Abstract
Background and purpose
We report the outcomes of carotid artery stenting for patients with angiographically visible occipital artery–vertebral artery anastomosis.
Methods
Among 47 consecutive patients who underwent carotid artery stenting from January 2007 to December 2010, seven patients for whom cerebral angiograms clearly showed occipital artery–vertebral artery anastomosis were selected. Four different protection methods were used: distal internal carotid artery protection; carotid flow reversal; seatbelt and airbag technique; and double protection method of protecting both the external and internal carotid artery.
Results
One patient with distal internal carotid artery protection showed a high-intensity lesion at the border of the upper thalamus, internal capsule and lateral ventricle wall after carotid artery stenting. The other patient with the double protection method did not show any high-intensity lesions on postoperative diffusion-weighted imaging in the vertebrobasilar territory. All seven patients with visible occipital artery–vertebral artery anastomosis showed ipsilateral vertebral artery severe stenosis or occlusion.
Conclusion
Large occipital artery–vertebral artery anastomosis may be a pathway for embolic materials during carotid artery stenting. External carotid artery protection is recommended for carotid artery stenting in such patients.
Keywords: Occipital vertebral anastomosis, carotid artery stenting, embolic protection technique
Introduction
Occipital artery (OA)–vertebral artery (VA) anastomosis is a well-known anastomosis between the external carotid artery (ECA) and VA, described in many reports over a long period.1–4 The OA gives rise to many branches, some of which directly anastomose with the VA while others anastomose indirectly by way of muscular or meningeal branches.5,6
While OA–VA anastomosis can be seen in almost all cases during autopsy, the frequency of visualising this anastomosis on cerebral angiography varies from eight in 1000 cases to four in 100 cases.4,7 OA–VA anastomoses are considered to be latent arterial anastomoses that can be barely recognised on cerebral angiography under normal haemodynamic conditions. These extracranial–intracranial anastomoses, however, may often develop under conditions of vascular occlusive disease of the carotid artery or VA that create an arterial pressure gradient between the carotid and vertebrobasilar circulations, forming significant collateral pathways on cerebral angiograms.4,7–9
OA–VA anastomosis should be taken into account for carotid artery stenting (CAS), as embolic materials released from the carotid plaque during CAS may run through the anastomosis and cause cerebral infarction in the vertebrobasilar circulation. We investigated the frequency of significant OA–VA anastomosis during CAS and discuss potential methods for risk management that should be considered during CAS for this specific population of patients.
Patient population and methods
Forty-seven consecutive case of CAS were retrospectively identified as having undergone CAS for carotid artery stenosis from January 2007 to December 2010. The local internal review board approved the conduct of this retrospective study. All patients underwent diagnostic cerebral angiography with anatomical details confirmed before performing the procedure. As the double transfemoral approach also poses a double puncture site complication risk, not only the degree of the stenosis of the affected carotid arteries, but also bilateral femoral arteries to be punctured were evaluated. All angiographic images were obtained by single-plane Phillips Allura Xper FD20 with three-dimensional capability. Plaque characteristics and coverage of the carotid artery were also evaluated and the peak systolic flow velocity by ultrasonography. Magnetic resonance imaging (MRI) was performed before and after CAS to identify additional new high-intensity lesions compared to preoperative MRI, with detailed neurological examination to elucidate any ischaemic symptoms related to the procedure.
