Abstract
Background
Carotid artery stenting requires antiplatelet therapy for prevention of in-stent thrombosis. Patients suffering from acute ischemic stroke undergoing intravenous thrombolysis and emergent carotid artery stenting (eCAS) are at high risk for intracranial bleeding. We assessed efficacy and safety of acute administration of intravenous tirofiban versus aspirin in these patients.
Methods
A retrospective, single center, cohort study was carried out of 32 patients who underwent eCAS (18 received tirofiban, 14 received aspirin) at our comprehensive stroke center (2008–2016).
Results
Of our 32 consecutive eCAS patients, favorable clinical outcomes (modified Rankin scale ≤ 2) were achieved in eight (47%) tirofiban patients and six (46%) aspirin patients (p = 0.96). Overall rates were similar for symptomatic intracranial bleeding (tirofiban 22%, aspirin 29%, p = 0.68) and mortality (tirofiban 18%, aspirin 23%, p = 0.71).
Conclusions
Tirofiban and aspirin demonstrated similar efficacy and safety in thrombolyzed stroke patients who underwent eCAS in our cohort. Intravenous tirofiban with its short half-life might represent an alternative to aspirin in select patients.
Keywords: Tirofiban, emergent carotid artery stenting, stroke
Introduction
In acute ischemic strokes with large intracranial vessel occlusions, additional extra-cranial high-grade internal carotid artery (ICA) stenosis or occlusion is present in 10–20% of cases.1 Intravenous thrombolysis, mechanical thrombectomy, and emergent carotid artery stenting (eCAS) are used for rapid recanalization in these patients. During stent placement, peri-interventional antiplatelet treatment is administered, typically along with aspirin and clopidogrel, to prevent acute in-stent thrombosis.2 However, eCAS is associated with higher risk of intracranial hemorrhage (ICH).3 In contrast with clopidogrel and aspirin, intravenous tirofiban is a fast-acting non-peptide glycoprotein IIb/IIIa (GPIIb/IIIa) receptor antagonist located on thrombocytes and with a short half-life that reverses bleeding time to normal within 4 h after discontinuation.4 Unlike clopidogrel, which is available only orally, its parental formulation allows for easy and fast administration. Thus, tirofiban might be an ideal agent for initial inhibition of platelets in eCAS treatment.
Despite these favorable characteristics, the use of tirofiban in acute stroke patients is controversial because of the increased rates of symptomatic ICH (sICH) reported in some studies,5 and similar ICH rates compared with standard treatment in others.6,7 Therefore, the aim of our study was to assess the clinical outcome and safety of tirofiban compared with classic antiplatelet therapy (APT) with aspirin in stroke patients undergoing eCAS.
Material and methods
Patient selection
In this retrospective, descriptive, single-center cohort study, data for this analysis was derived from our prospectively maintained local stroke database.
The local ethic committee approved data collection and analysis. Patients were included when the following criteria were met during the study period that began June 2008 through December 2016 at our comprehensive stroke center: age ≥ 18 years, diagnosis of acute ischemic stroke and eligible for intravenous thrombolysis, and eCAS within 9 h of symptom onset and acute administration of tirofiban, or loading dose aspirin (500 mg intravenously (IV)) before the CAS with a self-expendable stent-system (Cristallo Ideale stent from Invatec (Medtronic), Brescia, Italy). Of 32 consecutive patients included in our study, 18 patients were in the tirofiban group and 14 patients were in the aspirin group.
Medical, radiological, and endovascular treatment procedures
In both groups, the specific antiplatelet treatment was administered before carotid stent placement, with the choice of tirofiban or aspirin at the discretion of the treating physician. Tirofiban was initiated with an initial intra-arterial bolus (10 mcg/kg body weight (bw)), followed by continuous IV application (9 mcg/kg bw/h) for up to 60 h. Aspirin (500 mg) was given IV before stent placement. All patients before stent placement received an IV bolus of low-molecular heparin adjusted according to the activated clotting time of 90–100 ms. APT for eCAS was continued with aspirin 100 mg and clopidogrel 75 mg for at least 2 months in all cases. Because of the initial IV thrombolysis treatment, the administration of clopidogrel was started 24 h after the intervention.
Endovascular procedures were performed using a Philips Allura Xper FD20/20 biplane angiography system (Philips Medical System, Best, the Netherlands) according to the local protocol.
