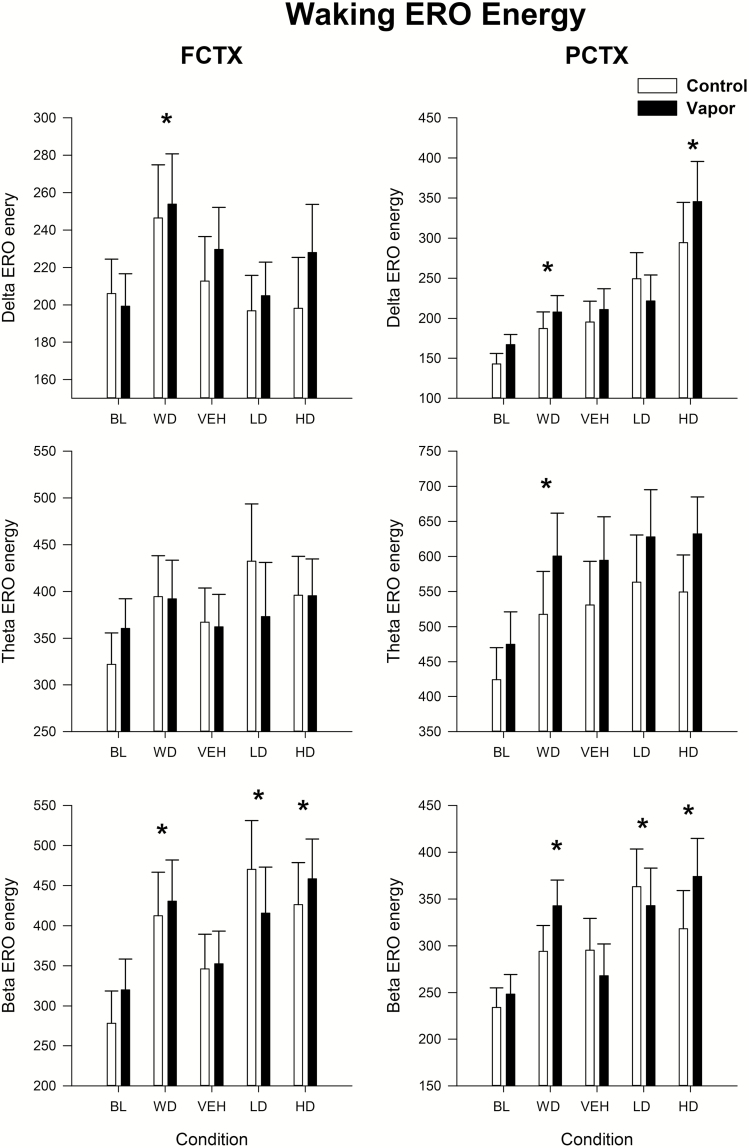
Our first aim evaluated the effects of alcohol withdrawal as well as response to vehicle and two doses of suvorexant on the waking EEG as indexed by ERO energy. Data were evaluated in the control and EtOH-exposed groups in frontal (FCTX) and parietal (PCTX) cortex, in the δ, θ, and β time–frequency regions of interest. Repeated measure ANOVA revealed no effect of group but an effect of condition (baseline [BL], withdrawal [WD], Vehicle [VEH], Suvorexant 10 mg/kg, 30 mg/kg) on ERO energy in δ frequency band, to the infrequent tone, in frontal cortex [condition: F(4,136) = 3.4, p < 0.019] and parietal cortex [condition: F(4,128) = 11.25, p < 0.001]. Post hoc revealed that the withdrawal condition had significantly more energy when compared with baseline [FCTX: F(1,35) = 9.9, p < 0.003, PCTX: F(1,33) = 12.4, p < 0.001] and the 30 mg/kg dose had higher energy than the VEH condition [PCTX: F(1,33) = 10.4, p < 0.003; see Figure 1]. Repeated measures ANOVA revealed a condition effect on ERO energy in the θ frequency band in PCTX [F(4,128) = 7.4, p < 0.0001], and post hoc showed that energy in the withdrawal condition was significantly higher than in the baseline condition [PCTX: F(1,33) = 20.6, p < 0.0001] but not when comparing the VEH and drug conditions. Significant effects of condition were also seen in the β frequencies in both frontal cortex [condition: F(4,136) = 6.5, p = 0.001] and parietal cortex [condition: F(4,128) = 6.3, p < 0.001]. Post hoc revealed that β energy was higher during withdrawal when compared with baseline [FCTX: F(1,35) = 20.2, p < 0.0001; PCTX: F(1,33) = 24.4, p < 0.0001] and also when VEH was compared with suvorexant at the 10 mg/kg dose [FCTX: F(1,35) = 5.7, p < 0.02; PCTX = F(1,33) = 7.3, p < 0.01] and the 30 mg/kg dose [FCTX: F(1,35) = 7.8, p < 0.008; PCTX: F(1,33) = 5.0, p < 0.03]. The cause for the increase seen in the withdrawal condition in both treatment groups is not clear but it most likely represents a brain maturation effect.
Figure 1.
Waking ERO energy following alcohol vapor exposure and administration of two doses of suvorexant. Means and standard errors are shown for the δ, θ, and β regions of interest for the following conditions: baseline prior to vapor/air exposure, during withdrawal of vapor exposure, and following vehicle and two doses of suvorexant (10, 30mg/kg) in vapor-exposed animals and their controls. Asterisk, * indicates a significant (p < 0.05) post hoc by condition finding when compared with baseline or vehicle condition. FCTX = frontal cortex; PCTX = parietal cortex; BL = baseline; WD = withdrawal; VEH = vehicle; LD = low-dose suvorexant 10 mg/kg; HD = high-dose suvorexant 30 mg/kg.