Stent placement procedures
Patients received oral dual antiplatelet therapy with either clopidogrel (75 mg), ticlopidine (100 mg) or cilostazol (200 mg) in addition to aspirin (100 mg) at least one week prior to CAS. All stent placement procedures were performed under local anaesthesia using the transfemoral approach. After sheath insertion, all patients received an intravenous bolus injection of heparin to increase activated clotting time to over 300 seconds. Either a self-expandable stent (Precise; Cordis, Johnson & Johnson, Fremont, CA, USA) or a carotid wall stent (Boston Scientific, Marlborough, MA, USA) was used for all 47 patients in the study. Four different methods of embolic protection were used in this case series. Three were cerebral protection methods reported by Parodi et al.: distal internal carotid artery (ICA) protection (balloon occlusion of the distal ICA, filters in the distal ICA); flow reversal technique; and the seat belt and air bag technique.10,11 Another protection method was a double protection method to protect both the ICA and the ECA. Either a filter wire device (Angioguard XP; Cordis, Johnson & Johnson) or a distal protection balloon (Percusurge guardwire; Medtronic, Minneapolis, MN, USA) was used for distal ICA protection.
Either a 10.5-Fr Patlive (Terumo Clinical Supply, Tokyo, Japan) or a 9-Fr Cello (Fuji Systems, Tokyo, Japan) with a distal elastomeric balloon was used as a proximal occlusion balloon catheter for the carotid flow reversal technique and seat belt and air bag technique. The guiding catheter with the balloon at its tip was placed into the common carotid artery (CCA), and a Percusurge guardwire was advanced into the ECA. Protection was activated with consecutive inflation of the balloons, first with the Percusurge guardwire, then with the balloon of the guiding catheter in the CCA. In cases of carotid flow reversal, a 0.014-inch micro-guidewire was passed through the stenosis and the following procedures were performed: pre-dilation, stent placement and post-dilation with protection continued. Blood was sufficiently aspirated through the guiding catheter after post-dilation. In the case of the seat belt and air bag technique, after carotid flow reversal, Angioguard XP was deployed in the distal ICA. The Percusurge guardwire in the ECA was deflated and pulled away. The balloon of the guiding catheter was deflated, completing protection. For the double protection method, a Percusurge guardwire was used for the purpose of protecting the OA as well as the ECA. Under a bilateral transfemoral approach, 8-Fr and 5-Fr guiding catheters were placed into the CCA together. The Percusurge guardwire was placed through the 5-Fr guiding catheters in the ECA proximal to the OA origin and the Angioguard XP was advanced and deployed at the distal ICA through the 8-Fr catheter. Subsequently, the Percusurge guardwire was inflated to protect both the OA and ECA. After stent placement procedures were completed, both the balloon and filter were deactivated while continuous blood aspiration was performed from the 8-Fr Launcher (Medtronic, Minneapolis, MN, USA) to eliminate debris pooling beneath the protection balloon. We pulled back the protection balloon outside the expanded stent.
Results
Stent placement procedures were successfully completed in all cases. The OA–VA anastomosis was angiographically visualised in seven of the 47 CAS patients. The background and anatomical characteristics of the patients with OA–VA anastomosis are presented in Table 1.
Table 1.
Background characteristics and summary of patients.
| Patient | Age | Sex | Side | Symptom | Artery anastomosed to VA | Anastomotic site | Size of anastomosis | Ipsilateral VA stenosis | Protection method in CAS | Protection device | Postoperative DWI hyperintensity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | L | Asymptomatic | OA | C1–2 | Large | Severe stenosis | Distal ICA protection | GW | Floor of left lateral ventricle |
| 2 | 68 | M | L | Amaurosis fugax | OA | C1–2 | Large | Severe stenosis | Flow reversal | Patlive, GW | No |
| APA | C2–3 | Small | |||||||||
| 3 | 68 | M | R | OA | Oc–C1 | Small | Occlusion | Distal ICA protection | AG 6 mm | Frontal lobe | |
| 4 | 62 | M | R | Asymptomatic | OA | Oc–C1 | Large | Occlusion | Distal ICA protection | AG 5 mm | No |
| 5 | 69 | M | R | Asymptomatic | OA | Oc–C1 | Small | Occlusion | Distal ICA protection | AG 6 mm | No |
| APA | C2–3 | Small | |||||||||
| 6 | 60 | M | L | Visual field defect | OA | C1–2 | Large | Occlusion | Double protection | AG 5 mm, GW | No |
| 7 | 71 | M | R | Asymptomatic | OA | Oc–C1 | Large | Severe stenosis | SB + AB | Cello + GW | * |
| OA | C1–2 | Large |
OA: occipital artery; VA: vertebral artery; DWI: diffusion-weighted imaging; CAS: carotid artery stenting; Oc: occipital bone; C1: atlas; C2: axis; C3: third cervical vertebra; asymptomatic: no ischaemic symptom associated with carotid stenosis more than 120 days before CAS; APA: ascending pharyngeal artery; SB + AB: seat belt and air bag technique; GW: PercuSurg guard wire; AG: AngioGuard XP.
