Abstract
Objective
The endovascular treatment strategy for acute tandem occlusion stroke is challenging, and controversy exists regarding which lesion should be treated first. This study addresses the uncertainty regarding the priority choice for thrombectomy in acute anterior circulation tandem occlusion stroke.
Methods
We analysed the clinical and angiographic data of tandem stroke patients who underwent interventional therapy from the endovAsCular Treatment of acUte Anterior circuLation ischaemic stroke (ACTUAL) registry. Recanalisation was assessed according to the modified thrombolysis in cerebral infarction score. Clinical outcome was evaluated at 90 days using the modified Rankin scale score.
Results
Sixty tandem occlusion stroke patients were enrolled. Thirty-one (51.7%) patients received anterograde therapy, while 29 (48.3%) patients underwent the retrograde approach. Successful recanalisation (modified thrombolysis in cerebral infarction score 2b–3) occurred in 78.3% (47/60) of patients, and 50.0% (30/60) of patients achieved a modified Rankin scale score of 0–2 at 90 days. Patients undergoing the retrograde approach spent less time in distal occlusion recanalisation (125 (86–167) vs. 95 (74–122) minutes; P = 0.04) and achieved better functional outcomes at 90 days (69.0% (20/29) vs. 32.3% (10/31); P = 0.004) than patients who received anterograde therapy. The retrograde approach was associated with favourable clinical outcomes (odds ratio 0.21; 95% confidence interval 0.07–0.64; P = 0.006).
Conclusion
For acute tandem occlusion stroke, favourable outcomes were better in patients undergoing retrograde therapy than in patients who received the anterograde approach. Future randomised trials are warranted to determine the optimal treatment.
Keywords: Tandem occlusion, intervention, stroke
Introduction
Acute ischaemic stroke due to tandem occlusions of the extracranial internal carotid artery (ICA) with the concomitant middle cerebral artery (MCA) was associated with major stroke leading to severe disability or death.1,2 Due to the large clot burden and likely limited delivery of the thrombolytic drug to the distal occlusion, tandem occlusions responded poorly to intravenous tissue plasminogen activator, and predicted poor outcome after intravenous thrombolysis.2,3 Therefore, endovascular treatment has become a more effective option for these lesions. Based on the evidence of recent clinical trials,4–9 acute ischaemic anterior circulation stroke patients could benefit from endovascular therapy, and the MR CLEAN,7 ESCAPE6 and REVASCAT9 trials included tandem stroke patients.
Despite the obvious benefit from mechanical thrombectomy, the best treatment strategy for tandem occlusion remains unclear. Different endovascular approaches have been proposed, including angioplasty with or without stenting of the cervical lesion first, followed by intracranial thrombectomy, or recanalisation of the intracranial occlusion prior to treatment of the cervical ICA occlusion.10–14 However, there has been no standardised recommendation. With the choice left up to the physician, controversy exists regarding which lesion should be addressed first. Therefore, we sought to evaluate the priority of the endovascular approach for recanalisation of tandem lesions from a multicentre, retrospective cohort study.
Methods
Study design and participants
We retrieved patients from the endovAsCular Treatment of acUte Anterior circuLation ischaemic stroke (ACTUAL) registry, which is a multicentre retrospective study of 21 hospitals in China from January 2014 to June 2016. The details of the registry have been described in our previous studies.15,16
Treatment strategy
A tandem occlusion was diagnosed based on the simultaneous presence of cervical ICA/MCA occlusions confirmed by computed tomographic angiography, magnetic resonance angiography or digital subtraction angiography. All procedures were performed by way of a femoral access, and after femoral access intravenous heparin was given immediately. Diagnostic angiography was performed to confirm the occlusion site and collateral supply. Two endovascular approaches were used in the management of patients with tandem occlusions. According to the sequence of extracranial or intracranial lesions addressed, the treatment strategies were classified as the anterograde approach and retrograde approach.