Effects of withdrawal and suvorexant on REM and SW sleep patterns
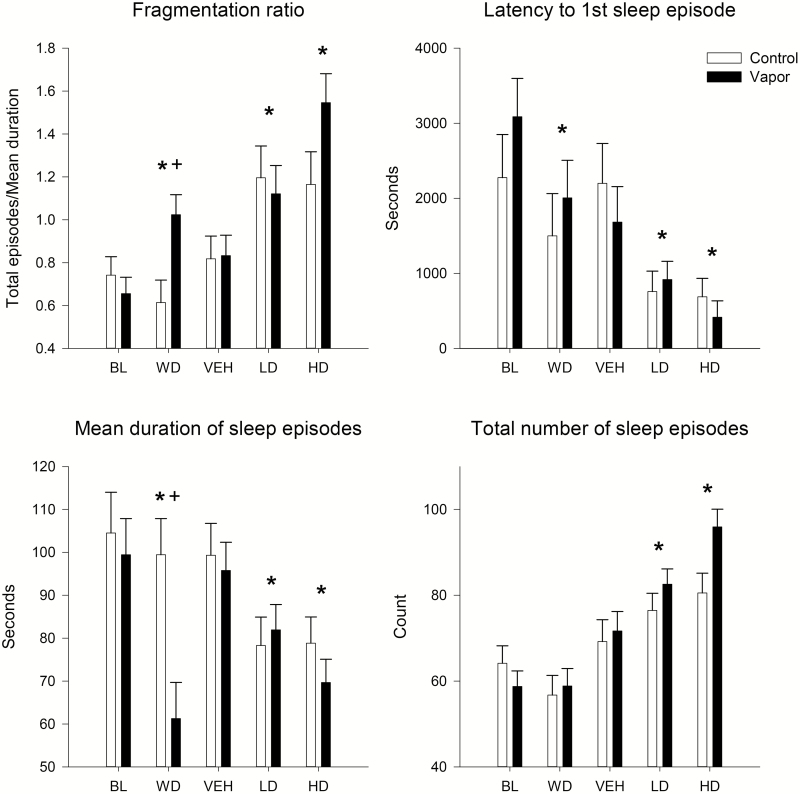
Our second aim evaluated the effects of alcohol withdrawal as well as response to vehicle and two doses of suvorexant on EEG sleep patterns as indexed by the following SWS and REM states: (1) onset latency of the first episode, (2) mean duration of all episodes, (3) total number of instances of each state, and (4) fragmentation ratio (total episodes/mean duration). To test for group and condition differences, a 2 (ethanol vs control) by 5 [baseline (BL), withdrawal (WD), vehicle (VEH), suvorexant 10mg/kg, suvorexant 30 mg kg] repeated measure ANOVA was used. An effect of condition, but not group, was found for the latency to onset of SW sleep [F(4,128) = 10.6, p < 0.0001]. Post hoc analyses revealed that a reduction in the latency to the onset of SW sleep was seen during the withdrawal condition when compared with BL [F(1,33) = 8.1, p < 0.008]. Suvorexant was found to reduce the latency to onset of SW sleep when VEH was compared with the 10 mg/kg dose [F(1,33) = 9.6, p < 0.004] and the 30 mg/kg dose (F = 12.3, p < 0.001). An effect of both condition [F(4,128) = 9.9, p < 0.001] and group × condition [F(4,128) = 10.7, p < 0.001] was observed for the mean duration of SW sleep episodes. During withdrawal SW sleep episodes were found to be overall shorter [F(1,33) = 4.6, p < 0.04] when compared with baseline. The ethanol-exposed group, while not different from controls at baseline, had significantly shorter SW episodes during withdrawal when compared with the controls [F(1,33) = 19.8, p < 0.0001]. Suvorexant was also found to shorten the length of SW sleep episodes when VEH was compared with the 10 mg/kg dose [F(1,33) = 12.8, p < 0.001] and the 30 mg/kg dose [F(1,33) = 31.0, p < 0.0001]. An effect of both condition [F(4,128) = 33.2, p < 0.0001] and group × condition [F(4,128) = 3.0, p < 0.03] was also observed for the number of SW sleep episodes. Post hoc analyses showed that suvorexant increased the number of SW sleep episodes when VEH was compared with the 10 mg/kg dose [F(1,33) = 10.5, p < 0.003] and the 30 mg/kg dose [F(1,33) = 26.2, p < 0.0001]. The decrease in the duration of SWS episodes concomitant with an increase in the number of episodes resulted in significant sleep fragmentation as a function of both condition [F(4,128) = 23.2, p < 0.0001] and group × condition [F(1,33) = 4.7, p < 0.003]. SW sleep was found to be significantly more fragmented in the ethanol-exposed animals during WD when compared with the controls [F(1,33) = 8.6, p < 0.006], and suvorexant was found to increase fragmentation when VEH was compared with the 10 mg/kg dose [F(1,33) = 14.0, p < 0.001] and the 30 mg/kg dose [F(1,33) = 37.7, p < 0.0001; see Figure 2].
Figure 2.
The effects of alcohol withdrawal and subsequent suvorexant administration on slow-wave sleep patterns. Latency to first slow-wave sleep episode, total number of sleep episodes, their average duration, and sleep fragmentation ratio (total episodes/average duration) are shown for the alcohol vapor–exposed animals and their controls. Means and standard errors shown, asterisk, * indicates significant (p < 0.05) post hoc by condition compared with baseline or vehicle condition, plus sign, + indicates significant (p < 0.05) post hoc by group treatment. FCTX = frontal cortex; PCTX = parietal cortex; BL = baseline; WD = withdrawal; VEH = vehicle; LD = low-dose suvorexant 10 mg/kg; HD = high-dose suvorexant 30 mg/kg.