Carotid artery stenting was performed on an emergency basis within 6 h of symptom onset or within 9 h with relevant computed tomography perfusion (CTP) mismatch. A ratio of time-to-peak (TTP):cerebral blood volume (CBV) of ≥2 was considered relevant. All emergent brain CT images were reviewed according to the Alberta Stroke Programme Early CT (ASPECT) score by a trained physician,8 and leptomeningeal collaterals were rated using the collateral grading score previously described by Maas et al.9
All symptomatic ICA stenoses were angiographically assessed according to North American Symptomatic Carotid Endarterectomy (NASCET) criteria.10 The ICA stenoses were treated with a self-expendable stent-system (Cristallo Ideale stent, Invatec) in all cases. In cases of a concomitant distal vessel occlusion, an endovascular thrombectomy was also performed. Cerebral artery recanalization was assessed according to the Thrombolysis in Cerebral Infarction (TICI) score;11 excellent endovascular reperfusion was defined as TICI ≥ 2 b. Follow-up imaging with cranial CT was performed in all patients during their hospitalization, and sICH and stroke volume were determined during follow-up imaging using the (ABC)/2 formula.12
Outcome measures
Demographic and clinical baseline characteristics were retrieved from the medical records and imaging data were assessed from radiological reports. The primary outcome measure was favorable clinical outcome defined as a modified Rankin scale (mRS) score ≤2 at 3-month follow-up. Secondary outcome measures were any ICH and sICH; the latter was defined as any new hemorrhage not seen on previous imaging in conjunction with additional neurological deterioration ≥4 on the National Institute of Health stroke scale (NIHSS) score (according to ECASS III criteria),13 peri-procedural stent patency, and thrombo-embolic events. Both clinical and imaging outcomes were assessed by a blinded reviewer.
Statistical analysis
Group comparison was calculated either by Chi-square test or Wilcoxon rank-sum test. Logistic regression analysis was performed for the primary and secondary outcome measures. A p-value ≤ 0.05 was considered statistically significant. All statistical analysis was performed using STATA/ IC 14.1 (StataCorp, Texas, USA).
Results
Of the 32 consecutive patients who underwent eCAS (June 2008 to December 2016), median age was 66 years (interquartile range (IQR) 61–77 years) in the 18 tirofiban patients and 68 years (IQR 61–75 years) in the 14 aspirin patients (Table 1). In both groups, the female:male ratio was 1:2. Median NIHSS score on admission was 14 (IQR 9–19) for the tirofiban and 18 (IQR 8–20) for the aspirin groups (p = 0.62). No differences were observed in clinical baseline characteristics, such as hypertension, dyslipidemia, diabetes mellitus, or active smoking (Table 1). Additionally, 17 (94%) of the tirofiban and 12 (86%) of the aspirin patients had a tandem-occlusion lesion. Median stenosis NASCET degree of the ICA was 91% (IQR 86–100%) for the tirofiban and 88% (IQR 79–99%) for the aspirin group (p = 0.28). Median ASPECT score on the initial CT scan was 8 (IQR 7–9) for the tirofiban and 9 (IQR 8–9) for the aspirin group, with a median ASPECT collateral score of 3 (IQR 3–4) in both groups (Table 2).
Table 1.
Demographic and baseline characteristics in 32 patients.
| Characteristics | Tirofiban (n = 18) | Aspirin (n = 14) | p-value |
|---|---|---|---|
| Age in years, median (IQR) | 66 (61–71) | 68 (61–75) | 0.73 |
| No. male (%) | 13 (72) | 10 (71) | 0.96 |
| NIHSS score on admission, median (IQR) | 14 (9–19) | 18 (8–20) | 0.62 |
| Location of stroke in left hemisphere, n (%) | 11 (61) | 6 (43) | 0.31 |
| Tandem occlusion, n (%) | 17 (94) | 12 (86) | 0.4 |
| Hypertension, n (%) | 8 (44) | 9 (64) | 0.27 |
| Diabetes mellitus, n (%) | 2 (11) | 2 (14) | 0.79 |
| Atrial fibrillation, n (%) | 1 (6) | 2 (14) | 0.4 |
| Dyslipidemia, n (%) | 12 (67) | 9 (64) | 0.89 |
| Active smoking, n (%) | 10 (55) | 6 (46) | 0.61 |
IQR = interquartile range; NIHSS score = National Institute of Health stroke scale score.
Table 2.