Anterograde approach
This approach was regarded as proximal-to-distal recanalisation, with angioplasty and/or stenting of the cervical lesion first, followed by intracranial thrombectomy (Figure 1). For this technique, a microwire followed by a microcatheter was advanced to pass the proximal occlusion at first. If difficult to cross the extracranial occlusion site, intra-arterial thrombolysis or balloon dilation was performed to relieve occlusion and establish a narrow passage so that the microwire or the microcatheter could pass through the occlusion when necessary. Then angioplasty and/or stenting was performed of the extracranial ICA lesion. The choice of stenting was up to the neurointerventionists. After cervical occlusion was recanalised, intracranial thrombectomy was performed in terms of stent retrieval, intra-arterial thrombolysis, angioplasty and aspiration.
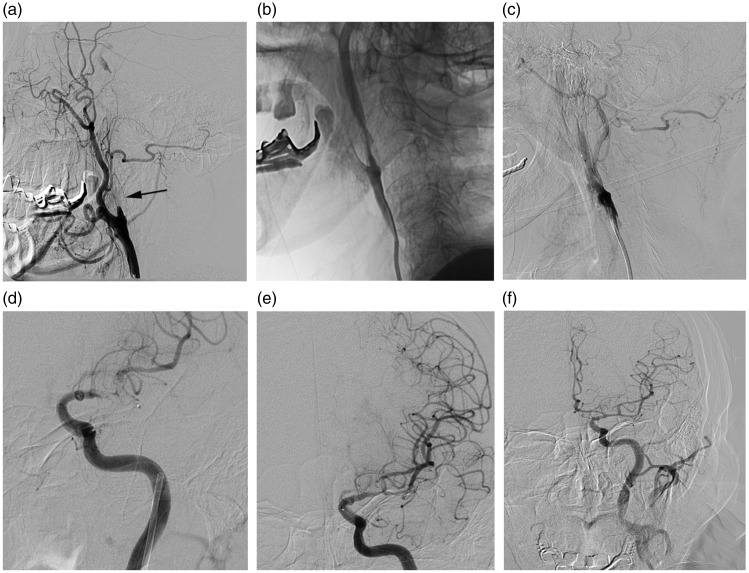
Figure 1.
The anterograde approach. A 74-year-old man, National Institutes of Health stroke scale onset score 15, distal reperfusion time 172 minutes and modified Rankin scale score at 90 days 6. (a) The angiogram showed complete occlusion of the left internal carotid artery (ICA) (arrow). (b, c) Angioplasty was performed with the ICA occlusion. (d, e) After proximal occlusion recanalisation, a stent retriever was deployed at the left middle cerebral artery (MCA) with revascularisation of the vessel. (f) The angiogram demonstrated complete revascularisation of the left ICA and MCA.
Retrograde approach
This approach was regarded as distal-to-proximal recanalisation, with treatment of the intracranial occlusion with mechanical thrombectomy first, followed by treatment of the cervical ICA occlusion (Figure 2). In these cases, once the proximal occlusion site was crossed with a microwire, intracranial occlusion thrombectomy was applied subsequently. Stent placement or balloon angioplasty of the extracranial ICA lesion was performed after intracranial thrombectomy.
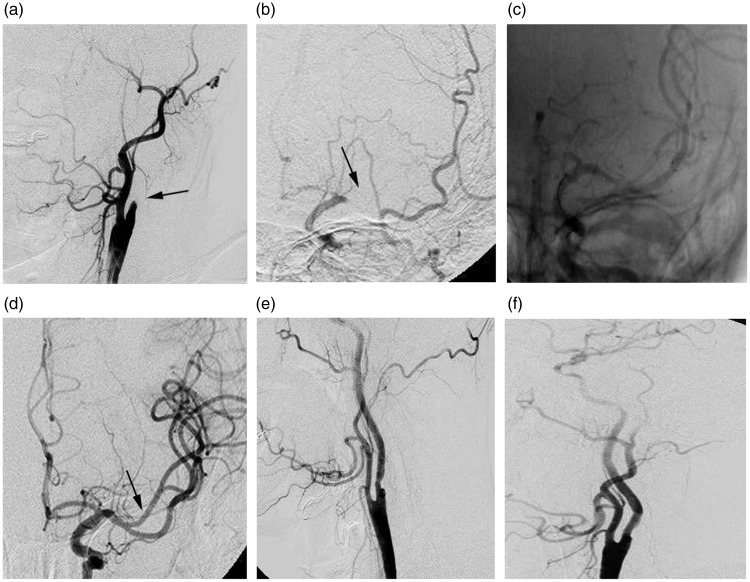
Figure 2.