No DWI because of pacemaker implantation.
OA–VA anastomosis occurred between the occipital bone and atlas (four patients), atlas and axis (three patients), or axis and third cervical vertebra (one patient). Patients 2 and 5 also presented with a visible connection from the ascending pharyngeal arteries toward the ipsilateral VAs, both of which were small anastomoses. Among the seven patients with OA–VA anastomosis, three patients showed severe VA stenosis and four patients had VA occlusion at the origin or extracranial segment of the VA on the ipsilateral side of the affected carotid artery. The blood flow direction was from OA towards VA in all seven patients with OA–VA anastomosis. Among the remaining 40 patients without OA–VA anastomosis, only one patient showed severe extracranial vertebral artery stenosis ipsilateral to the affected carotid artery. During CAS for patients with OA–VA anastomosis, distal ICA protection was applied in four patients; Percusurge guardwire for one patient, and Angioguard XP for three patients. The carotid flow reversal technique and ‘seat belt and air bag’ technique were applied for patients 2 and 7. Because these two patients had extremely severe carotid stenosis, a distal ICA protection device was not expected to pass the stenosis but rather to break the plaques, causing peri-procedural ischaemic complications. These expectations justified the use of the carotid flow reversal technique and ‘seat belt and air bag’ technique. The double protection method was applied for patient 6 who developed a right homonymous visual field deficit as the presenting symptom. Diffusion-weighted imaging (DWI) at onset showed cerebral infarction in the left occipital lobe and three-dimensional rotation angiography revealed severe ICA stenosis and a large left-sided OA–VA anastomosis. Occipital infarction was possibly caused by embolic plaques from the CCA traveling into the OA–VA anastomosis, requiring the double protection method to be applied for this patient.
Postoperative DWI was performed for six patients with OA–VA anastomosis. In one patient, postoperative DWI was not obtained because of its pacemaker implantation. Patient 1 presented with a large OA–VA anastomosis with CAS being completed only using the distal ICA protection balloon. Postoperative DWI showed a small, high-intensity lesion at the border of the upper surface of the thalamus, internal capsule and wall of the lateral ventricle body, fortunately without ischaemic symptoms (Figure 1). Patient 3 also presented with high-intensity lesions on postoperative DWI in the right frontal lobe with no ischaemic symptoms after CAS. Patient 6 underwent the double protection method during CAS and showed no additional high-intensity lesion on postoperative DWI (Figure 2). The results of DWI obtained from the other 40 patients without a visible OA–VA anastomosis are shown in Table 2. In one patient, DWI was not performed because of pacemaker implantation. Ten patients (25.6%) showed postoperative clinically silent DWI high-intensity lesions. Eight of the 10 patients showed high-intensity lesions in the anterior circulation, and the other two patients showed DWI high-intensity lesions in the ipsilateral occipital lobe. The posterior communicating artery was clearly identified by cerebral angiogram in those two patients, whereas it was not present in patients 1 and 6 with OA–VA anastomosis.