Potential bleeding risk factors.
| Risk factor | Tirofiban (n = 18) | Aspirin (n = 14) | p-value |
|---|---|---|---|
| Vitals and laboratory parameters | |||
| Systolic blood pressure on admission in mmHg, median (IQR) | 140 (130–160) | 161 (140–181) | 0.12 |
| Creatinine on admission (µL/mL), median (IQR) | 87 (71–94) | 79 (61–94) | 0.23 |
| Glucose level on admission, median (IQR) | 6.5 (5.6–7.2) | 6.1 (5.6–7.7) | 1 |
| Platelet count on admission (G/L), median (IQR) | 248 (207–282) | 211 (163–278) | 0.39 |
| INR on admission, median (IQR) | 1 (1–1) | 1.1 (1–1.2) | 0.033 |
| Medication | |||
| Prior antiplatelet therapy, n (%) | 2 (11%) | 3 (23%) | 0.37 |
| Peri-interventional heparin (dose in U/L), median (IQR) | 1625 (1000–3200) | 4800 (1300–5000) | 0.18 |
| Radiological parameters | |||
| ASPECT score, median (IQR) | 8 (7–9) | 9 (8–9) | 0.53 |
| Collateral score according Maas et al.,9 median (IQR) | 3 (3–4) | 3 (3–4) | 0.54 |
| ICA NASCET score, median (IQR) | 91 (86–100) | 88 (76–99) | 0.28 |
| TICI scale grade ≥ 2 b | 13 (72%) | 11 (85%) | 0.42 |
| Stroke volume (mL), median (IQR) | 43 (12.2–126.7) | 40.5 (12.6–99.2) | 0.97 |
| Temporal parameters | |||
| Time of stroke onset to intravenous thrombolysis in minutes, median (IQR) | 136 (105–150) | 115 (81–120) | 0.41 |
| Time of stroke onset to groin puncture in minutes, median (IQR) | 237 (187–285) | 243 (187–295) | 0.83 |
| Duration of endovascular intervention in minutes, median (IQR) | 129 (116–154) | 163 (92–205) | 0.29 |
ASPECT score = Alberta Stroke Programme Early CT score; ICA = internal carotid artery; IQR = interquartile range; NASCET = North American Symptomatic Carotid Endarterectomy Score; TICI Scale grade = Thrombolysis in Cerebral Infarction Scale grade.
All patients received intravenous thrombolysis with recombinant tissue plasminogen activator before the above-mentioned treatments with a median event-to-needle time of 136 min (IQR 187–285 min) in the tirofiban versus 115 min (IQR 81–120 min) in the aspirin group (p = 0.41). Peri-procedural, unfractioned heparin was also administered in all patients (Table 2). Median time of symptom onset to groin puncture was similar in all patients: 237 min (IQR 187–285 min) for tirofiban and 243 min (IQR 187–295 min) for aspirin groups, respectively (p = 0.83) (Table 2).
There were no significant differences between patients with or without ICH in terms of symptom onset to groin puncture or symptom onset to recanalization. Moreover, no statistical differences were present in terms of symptom onset to recanalization between the two study groups. Good recanalization rates (i.e., TICI score ≥2b) were achieved in 75% of the tirofiban and 85% of the aspirin groups (p = 0.42) (Table 2). On follow-up imaging, median stroke volumes of 40.5 mL (IQR 12.6–99.2 mL) in patients treated with tirofiban and 43 mL (IQR 12.2–126.7 mL) in aspirin treated patients were not significantly different (p = 0.97). Median stroke volume also did not significantly differ between patients with or without sICH (p = 0.22).
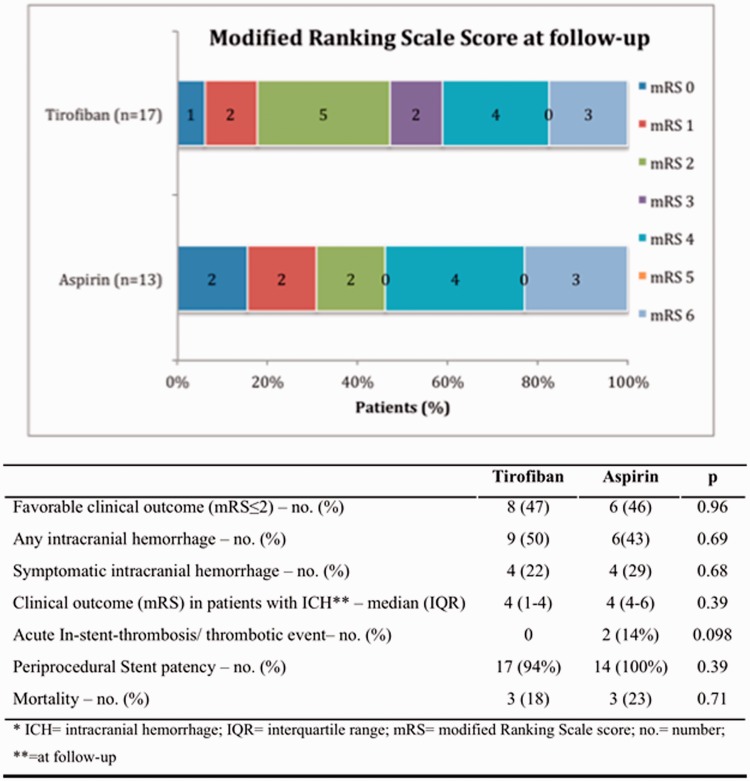
There were no significant differences in primary and secondary outcome measures between groups. Favorable clinical outcomes (mRS ≤ 2) were achieved in 47% of tirofiban and 46% of aspirin patients (p = 0.96) (Figure 1), independent of the initial APT (odds ratio (OR) 1.03, 95% confidence interval ((CI) 0.24–4.41, p = 0.96). Two patients were lost to follow-up (1 aspirin, 1 tirofiban). Both treatment regimens revealed similar mortality rates and no ischemic event occurred during the 3-month observation period.