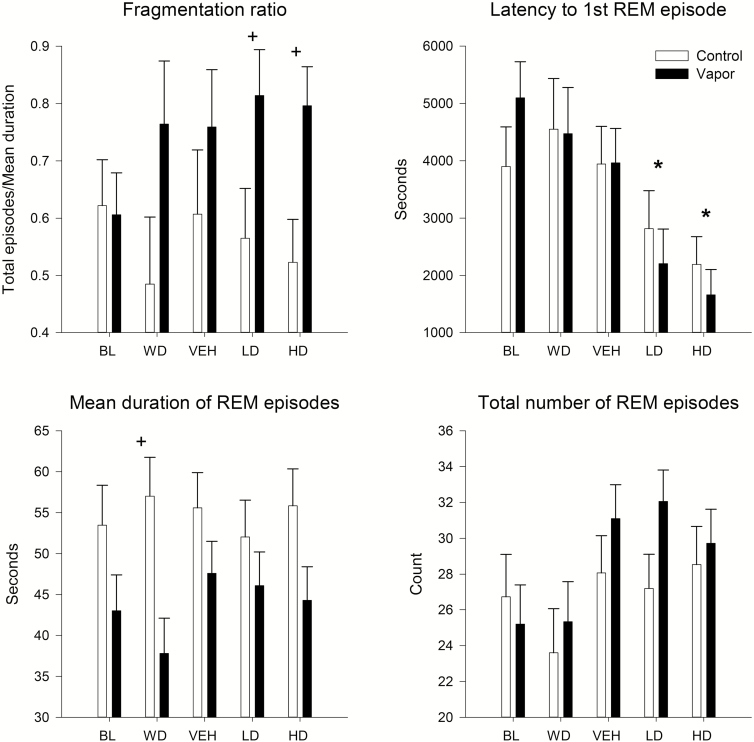
Tests for group and condition differences in REM sleep patterns were also conducted using a 2 (ethanol vs control) by 5 [baseline (BL), withdrawal (WD), vehicle (VEH), suvorexant 10 mg/kg, suvorexant 30 mg kg] repeated measure ANOVA. A significant effect of condition, but not group, was found for the latency to onset of REM sleep [F(4,124) = 9.1, p < 0.0001]. Post hoc analyses revealed that suvorexant reduced the latency to the onset of REM sleep when VEH was compared with the 10 mg/kg dose [F(1,32) = 7.5, p < 0.01] and the 30 mg/kg dose [F(1,32) = 16.7, p < 0.0001]. A significant effect of group was found for the overall mean duration of REM sleep episodes [F(1,31) = 5.0, p < 0.03], and post hoc showed that the ethanol-treated rats had shorter REM episodes during withdrawal when compared with controls [F(1,32) = 8.9, p < 0.006] but not at other time points. An effect of condition [F(4,124) = 3.9, p < 0.008] was also observed for the number of REM sleep episodes, but the post hoc analyses were not significant. Significant REM sleep fragmentation was observed as a function of group [F(1,31) = 4.2, p < 0.05] with ethanol animals having overall more fragmentation, as well as more fragmentation at the suvorexant 10 mg/kg dose [F(1,32) = 4.5, p < 0.04] and 30 mg/kg dose [F(1,32) = 7.2, p < 0.01] in post hoc analyses (see Figure 3).
Figure 3.
Effects of alcohol withdrawal and subsequent suvorexant administration on REM patterns. Latency to first REM episode, total number of REM episodes, their average duration, and REM sleep fragmentation ratio (total episodes/average duration) are shown for the alcohol vapor–exposed animals and their controls. Means and standard errors shown, asterisk, * indicates significant (p < 0.05) post hoc by condition compared with baseline or vehicle condition, plus sign, + indicates significant (p < 0.05) post hoc by group treatment. FCTX = frontal cortex; PCTX = parietal cortex; BL = baseline; WD = withdrawal; VEH = Vehicle; LD = low-dose suvorexant 10 mg/kg; HD = high-dose suvorexant 30 mg/kg.