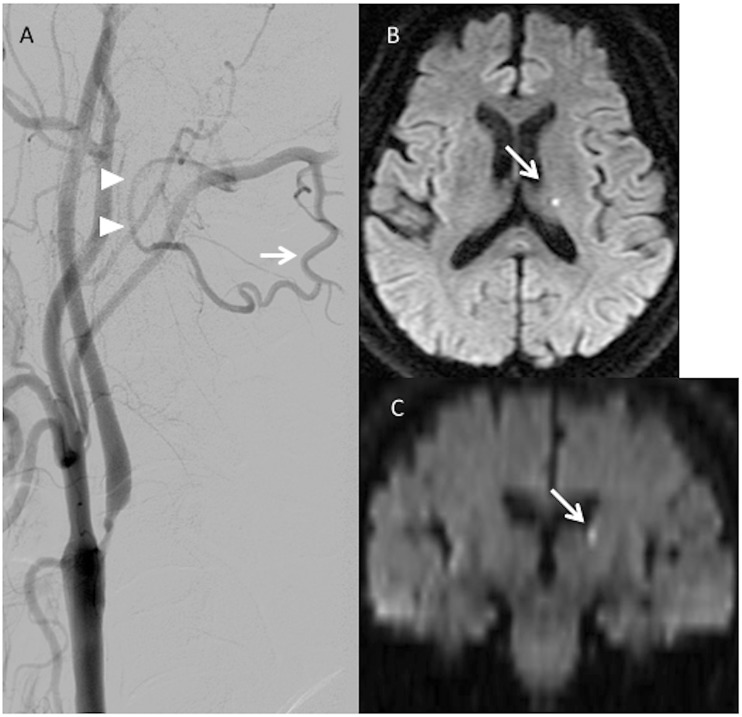
Figure 1.
(a) Patient 1 has a large descending muscular branch (arrow) of the occipital artery, which anastomoses with the vertebral artery (arrowhead) between the atlas and axis. (b, c) Axial and coronal sections of postoperative diffusion-weighted imaging show a small, high-intensity spot in the left lateral ventricle wall above the thalamus (arrow).
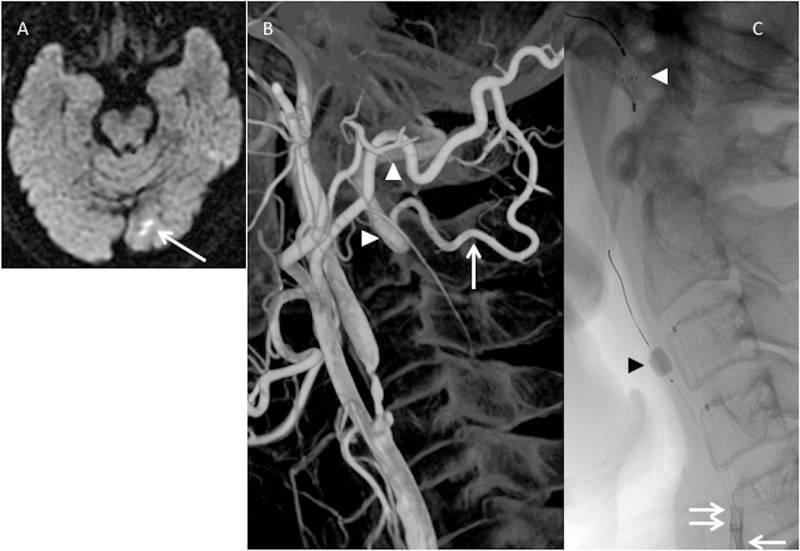
Figure 2.
(a) Preoperative diffusion-weighted imaging shows cerebral infarction (arrow) on the left occipital lobe in patient 6. (b) Three-dimensional rotational angiography shows severe internal carotid artery (ICA) stenosis and a large visible occipital artery (OA)–vertebral artery (VA) anastomosis on the left side. The OA muscular branch (white arrow) anastomoses with the extracranial VA (white arrowheads). (c) A double protection method is applied in the carotid artery stenting procedure. Both 8-Fr (double arrow) and 5-Fr (arrow) guiding catheters are placed into the common carotid artery together. A Percusurge guardwire (black arrowhead) is placed through the 5-Fr catheter into the external carotid artery and Angioguard XP (white arrowhead) is passed through the 8-Fr catheter in the distal ICA.