Figure 1.
Clinical outcome and safety.
No difference regarding stent patency were observed between groups (p = 0.39) before changing antiplatelet treatment to aspirin or clopidogrel. One tirofiban patient developed a distal re-occlusion of the symptomatic ICA and two patients in the aspirin group suffered from intra-procedural in-stent thrombosis that quickly resolved by local tirofiban bolus application (Figure 1).
Both tirofiban and aspirin groups had similar rates of overall intracranial bleeding with 9 (50%) and 6 (43%) patients, (p = 0.69), respectively, and sICH in 4 (22%) and 4 (29%) patients (p = 0.68), respectively. Median sICH hemorrhage volume of the tirofiban group (29.3 mL, IQR 7.7–71.5 mL) was similar to the aspirin group (44.15 mL, IQR 29.1–73.6 mL) (p = 0.39). The most common bleeding side was the anterior part of basal ganglia. For the acute management of sICH patients, the antiplatelet therapies were immediately stopped. In two cases, an extraventricular drainage was inserted, including one case in which a hemicraniectomy with hematoma evacuation was performed. All sICH patients suffered unfavorable clinical outcomes; median mRS 4 at 90-day follow-up did not significantly differ between the two groups. Two patients died from sICH immediately after diagnosis (1 per group). Usually, after 3 days, low dose heparin and aspirin 100 mg was re-initiated, and 3 weeks later, clopidogrel 75 mg was added.
No significant associations were seen between ICH or sICH and demographic, clinical, or interventional parameters. In particular, there was no association of sICH (OR 0.71, 95% CI 0.14–3.55, p = 0.68) or any ICH (OR 1.33, 95% CI 0.33–5.43, p = 0.69) and tirofiban treatment. There was also no association between stroke volume and sICH (OR 1.003, 95% CI 0.99–1.01, p = 0.376). There was a trend for an association of higher NIHSS score on admission and sICH (OR 1.13, 95% CI 0.98–1.31, p = 0.085).
Discussion
We found similar clinical outcomes and safety profile between tirofiban and aspirin treated patients with eCAS in our cohort. Following current guidelines, dual APT (aspirin plus clopidogrel) is recommended for CAS.2 However, this initiates irreversible platelet dysfunction in acute stroke patients with eCAS who are predisposed for hemorrhagic complications.3 Consequently, tirofiban may be an appropriate alternative with its rapid effect onset and short half-life.
To date, the safety of GPIIb/IIIa antagonists has been investigated in several studies of acute stroke patients with controversial results. Promising data from the SaTIS trial showed that tirofiban in acute stroke patients was safe,7 whereas the AbESTT-II trial revealed negative results for abciximab in acute stroke.14 A large registry of CAS patients found neither a clear benefit nor increased risk for treatment with or without GPIIb/IIIa antagonists.15
However, these large trials were not limited solely to patients with eCAS and there are no large randomized trials on GPIIb/IIIa inhibitors in eCAS. In line with a recent meta-analysis (44%),16 and in the upper range compared to similar studies (29–52%),17,18 our results showed favorable clinical outcome in 47% and 46% of patients, respectively. Despite the relatively high NIHSS on admission, mortality rates were rather low and comparable with the 13–24.7% in other studies. Recanalization was successful in all cases. However, two aspirin-treated patients had intra-procedural acute in-stent thrombosis that quickly resolved by application of intra-arterial tirofiban bolus. Concerning stent patency, no differences were observed between the study groups and all stents remained patent before changing the treatment to aspirin or clopidogrel in the group. Our intracranial reperfusion rate with TICI ≥ 2b with 72% vs. 85% is comparable to other recent studies that ranged from 77% to 81%.