Effects of ethanol withdrawal and suvorexant on sleep EEG spectra during SW and REM sleep
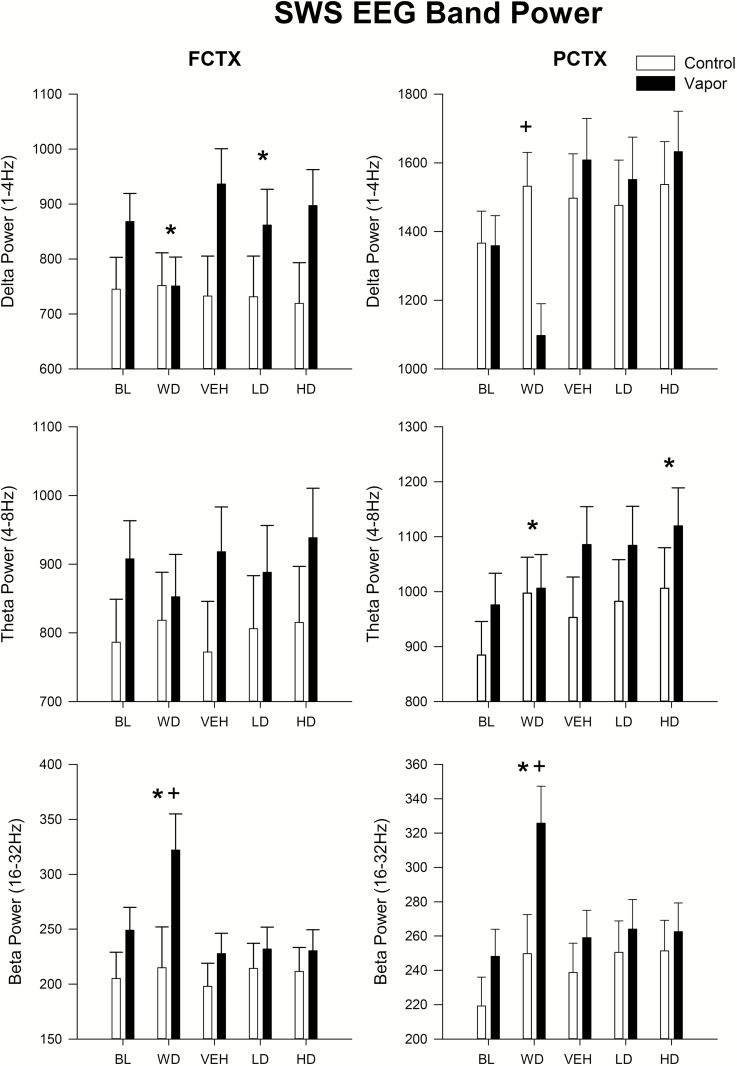
An evaluation of the EEG sleep spectra during SW sleep episodes demonstrated that a significant effect of both condition [FCTX: F(4,120) = 3.8, p < 0.03; PCTX: F(4,120) = 14.1, p < 0.0001] and group × condition [FCTX: F(4,120) = 6.4, p < 0.002; PCTX: F(4,120) = 13.0, p < 0.0001] was seen in EEG δ power (see Figure 4). Post hoc analyses showed that power in the δ frequencies was lower during SW sleep in the withdrawal condition when compared with baseline [FCTX: F(1,31) = 9.1, p < 0.005] and that the ethanol-treated animals had lower power than controls in the withdrawal condition [PCTX: F(1,31) = 10.4, p < 0.003]. Suvorexant was also found to lower δ power when the VEH condition was compared with 10 mg/kg [FCTX: F(1,31) = 6.8, p < 0.014], but other time points were not found to be significant.
Figure 4.
Spectra power during slow-wave sleep episodes following alcohol vapor exposure and the subsequent administration of two doses of suvorexant. Means and standard errors shown for in the δ, θ, and β bands for the following conditions: baseline prior to vapor exposure, during withdrawal of vapor exposure and following administration of vehicle and two doses of suvorexant (10, 30mg/kg) in vapor-exposed animals and their controls. Asterisk, * indicates a significant (p < 0.05) post hoc by condition effect when comparing baseline or vehicle condition, plus sign, + indicates a significant (p < 0.05) post hoc by group treatment. FCTX = frontal cortex; PCTX = parietal cortex; BL = baseline; WD = withdrawal; VEH = vehicle; LD = low-dose suvorexant 10 mg/kg; HD = high-dose suvorexant 30 mg/kg.
An evaluation of the EEG sleep spectra during SW sleep episodes demonstrated that a significant effect of condition occurred in EEG θ power in parietal cortex [F(4,120) = 9.8, p < 0.001]. Post hoc analyses showed that more θ activity was seen during withdrawal in parietal cortex [F(1,31) = 9.1, p < 0.005] and at the 30 mg/kg dose when compared with VEH [F(1,31) = 11.7, p < 0.002]. Significant effects of both condition [FCTX: F(4,120) = 9.9, p < 0.001; PCTX: F(4,120) = 19.0, p < 0.0001] and group × condition [FCTX: F(4,120) = 7.1, p < 0.004; PCTX: F(4,120) = 8.7, p < 0.001] were also seen in EEG β power during SW episodes as seen in Figure 4. An overall increase in β power was seen during withdrawal when compared with baseline [FCTX: F(1,31) = 11.7, p < 0.002; PCTX: F(1,31) = 36.1, p < 0.0001]. Ethanol-treated rats also had significantly more β power in their SW sleep than their controls at the withdrawal time point [FCTX: F(1,31) = 4.6, p < 0.04; PCTX: F(1,31) = 5.8, p < 0.02], but no other time points were found to be significant.
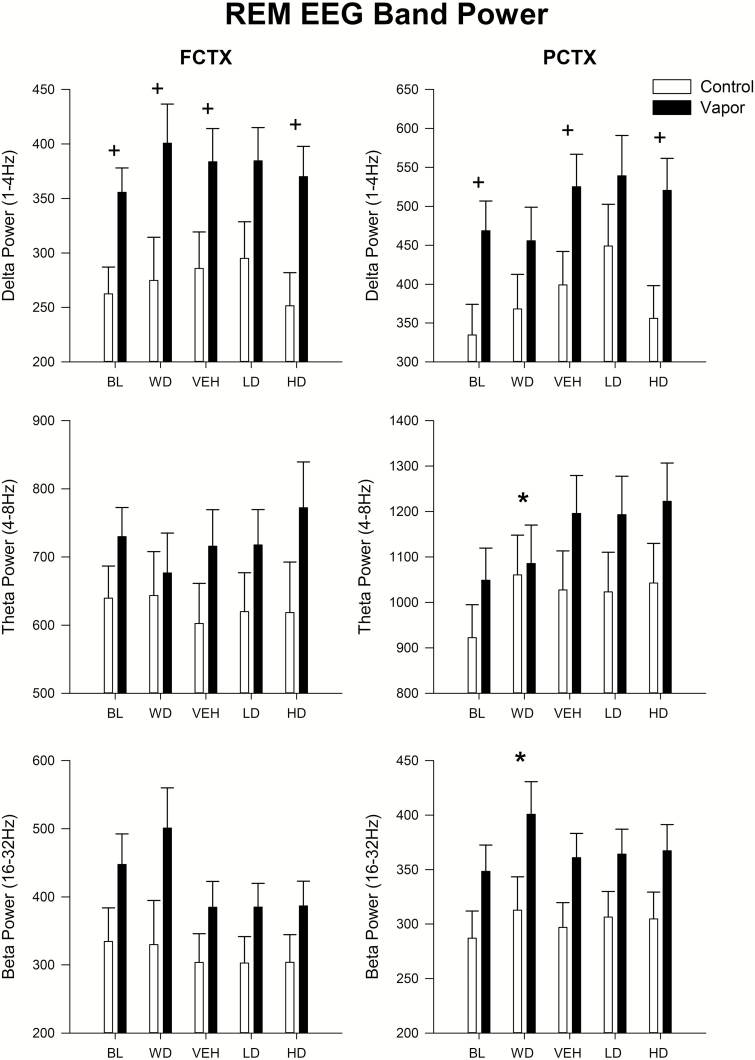
Spectral changes in REM sleep as a function of ethanol exposure and suvorexant administration were also determined. A significant overall effect of group was seen in the δ frequencies in both frontal [F(1,29) = 7.8, p < 0.009] and parietal cortex [F(1,29) = 5.34, p < 0.03]. Post hoc analyses showed that δ power was significantly higher during REM episodes in the ethanol-treated group at baseline [FCTX: F(1,30) = 7.9, p < 0.01; PCTX: F(1,30) = 6.0, p < 0.02) and withdrawal [FCTX: F(1,30) = 5.5, p < 0.03] and at the 30 mg/kg dose of suvorexant [FCTX: F(1,30) = 8.3, p < 0.008; PCTX: F(1,30) = 7.8, p < 0.009]. An evaluation of the EEG sleep spectra during REM sleep episodes demonstrated that a significant effect of condition was found in EEG θ power in parietal cortex [F(4,116) = 10.75, p < 0.001] as well as a group × condition interaction [F(4,116) = 3.3, p < 0.03]. Post hoc analyses showed that more θ activity was seen during withdrawal in parietal cortex [F(1,30) = 10.6, p < 0.003) compared with baseline. Significant effects of condition [FCTX (4,116): F = 8.3, p < 0.001; PCTX: F(4,116) = 5.0, p < 0.002] were also seen in EEG β power during REM episodes as seen in Figure 5. An overall increase in β power was seen during withdrawal when compared with baseline [PCTX: F(1,30) = 16.1, p < 0.0001), but no other time points were significant.