Table 2.
DWI high-intensity lesions after CAS.
| Lesions of DWI high intensity | Patients with a visible OA–VA anastomosis (n=6*) | Patients without a visible OA–VA anastomosis (n=39*) |
|---|---|---|
| Anterior circulation | 1 | 8 |
| Posterior circulation | 1 | 2 |
| Total | 2 (33.3%) | 10 (25.6%) |
OA: occipital artery; VA: vertebral artery; DWI: diffusion-weighted imaging; CAS: carotid artery stenting.
Magnetic resonance imaging was not obtained in each patient in both groups because of pacemaker implantation.
Discussion
OA–VA anastomosis is one of the better known anastomoses between the ECA and VA. Lasjaunias et al. reported in detail on the anatomy of the OA.5 This artery is potentially connected to the VA by many channels, such as meningeal collaterals, muscular branches, and direct anastomotic channels.5,6 In our study, all seven patients with OA–VA anastomosis showed severe stenosis or occlusion at the ipsilateral VA. This contrasts with the fact that only one patient showed severe stenosis of the ipsilateral VA among the 40 patients without OA–VA anastomosis. This suggests that decreased perfusion pressure caused by severe stenosis or occlusion of the VA may generate a pressure gradient from the ECA towards the VA, and this pressure gradient may result in a more prominent appearance of the OA–VA anastomosis on cerebral angiograms. Actually, the blood flow direction in our patients with OA–VA anastomosis was all from the OA to the VA. Furthermore, the frequency of visualised OA–VA anastomosis was 14.9% (seven of 47) in our case series, much higher than that reported from normal cerebral angiograms.2–4 A normal cerebral angiogram is usually applied not only for atherosclerotic disease but also for non-atherosclerotic disease, such as cerebral aneurysms and brain tumours. On the other hand, our case series was limited to patients who had atherosclerotic carotid artery stenosis and were candidates for CAS. As atherosclerosis causes systemic steno-occlusive disease, CAS candidates may harbour not only carotid stenosis, but also other arterial occlusive diseases. In such a patient population, it is likely that more patients have multiple stenoses or occlusions in their systemic arteries, including VA stenosis, than patients without atherosclerotic disease. This unique patient background could potentially increase the rate of developing visualised extracranial–intracranial anastomosis, explaining a much higher frequency of visualised OA–VA anastomosis in our case series than that reported from normal cerebral angiograms.
From a surgical perspective, OA–VA anastomosis, especially from OA to VA, may become a pathway for embolic materials generated during the CAS procedure to cause cerebral ischaemia. Yamagata et al. reported a case of symptomatic ECA stenosis with ipsilateral ICA occlusion and with visible OA–VA, OA–ascending pharyngeal artery (APA) anastomosis by cerebral angiogram.12 They treated ECA stenosis with angioplasty and stenting and used distal balloon protection to protect not only the ECA, but also the OA and APA connecting to the VA. They concluded that ECA stenting without protection of the OA and APA may risk carrying debris into the vertebrobasilar territory. Embolic particles are released from the plaque near the carotid bifurcation during CAS procedures and a proportion travel into the ECA. Visible OA–VA anastomosis may become a pathway for debris to enter the vertebrobasilar territory. In fact, postoperative DWI showed a new, small, high-intensity lesion at the border of the upper surface of the thalamus, internal capsule and wall of the lateral ventricle body in patient 1. This lesion is possibly supplied by either the anterior choroidal artery (AChA), lateral posterior choroidal artery (LPChA), or medial posterior choroidal artery (MPChA). There are two possible causes of the high-intensity lesion: an embolic particle entering the AChA during the CAS procedure, especially when the guiding catheter was introduced into the CCA or the distal protection balloon passed through the carotid stenosis before activation; or an embolic particle passing through the OA–VA anastomosis causing ischaemic stroke in the LPChA or MPChA territory. As the latter possibility cannot be eliminated, the double protection method was applied for patient 6 aiming to avoid embolic particles travelling into the vertebrobasilar system through the OA–VA anastomosis. Severe carotid stenosis was present in this case accompanying large OA–VA anastomosis ipsilaterally, with the VA occluded at this origin.