The retrograde approach. A 60-year-old man, National Institutes of Health stroke scale onset score 14, distal reperfusion time 72 minutes and modified Rankin scale score at 90 days 2. (a) Extracranial angiogram showed complete occlusion of the left internal carotid artery (ICA) (arrow). (b) Intracranial angiogram showed a distal middle cerebral artery (MCA) occlusion (arrow) and good leptomeningeal collateralisation. (c) A stent retriever was deployed at the left distal MCA occlusion. (d) After thrombectomy with the stent retriever, successful recanalisation of the MCA segments was achieved (arrow). (e, f) The angiogram demonstrated successful reconstruction of the left ICA lumen after stent implantation and angioplasty.
For both approaches, after endovascular treatment, clopidogrel (75 mg) and aspirin (100 mg) were given after 24 hours of alteplase administration to patients who received intravenous alteplase, while for those without prior intravenous alteplase, loading doses of clopidogrel (300 mg) and aspirin (300 mg) were given. Patients who underwent stent placement during the procedure would receive antithrombotic medication. Further medication of clopidogrel (75 mg/day) and aspirin (100 mg/day) were prescribed to all the patients for the next 3 months.
Outcome measures
We used modified Rankin scale (mRS)17 score at 90 days to evaluate independent functional outcomes. A favourable outcome was defined as mRS 0–2 while a poor outcome was defined as mRS 3–6. Vessel recanalisation was assessed according to the modified thrombolysis in cerebral infarction (mTICI)18 scale, of which 0–2a was regarded as failed recanalisation and 2b–3 was defined as successful reperfusion. Symptomatic intracerebral haemorrhage (sICH) within 72 hours was evaluated based on the Heidelberg bleeding classification.19 Other variables including mortality, National Institutes of Health stroke scale (NIHSS) score, and procedure-related complications were also recorded.
Statistical analysis
Mean (standard deviation) or median (interquartile range; IQR) was performed to describe characteristics for continuous variables, and frequency (percentage) was used to describe categorical variables. Patients were divided into two groups described as the anterograde and retrograde approach. Comparisons of baseline and outcomes between both groups were based on a t-test or Mann–Whitney U-test for continuous variables, and χ2 or Fisher’s exact tests for categorical variables. A binary logistic regression model was conducted to evaluate independent predictors of functional outcome with entered factors including treatment strategy and other marginally significant variables (P < 0.1) on univariate analysis. Two-sided P < 0.05 was statistically significant. Data were analysed using Statistical Product and Service Solutions 22.0 (SPSS 22.0, IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics
Overall, 60 tandem stroke patients undergoing endovascular treatment were enrolled. Baseline characteristics are described in Table 1. The average age was 64 (IQR 52–69) years, and 85.0% (51/60) of the patients were men. Thirty-one (51.7%) patients received anterograde approach therapy, while 29 (48.3%) patients were given the retrograde approach. Treatment methods for proximal ICA occlusion included angioplasty 56.7% (34/60), stenting 41.7% (25/60) and aspiration 1.7% (1/60). Of the 25 stent placement cases, the stroke aetiology included atherosclerosis (60%, 15/25), cardioembolism (8%, 2/25), dissection (28%, 7/25) and other aetiology (4%, 1/25). Therapy strategies of MCA occlusion recanalisation consisted of stent retriever (66.7%, 40/60), intra-arterial thrombolysis (21.7%, 13/60), angioplasty (5.0%, 3/60) and aspiration (1.7%, 1/60). There was no difference between the anterograde and retrograde groups except artery occlusion aetiology (atherosclerosis 67.7% (21/31) vs. 65.5% (19/29); cardioembolism 22.6% (7/31) vs. 3.4% (1/29); dissection 6.5% (2/31) vs. 31.0% (9/29); and undetermined aetiology, 3.2% (1/31) vs. 0; P = 0.02).