Figure 5.
Spectra power during REM episodes following alcohol vapor exposure and two doses of suvorexant. Means and standard errors shown for in the δ, θ, and β bands prior to vapor exposure, during withdrawal of vapor exposure and following vehicle and two doses of suvorexant (10, 30 mg/kg) in vapor-exposed animals and their controls. *Significant (p < 0.05) post hoc by condition compared with baseline or vehicle condition, + significant (p < 0.05) post hoc by group treatment. FCTX = frontal cortex; PCTX = parietal cortex; BL = baseline; WD = withdrawal; VEH = vehicle; LD = low-dose suvorexant 10 mg/kg; HD = high-dose suvorexant 30 mg/kg.
Discussion
During chronic alcohol exposure, key neurobiological mechanisms underlying homeostatic regulation become dysregulated, that following cessation of exposure results in a protracted alcohol “withdrawal-like state” that includes changes in sleep [22]. We have shown that there is a critical length of time necessary for an animal to be exposed to alcohol vapor in order to induce neuroadaptive changes in both behavior and neurochemistry that persist into protracted withdrawal [80] which is between 6 and 8 weeks of vapor exposure. Here we show that after 8 weeks of chronic alcohol exposure, during acute withdrawal, there was a significant decrease in the duration of SWS episodes in the ethanol-exposed group compared with control rats. The analysis of the cortical power in the ethanol-exposed group revealed an increase in the θ and β frequency bands both during waking EROs and in SW and REM sleep spectra. Since EEG θ and β power are considered an index of cortical arousal [81–83], this indicates that the ethanol-exposed rats may have a decreased tendency to sleep and increased tendency to be awake. This finding supports the hypothesis that AUD-associated insomnia may be the result of a mismatch involving persistent activity in wake-promoting structures during SWS (also referred as nonrapid eye movement, NREM) [84]. This phenomena may also be responsible for the increase in REM and SW sleep fragmentation (shorter duration and increased frequency of SW and REM sleep episodes) seen in the ethanol-exposed animals after withdrawal of the ethanol.
The anatomical localization of the Hct/OX system, and its receptors, is in key wake-promoting structures of the brain, suggests a role for the involvement of Hct/OX system in the promotion of wakefulness [25, 26, 85]. Hct/OX-R1 is expressed in high levels in the locus coeruleus, in the latero-dorsal tegmentum, and the pedunculopontine nucleus (brain stem cholinergic regions), whereas the Hct/OX-R2 is predominant in the tuberomammillary nuclei (TMN), and both receptors are expressed at moderately high levels in the dorsal and medial raphe and in the cholinergic regions of the basal forebrain [85, 86], all of which are important areas in the regulation of the sleep–wake cycle [27–29].
As expected, the dual Hct/OX-R1 / Hct/OX-R2 antagonist, suvorexant, was effective in altering SW and REM sleep following IP dosing (10 and 30 mg/kg) when EEG activity was recorded at the onset of the light phase in the ethanol-exposed rats and control group. Suvorexant was found to produce a significant, dose-dependent, decrease in the latency to REM and SW sleep onsets.