It should be noted, however, that complicating embolic protection methods themselves, such as the seatbelt and airbag technique or the double protection method, could cause peri-procedural ischaemic stroke by detached plaques from the CCA or the aortic arch. Szikra et al. reported that soft plaques in the aortic arch and CCA could pose a substantial risk of embolisation during CAS.13 Kim et al. reported that manoeuvres in the aortic arch during CAS play an important role in the occurrence of new ischaemic lesions in the posterior fossa and contralateral ICA territory.14 These reports may suggest that simpler endoluminal device manipulation in the CCA and aortic arch may be safer regarding the peri-procedural ischaemic stroke risk. The double protection method requires the insertion of two guiding catheters into the CCA, increasing the risk of ischaemic stroke from the plaques situated in the CCA or the aortic arch. Furthermore, there is no available level 1 evidence to support the routine use of embolic protection devices (EPDs).15 Szikra et al. reported that while an EPD may provide partial protection of the ICA to be treated, it does not provide protection against contralateral or posterior fossa embolisation originating from the plaques of the aortic arch.13 In our cases, postoperative DWI high-intensity lesions in the posterior circulation were identified in the two patients without visible OA–VA anastomosis. Although the postoperative DWI high-intensity lesions might have been caused by embolic agents through the posterior communicating artery, it is also possible that they were caused by plaque fragments from the aortic arch through the VA during catheter manipulation. Carotid endarterectomy (CEA) has intrinsically been the preferential treatment option for carotid steno-occlusive disease.16–18 The 2018 European Society for Vascular Surgery recommendation for the management of symptomatic carotid disease advised that both CEA and CAS are candidate treatment options for this medical condition, with the levels of evidence being slightly lower for CAS than for CEA19 due to the fact that the risks of death and stroke within 30 days after intervention were significantly higher after CAS than after CEA20–23 in the major randomised controlled trials. On the other hand, CAS is chosen as the first-line treatment option for severe carotid artery stenosis in our institution considering its less invasiveness compared with CEA. Sufficient preoperative evaluation of plaques in the aortic arch, however, has not been performed. As complicating embolic protection techniques of CAS may increase the risk of peri-procedural stroke not only by carotid plaques but also by the plaques in the aortic arch, preoperative evaluation of plaques should have been performed in detail. Should numerous severe plaques be revealed throughout the patient’s systemic vessels by preoperative evaluation, CEA may be preferred over CAS.
A lesson that can learned from these cases is that VA stenosis ipsilateral to the carotid stenosis requires careful observation and identification for the presence of OA–VA anastomosis. When CAS is planned for such patients with obvious OA–VA anastomosis only under the assistance of distal ICA protection and with the aid of simultaneous ECA protection, ischaemic complication may occur in the vertebrobasilar territory by embolic materials travelling through the large OA–VA anastomosis. Our experience suggests that CAS can be safely performed by protecting not only the ICA, but also the ECA, including the OA for patients with OA–VA anastomosis to avoid ischaemic stroke in the vertebrabasilar circulation, only under the circumstance in which the plaques on the route of the guiding catheter, especially in the CCA or aortic arch, were carefully evaluated and the complicating embolic protection technique could be considered to be safely carried out.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Schulze HA, Sauerbrey A. [Anastomosis of vertebral and occipital arteries]. Zentralbl Neurochir 1956; 16: 76–80. [PubMed] [Google Scholar]