Table 1.
Baseline and laboratory characteristics.
| Variable | All (n = 60) | Anterograde (n = 31) | Retrograde (n = 29) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR), years | 64 (52–69) | 64 (60–70) | 60 (41–68) | 0.20 |
| Men, n (%) | 51 (85.0%) | 24 (77.4%) | 27 (93.1%) | 0.15 |
| Medical history | ||||
| Atrial fibrillation, n (%) | 8 (13.3%) | 7 (22.6%) | 1 (3.4%) | 0.05 |
| Hypertension, n (%) | 30 (50.0%) | 19 (61.3%) | 11 (37.9%) | 0.07 |
| Diabetes mellitus, n (%) | 11 (18.3%) | 8 (25.8%) | 3 (10.3%) | 0.12 |
| Hyperlipidemia, n (%) | 5 (8.3%) | 4 (12.9%) | 1 (3.4%) | 0.36 |
| Current smoker, n (%) | 25 (41.7%) | 11 (35.5%) | 14 (48.3%) | 0.32 |
| Artery occlusion aetiology | 0.02 | |||
| Atherosclerosis, n (%) | 40 (66.7%) | 21 (67.7%) | 19 (65.5%) | |
| Cardioembolism, n (%) | 8 (13.3%) | 7 (22.6%) | 1 (3.4%) | |
| Dissection, n (%) | 11 (18.3%) | 2 (6.5%) | 9 (31.0%) | |
| Undetermined aetiology, n (%) | 1 (1.7%) | 1 (3.2%) | 0 | |
| Laboratory measures | ||||
| TG, median (IQR), mmol/L | 1.03 (0.71–1.89) | 1.19 (0.76–1.88) | 0.95 (0.64–2.10) | 0.38 |
| TC, median (IQR), mmol/L | 4.16 (3.59–5.09) | 4.16 (3.71–5.04) | 4.07 (3.45–5.19) | 0.88 |
| HDL, mean (IQR), mmol/L | 1.21 (1.01–1.52) | 1.28 (0.96–1.54) | 1.15 (1.01–1.40) | 0.44 |
| LDL, mean (SD), mmol/L | 2.56 (0.86) | 2.54 (0.74) | 2.57 (0.98) | 0.91 |
| Glu, median (IQR), mmol/L | 6.90 (5.46–8.80) | 7.53 (5.73–9.30) | 6.48 (5.45–7.40) | 0.19 |
| Clinical characteristics | ||||
| SBP, mean (SD), mmHg | 142 (21) | 142 (20) | 142 (23) | 0.99 |
| Baseline ASPECTS, median (IQR) | 9 (8–10) | 9 (8–10) | 9 (8–10) | 0.72 |
| Baseline NIHSS score, mean (SD) | 17 (6) | 17 (8) | 16 (3) | 0.92 |
SD: standard deviation; IQR: interquartile range; TG: triglyceride; TC: total cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Glu: glucose; SBP: systolic blood pressure; ASPECTS: Alberta Stroke Program early CT score; NIHSS: National Institutes of Health stroke scale.
Angiographic data and clinical outcome
The median procedural time was 122 (90–167) minutes and time to distal occlusion recanalisation was 114 (81–153) minutes. Successful recanalisation (mTICI 2b–3) was achieved by 78.3% (47/60) of patients. Two (3.3%) cases of spontaneous intracranial recanalisation occurred immediately after ICA therapy. sICH post-endovascular treatment at 72 hours was found in 11.7% (7/60) of patients, and two (3.3%) patients presented with dissection during the procedure. We did not observe arterial perforation, stent fracture, stent deployment failure and other technical complications. At 90 days follow-up, 50.0% (30/60) of patients achieved mRS 0–2 and the mortality rate was 20.0% (12/60). Patients undergoing the retrograde approach spent less time in distal occlusion recanalisation (125 (86–167) minutes vs. 95 (74–122) minutes; P = 0.04) and achieved better functional outcomes at 90 days (69.0% (20/29) vs. 32.3% (10/31); P = 0.004), as well as lower mortality (6.9% (2/29) vs. 32.3% (10/31); P = 0.01) than patients who received anterograde therapy. Multivariate logistic regression analysis showed that the retrograde approach (odds ratio (OR) 0.21, 95% confidence interval (CI) 0.07–0.64; P = 0.006) was the only independent predictor of favourable clinical outcomes at 90 days. Tables 2 and 3 show the endovascular characteristics and clinical outcomes.