Regarding safety, sICH rates of 22% in our tirofiban group were similar to the previously reported 18% by Stampfl et al. and lower than the 31% in abciximab-treated patients.17,19 However, it was higher than the 7% rate in a recent meta-analysis for tandem occlusion with 95% CI 2–13 in eight studies.16 In line with observations made by Malik et al.,20 we found no significant differences in sICH rates between the two groups; all of our patients were also treated with intravenous thrombolysis. Although conflicting results have been reported for intravenous thrombolysis and concomitant APT in acute stroke patients,21 a recent study showed that additional tirofiban treatment for early re-occlusion prevention in acute stroke patients with intravenous thrombolysis did not increase ICH rates.6 Despite a relatively high overall bleeding rate versus other studies,16 there were no significant difference between the two groups and no obvious impact on favorable clinical outcome. Therefore, additional APT in the setting of eCAS is feasible and may be considered given the otherwise poor outcomes in these patients without treatment. Emergent percutaneous transluminal angioplasty of ICA-stenosis and intracranial thrombectomy with stenting of ICA-stenosis as a second elective step might also be considered an alternative treatment strategy.
Important limitations are our study’s retrospective, single center design and its small sample size. Given our overall relatively high bleeding rates compared with reports in the literature, we speculate that significant differences between both study groups might become evident in larger studies. Nevertheless, there is currently sparse evidence on the use of GPIIb/IIIa inhibitors in eCAS.
Conclusion
Our study showed comparable results regarding clinical outcome and intracranial bleeding rates for acute stroke patients whose carotid artery occlusions were treated with either tirofiban or aspirin during emergent carotid stenting. Tirofiban might serve as an alternative to aspirin in high-risk stroke patients with eCAS. Further randomized clinical trials should be prompted.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32: 2559–2566. [DOI] [PubMed] [Google Scholar]
- 2.Brott TG, Halperin JL, Abbara S, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke 2011; 42: e464–e540. [DOI] [PubMed] [Google Scholar]
- 3.Dorado L, Castano C, Millan M, et al. Hemorrhagic risk of emergent endovascular treatment plus stenting in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2013; 22: 1326–1331. [DOI] [PubMed] [Google Scholar]
- 4.Harker LA. Therapeutic inhibition of platelet function in stroke. Cerebrovasc Dis 1998; 8(Suppl 5): 8–18. [DOI] [PubMed] [Google Scholar]
- 5.Kellert L, Hametner C, Rohde S, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke 2013; 44: 1453–1455. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Lin L, Zhang M, et al. Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients. Stroke 2016; 47: 2649–2651. [DOI] [PubMed] [Google Scholar]
- 7.Siebler M, Hennerici MG, Schneider D, et al. Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke 2011; 42: 2388–2392. [DOI] [PubMed] [Google Scholar]
- 8.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group: Alberta Stroke Programme Early CT Score. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 9.Maas MB, Lev MH, Ay H, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009; 40: 3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339: 1415–1425. [DOI] [PubMed] [Google Scholar]
- 11.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 12.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009; 72: 2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP Jr, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke 2008; 39: 87–99. [DOI] [PubMed] [Google Scholar]
- 15.Zahn R, Ischinger T, Hochadel M, et al. Glycoprotein IIb/IIIa antagonists during carotid artery stenting: results from the carotid artery stenting (CAS) registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Clin Res Cardiol 2007; 96: 730–737. [DOI] [PubMed] [Google Scholar]
- 16.Sivan-Hoffmann R, Gory B, Armoiry X, et al. Stent-retriever thrombectomy for acute anterior ischemic stroke with tandem occlusion: a systematic review and meta-analysis. Eur Radiol 2017; 27: 247–254. [DOI] [PubMed] [Google Scholar]
- 17.Stampfl S, Ringleb PA, Mohlenbruch M, et al. Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. Am J Neuroradiol 2014; 35: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behme D, Mpotsaris A, Zeyen P, et al. Emergency stenting of the extracranial internal carotid artery in combination with anterior circulation thrombectomy in acute ischemic stroke: a retrospective multicenter study. Am J Neuroradiol 2015; 36: 2340–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg 2015; 7: 170–175. [DOI] [PubMed] [Google Scholar]
- 20.Malik AM, Vora NA, Lin R, et al. Endovascular treatment of tandem extracranial/intracranial anterior circulation occlusions: preliminary single-center experience. Stroke 2011; 42: 1653–1657. [DOI] [PubMed] [Google Scholar]
- 21.Zinkstok SM, Roos YB, investigators A. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet 2012; 380: 731–737. [DOI] [PubMed] [Google Scholar]