The orexin system consists of two G-protein-coupled receptors: Hct/OX-R1 and Hct/OX-R2. There have been a number of studies that have investigated the potential effects of blockade of R1, R2 or both receptors in an attempt to dissect their effects on sleep in animal models. Suvorexant has been found to be slightly selective for Hct/OX-R1 over Hct/OX-R2 [87] and shows a fast onset of action during the first hour after dosing which persists for 4–5 hr [87] that correlates with the plasma concentration-time profile in both rats [88] and humans [89]. The relative contributions of Hct/OX-R1 and Hct/OX-R2 on sleep are based on the observations that Hct/OX-R2 knockout mice show the phenotypic characteristics of narcolepsy [90], whereas Hct/OX-R1 knockout mice show an almost normal sleep–wake cycle [91]. There are data to suggest that REM sleep is also regulated by Hct/OX receptors. This is supported by the findings that REM sleep is attenuated in both Hct/OX-R1 and Hct/OX-R2 knockout mice after administration of Hct/OX, thus suggesting a comparable contribution of the two receptors to REM sleep [92, 93]. Studies using a subtype selective receptor antagonist for Hct/OX receptors have also shown that the selective blockade of Hct/OX-R2 results in a reduction in SWS latency and an increase in SWS and REM duration [56, 86, 94, 95]; suvorexant has been shown to induce sleep largely by increasing REM sleep [95]. Selective blockade of Hct/OX-R1 by the antagonist SB-408124 was found to have no effect on sleep, although it was found to reduce locomotor activity (LMA) [36]. However, another Hct/OX-R1 antagonist, SB-334867, was found to reduce, in a dose-dependent manner, LMA and increase cumulative NREM activity [86]. Finally, selective blockade of Hct/OX-R1 has been found to reduce REM sleep latency and increases REM sleep duration at the expense of the time spent in NREM sleep when an Hct/OX-R2 antagonist JNJ-10397049 is administered [96]. Thus, taken together these studies suggest that Hct/OX-R2 selective antagonists could potentially offer more beneficial effects for the treatment of insomnia than R1 antagonists or dual receptor antagonists [56, 86].
Despite the abundance of data on the importance of alcohol-induced sleep disturbance to the clinical course of alcoholism [97], there have been few studies that have specifically evaluated therapeutic agents for comorbid insomnia disorder in animal models of protracted alcohol withdrawal [15, 22, 24]. In a recent review [15], the current pharmacological and behavioral treatment for insomnia associated with alcohol dependence is listed, and some encouraging results were reported for gabapentin [98, 99], quetiapine [100, 101], and cognitive behavioral therapy for insomnia (CBT-I) [102, 103]. We have demonstrated previously, in animal models, that gabapentin can ameliorate some of the sleep pathology that is seen following chronic ethanol vapor exposure by reducing sleep fragmentation [104, 105].
The present study is the first in the literature that experimentally targets the Hct/OX system to promote sleep in an AUD model. We found that while suvorexant was effective in hastening the onset of sleep, it also produced an increase in REM and SW sleep fragmentation and increased β energy in waking EROs when compared with VEH. In one clinical trial of insomnia, suvorexant, particularly at high doses, was found to reduce wakefulness after sleep onset although it increased the number and time spent in short wakefulness bouts during sleep when compared with placebo and baseline conditions [106]. Similar findings were reported in an animal model where a progressive loss of orexin neurons was also found to alter the appearance of wakefulness within sleep [107]. These findings suggest that the modulatory effect of the orexin system and its receptors (Hct/OX-R1/Hct/OX-R2) may be more complex than previously thought and may help to explain the fragmented sleep observed in patients with narcolepsy [108]. Thus, fragmented sleep with either the loss of the orexin neurons [109, 110] or pharmacological treatment with Hct/OX-R1/R2 antagonist may induce complex compensatory changes in other wake-promoting populations that may result in disrupted sleep and/or wakefulness as seen in narcolepsy [111].
These findings suggest that suvorexant, while hastening the onset of sleep, may not increase the quality of sleep and may in fact produce some changes in SWS that mimics what is seen following chronic ethanol exposure. Although human clinical trials with suvorexant and other DORAs have documented efficacy in both healthy subjects and in patients with primary insomnia [60, 112, 113], the AASM (American Academy of Sleep Medicine) advises that clinicians use suvorexant as a treatment for sleep maintenance insomnia (versus no treatment) in adults [18]. However, recently several authors have suggested that DORAs have the potential to be “perfect insomnia drugs” [114] for many types of insomnia including for the treatment of insomnia associated with AUDs [60]. It has also been suggested that suvorexant has not yet been shown to have those advantages [115]. Our studies suggest that, in the rat, suvorexant does not appear to be superior to gabapentin in ameliorating alcohol-induced sleep pathology [104, 105]. However, our studies do not address whether suvorexant has efficacy in reducing the reinforcing properties of alcohol, or attenuating alcohol craving, both potential targets for its use in the treatment of AUDs that might be independent of its effects on sleep.
Acknowledgments
The authors thank Phil Lau for help in statistical analyses. James Havstad developed the software used for EEG assessments.
Funding
This work was supported by National Institute of Health (NIH) grants (U01 AA019969; R01 AA006059 to Cindy L. Ehlers) from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Conflict of interest statement. None declared.