- 2.Takahashi H, Yamaguchi K, Uemura K, et al. [Four cases of occipital–vertebral anastomosis]. No To Shinkei 1971; 23: 1315–1319. [PubMed] [Google Scholar]
- 3.Gito Y, Kowada M, Miyasaka K, et al. [Occipital–vertebral anastomosis]. Rinsho Hoshasen 1975; 20: 17–22. [PubMed] [Google Scholar]
- 4.Schechter MM. The occipital–vertebral anastomosis. J Neurosurg 1964; 21: 758–762. [DOI] [PubMed] [Google Scholar]
- 5.Lasjaunias P, Theron J, Moret J. The occipital artery. Anatomy – normal arteriographic aspects – embryological significance. Neuroradiology 1978; 15: 31–37. [DOI] [PubMed] [Google Scholar]
- 6.Alvernia JE, Fraser K, Lanzino G. The occipital artery: a microanatomical study. Neurosurgery 2006; 58: ONS-114–ONS-122. [DOI] [PubMed] [Google Scholar]
- 7.Miyachi S, Negoro M, Sugita K. The occipital–vertebral anastomosis as a collateral pathway: hemodynamic patterns – case report. Surg Neurol 1989; 32: 350–355. [DOI] [PubMed] [Google Scholar]
- 8.Oguzkurt L, Kizilkilic O, Tercan F, et al. Vertebrocarotid collateral in extracranial carotid artery occlusions: digital subtraction angiography findings. Eur J Radiol 2005; 53: 168–174. [DOI] [PubMed] [Google Scholar]
- 9.Vasović L, Mojsilović M, Anđelković Z, et al. Proatlantal intersegmental artery: a review of normal and pathological features. Child’s Nervous System 2009; 25: 411–421. [DOI] [PubMed] [Google Scholar]
- 10.Parodi JC, La Mura R, Ferreira LM, et al. Initial evaluation of carotid angioplasty and stenting with three different cerebral protection devices. J Vasc Surg 2000; 32: 1127–1136. [DOI] [PubMed] [Google Scholar]
- 11.Parodi JC, Schonholz C, Ferreira LM, et al. “Seat belt and air bag” technique for cerebral protection during carotid stenting. J Endovasc Ther 2002; 9: 20–24. [DOI] [PubMed] [Google Scholar]
- 12.Yamagata T, Mitsuhashi Y, Nishio A, et al. Protection of anastomotic pathways to the vertebral artery during stenting of external carotid artery stenosis. Neurol Med Chir (Tokyo) 2010; 50: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 13.Szikra P, Boda K, Rarosi F, et al. Aortic arch and common carotid artery plaques with soft components pose a substantial risk of cerebral embolization during carotid stenting. Interv Neuroradiol: Journal of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences 2016; 22: 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Lee HJ, Yang JH, et al. The influence of carotid artery catheterization technique on the incidence of thromboembolism during carotid artery stenting. AJNR Am J Neuroradiol 2010; 31: 1732–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schonholz CJ, Uflacker R, Parodi JC, et al. Is there evidence that cerebral protection is beneficial? Clinical data. J Cardiovasc Surg 2006; 47: 137–141. [PubMed] [Google Scholar]
- 16.Barnett HJM, Taylor DW, Haynes RB, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 17.MRC European Carotid Surgery Trial. Interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet (London, England) 1991; 337: 1235–1243. [PubMed] [Google Scholar]
- 18.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet (London, England) 2004; 363: 1491–1502. [DOI] [PubMed] [Google Scholar]
- 19.Naylor AR. Endarterectomy versus stenting for stroke prevention. Stroke Vasc Neurol 2018; 3: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein HH, Ringleb P, Allenberg JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008; 7: 893–902. [DOI] [PubMed] [Google Scholar]
- 21.Mas JL, Chatellier G, Beyssen B. Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke 2004; 35: e18–e20. 2003/12/06. [DOI] [PubMed] [Google Scholar]
- 22.Ederle J, Dobson J, Featherstone RL, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet (London, England) 2010; 375: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantese VA, Timaran CH, Chiu D, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke 2010; 41: S31–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]