References
- 1. Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7(6):523–539. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhary NS, et al. Insomnia in alcohol dependent subjects is associated with greater psychosocial problem severity. Addict Behav. 2015;50:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drummond SP, et al. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22(8):1796–1802. [PubMed] [Google Scholar]
- 4. Brower KJ, et al. Prevalence and correlates of withdrawal-related insomnia among adults with alcohol dependence: results from a national survey. Am J Addict. 2010;19(3):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association; DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 6. Brower KJ. Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol. 2015;49(4):417–427. [DOI] [PubMed] [Google Scholar]
- 7. Conroy DA, et al. Perception of sleep in recovering alcohol-dependent patients with insomnia: relationship with future drinking. Alcohol Clin Exp Res. 2006;30(12):1992–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark CP, et al. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biol Psychiatry. 1998;43(8):601–607. [DOI] [PubMed] [Google Scholar]
- 9. Brower KJ, et al. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158(3):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foster JH, et al. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res. 1999;23(6):1044–1051. [PubMed] [Google Scholar]
- 11. Feige B, et al. Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res. 2007;31(1):19–27. [DOI] [PubMed] [Google Scholar]
- 12. Gillin JC, et al. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51(3):189–197. [DOI] [PubMed] [Google Scholar]
- 13. Brower KJ, et al. Persistent insomnia, abstinence, and moderate drinking in alcohol-dependent individuals. Am J Addict. 2011;20(5):435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolla BP, et al. The association between sleep disturbances and alcohol relapse: A 12-month observational cohort study. Am J Addict. 2015;24(4):362–367. [DOI] [PubMed] [Google Scholar]
- 15. Chakravorty S, et al. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40(11):2271–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mark TL, et al. Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009;99(1-3):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qaseem A, et al. ; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 18. Sateia MJ, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coleman PJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509–533. [DOI] [PubMed] [Google Scholar]
- 20. Atkin T, et al. Drugs for insomnia beyond Benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70(2):197–245. [DOI] [PubMed] [Google Scholar]
- 21. Sateia MJ, et al. Payer perspective of the American academy of sleep medicine clinical practice guideline for the pharmacologic treatment of chronic insomnia. J Clin Sleep Med. 2017;13(2):155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ehlers CL, et al. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20(2):173–179. [DOI] [PubMed] [Google Scholar]
- 23. Thakkar MM, et al. Sleep-wakefulness in alcohol preferring and non-preferring rats following binge alcohol administration. Neuroscience. 2010;170(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veatch LM. Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res. 2006;30(7):1214–1222. [DOI] [PubMed] [Google Scholar]
- 25. de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 27. de Lecea L. Optogenetic control of hypocretin (orexin) neurons and arousal circuits. Curr Top Behav Neurosci. 2015;25:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saper CB, et al. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tyree SM, de Lecea L. Optogenetic investigation of arousal circuits. Int J Mol Sci. 2017;18(8):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. [DOI] [PubMed] [Google Scholar]
- 31. Lee MG, et al. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25(28):6716–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kalogiannis M, et al. Cholinergic modulation of narcoleptic attacks in double orexin receptor knockout mice. PLoS One. 2011;6(4):e18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kohlmeier KA, et al. Differential actions of orexin receptors in brainstem cholinergic and monoaminergic neurons revealed by receptor knockouts: implications for orexinergic signaling in arousal and narcolepsy. Front Neurosci. 2013;7:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. [DOI] [PubMed] [Google Scholar]
- 35. Roecker AJ, et al. Orexin receptor antagonists: new therapeutic agents for the treatment of insomnia. J Med Chem. 2016;59(2):504–530. [DOI] [PubMed] [Google Scholar]
- 36. Dugovic C, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142–151. [DOI] [PubMed] [Google Scholar]
- 37. Haynes AC, et al. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96(1-2):45–51. [DOI] [PubMed] [Google Scholar]
- 38. Smart D, et al. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132(6): 1179–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matzeu A, et al. Drug seeking and relapse: new evidence of a role for orexin and dynorphin co-transmission in the paraventricular nucleus of the thalamus. Front Neurol. 2018;9:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tyree SM, et al. Hypocretin as a hub for arousal and motivation. Front Neurol. 2018;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harris GC, et al. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29(10):571–577. [DOI] [PubMed] [Google Scholar]
- 42. Mahler SV, et al. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boutrel B, et al. The hypocretins and the reward function: what have we learned so far? Front Behav Neurosci. 2013;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsujino N, et al. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahler SV, et al. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baimel C, et al. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2015;172(2):334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barson JR, et al. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20(3):469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brown RM, et al. Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol. 2016;21(3):603–612. [DOI] [PubMed] [Google Scholar]
- 49. Moorman DE, et al. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague–Dawley rats. Alcohol. 2009;43(5):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson RI, et al. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci. 2014;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown RM, et al. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013;16(9):2067–2079. [DOI] [PubMed] [Google Scholar]
- 52. Borgland SL, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cason AM, et al. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100(5):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith RJ, et al. Orexin / hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35(5):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lopez MF, et al. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016;1636:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cox CD, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320–5332. [DOI] [PubMed] [Google Scholar]
- 57. Kuriyama A, et al. Suvorexant for the treatment of primary insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2017;35:1–7. [DOI] [PubMed] [Google Scholar]
- 58. Winrow CJ, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52–61. [DOI] [PubMed] [Google Scholar]
- 59. Gotter AL, et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Campbell EJ, et al. A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Res. 2018, in press. [DOI] [PubMed] [Google Scholar]
- 61. Criado JR, et al. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008;42(8):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ehlers CL, et al. Developmental differences in EEG and sleep responses to acute ethanol administration and its withdrawal (hangover) in adolescent and adult Wistar rats. Alcohol. 2013;47(8):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thakkar MM, et al. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49(4):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Başar E, et al. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35(2-3):95–124. [DOI] [PubMed] [Google Scholar]
- 65. Roach BJ, et al. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34(5):907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Klimesch W, et al. Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev. 2007;31(7):1003–1016. [DOI] [PubMed] [Google Scholar]
- 67. Anokhin AP. Genetic psychophysiology: advances, problems, and future directions. Int J Psychophysiol. 2014;93(2):173–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schack B, et al. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331(2):107–110. [DOI] [PubMed] [Google Scholar]
- 69. Başar E, et al. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259(3):165–168. [DOI] [PubMed] [Google Scholar]
- 70. Ehlers CL, et al. Low voltage alpha EEG phenotype is associated with reduced amplitudes of alpha event-related oscillations, increased cortical phase synchrony, and a low level of response to alcohol. Int J Psychophysiol. 2015;98(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pandey AK, et al. Event-related oscillations in alcoholism research: a review. J Addict Res Ther. 2012;Suppl 7(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rangaswamy M, et al. Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol. 2014;125:383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Paxinos G, Watson C.. The Rat Brain in Stereotaxic Coordinates. 2nd ed. Sydney, Australia: Academic Press; 1986. [Google Scholar]
- 74. Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26(2):246–254. [PubMed] [Google Scholar]
- 75. Ehlers CL, et al. Event-related potential responses to the acute and chronic effects of alcohol in adolescent and adult Wistar rats. Alcohol Clin Exp Res. 2014;38(3):749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ehlers CL, et al. Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res. 2012;1450:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stockwell RG, et al. Localization of the complex spectrum: The S transform. IEEE Trans Signal Process. 1996;44(4): 998–1001. [Google Scholar]
- 78. Thatcher RW. Coherence, phase differences, phase shift, and phase lock in EEG/ERP analyses. Dev Neuropsychol. 2012;37(6):476–496. [DOI] [PubMed] [Google Scholar]
- 79. Ehlers CL, et al. Characterization of drug effects on the EEG by power spectral time series analysis. Psychopharmacology Bull. 1982;18(3):43–47. [Google Scholar]
- 80. Walker BM, et al. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44(6):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kamiński J, et al. β band oscillations engagement in human alertness process. Int J Psychophysiol. 2012;85(1): 125–128. [DOI] [PubMed] [Google Scholar]
- 82. Porjesz B, et al. Alcoholism and human electrophysiology. Alcohol Res Health. 2003;27(2):153–160. [PMC free article] [PubMed] [Google Scholar]
- 83. Rangaswamy M, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52(8):831–842. [DOI] [PubMed] [Google Scholar]
- 84. Buysse DJ, et al. A neurobiological model of insomnia. Drug Discov Today Dis Models. 2011;8(4):129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Marcus JN, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. [DOI] [PubMed] [Google Scholar]
- 86. Morairty SR, et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7(7):e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Betschart C, et al. Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56(19):7590–7607. [DOI] [PubMed] [Google Scholar]
- 88. Iqbal M, et al. Simple and highly sensitive UPLC-ESI-MS/MS assay for rapid determination of suvorexant in plasma. J Anal Toxicol. 2017;41(2):114–120. [DOI] [PubMed] [Google Scholar]
- 89. Yee KL, et al. Safety, Tolerability, and pharmacokinetics of suvorexant: a randomized rising-dose trial in healthy men. Clin Drug Investig. 2018;38(7):631–638. [DOI] [PubMed] [Google Scholar]
- 90. Willie JT, et al. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38(5):715–730. [DOI] [PubMed] [Google Scholar]
- 91. Willie JT, et al. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. [DOI] [PubMed] [Google Scholar]
- 92. Mieda M, et al. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31(17):6518–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mieda M, et al. Orexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders. Rationale for development and current status. CNS Drugs. 2013;27(2):83–90. [DOI] [PubMed] [Google Scholar]
- 94. Coleman PJ, et al. Discovery of 3,9-diazabicyclo[4.2.1]nonanes as potent dual orexin receptor antagonists with sleep-promoting activity in the rat. Bioorg Med Chem Lett. 2010;20(14):4201–4205. [DOI] [PubMed] [Google Scholar]
- 95. Hoyer D, et al. Distinct effects of IPSU and suvorexant on mouse sleep architecture. Front Neurosci. 2013;7:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dugovic C, et al. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. 2014;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kolla BP, et al. The course of sleep disturbances in early alcohol recovery: an observational cohort study. Am J Addict. 2014;23(1):21–26. [DOI] [PubMed] [Google Scholar]
- 98. Brower KJ, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mason BJ, et al. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Litten RZ, et al. ; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Litten RZ, et al. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17(3):513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brooks AT, et al. Sleep disturbances in individuals with alcohol-related disorders: a review of cognitive-behavioral therapy for insomnia (CBT-I) and associated non-pharmacological therapies. Subst Abuse. 2014;8:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Miller MB, et al. Insomnia treatment in the context of alcohol use disorder: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;181:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sanchez-Alavez M, et al. Effect of gabapentin on sleep and event-related oscillations (EROs) in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Alcohol Clin Exp Res. 2018;42(3):624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ehlers CL, et al. Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology (Berl). 2018;235(6):1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Svetnik V, et al. Insight into reduction of wakefulness by suvorexant in patients with insomnia: analysis of wake bouts. Sleep. 2018;41(1):1–9. [DOI] [PubMed] [Google Scholar]
- 107. Branch AF, et al. Progressive loss of the orexin neurons reveals dual effects on wakefulness. Sleep. 2016;39(2):369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Alakuijala A, et al. Hypocretin-1 levels associate with fragmented sleep in patients with narcolepsy type 1. Sleep. 2016;39(5):1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Valko PO, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74(6):794–804. [DOI] [PubMed] [Google Scholar]
- 110. John J, et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol. 2013;74(6):786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Heiss JE, et al. Parallel arousal pathways in the lateral hypothalamus. eNeuro. 2018;5(4):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Herring WJ, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. [DOI] [PubMed] [Google Scholar]
- 113. Sun H, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2): 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hoyer D, et al. Orexin in sleep, addiction and more: is the perfect insomnia drug at hand? Neuropeptides. 2013;47(6):477–488. [DOI] [PubMed] [Google Scholar]
- 115. Kripke DF. Is suvorexant a better choice than alternative hypnotics? F1000Res. 2015;4:456. [DOI] [PMC free article] [PubMed] [Google Scholar]