Table 2.
Characteristics of endovascular procedures.
| Variables | All (n = 60) | Anterograde (n = 31) | Retrograde (n = 29) | P value |
|---|---|---|---|---|
| Collateral (ASITN/SIR), no. (%) | ||||
| 0–1 | 31 (51.7%) | 17 (54.8%) | 14 (48.3%) | 0.61 |
| 2–3 | 29 (48.3%) | 14 (45.2%) | 15 (51.7%) | |
| Intravenous thrombolysis, n (%) | 21 (35.0%) | 8 (25.8%) | 13 (44.8%) | 0.12 |
| Time intervals | ||||
| Time from onset to emergency room, min, mean (SD) | 166 (105) | 172 (114) | 160 (97) | 0.66 |
| Time from door to puncture, min, mean (SD) | 131 (61) | 137 (63) | 125 (60) | 0.45 |
| Time from onset to treatment,a median (IQR), min | 297 (221–355) | 298 (220–365) | 295 (221–338) | 0.58 |
| Time from groin puncture to reperfusion, median (IQR), min | 122 (90–167) | 125 (86–167) | 116 (91–179) | 0.99 |
| Distal occlusion recanalisation, median (IQR), min | 114 (81–153) | 125 (86–167) | 95 (74–122) | 0.04 |
| Time from onset to recanalisation, median (IQR), min | 410 (351–514) | 405 (336–521) | 410 (367–487) | 0.94 |
| Treatment strategy | ||||
| ICA stenting, n (%) | 25 (41.7%) | 10 (32.3%) | 15 (51.7%) | 0.13 |
| MCA recanalisation | 0.16 | |||
| Retrievable stent, n (%) | 41 (68.3%) | 20 (64.5%) | 21 (72.4%) | |
| Intra-arterial thrombolysis, n (%) | 13 (21.7%) | 8 (25.8%) | 5 (17.2%) | |
| Angioplasty, n (%) | 3 (5.0%) | 0 | 3 (10.3%) | |
| Aspiration, n (%), | 1 (1.7%) | 1 (3.2%) | 0 | |
| Spontaneous recanalisation, n (%) | 2 (3.3%) | 2 (6.5%) | 0 | |
| mTICI, n (%) | 0.15 | |||
| 0–2a | 13 (21.7%) | 9 (29.0%) | 4 (13.8%) | |
| 2b–3 | 47 (78.3%) | 22 (71.0%) | 25 (86.2%) | |
Time from symptom onset to groin puncture.
ASITN/SIR: American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; ICA: internal carotid artery; MCA: middle cerebral artery; mTICI: modified thrombolysis in cerebral infarction.
Table 3.
Clinical and safety outcomes.
| Variables | All (n = 60) | Anterograde (n = 31) | Retrograde (n = 29) | P value |
|---|---|---|---|---|
| mRS score at 90 days 0–2, n (%) | 30 (50.0%) | 10 (32.3%) | 20 (69.0%) | 0.004 |
| Mortality at 90 days, n (%) | 12 (20.0%) | 10 (32.3%) | 2 (6.9%) | 0.01 |
| Safety outcomes, n (%) | ||||
| sICH | 7 (11.7%) | 5 (16.1%) | 2 (6.9%) | 0.43 |
| aICH | 29 (48.3%) | 16 (51.6%) | 13 (44.8%) | 0.60 |
| Procedure and device related complications, n (%) | ||||
| Arterial dissection | 2 (3.3%) | 2 (6.5%) | 0 | 0.49 |
| Arterial perforation | 0 | 0 | 0 | N/A |
| Stent fracture | 0 | 0 | 0 | N/A |
| Stent deployment failure | 0 | 0 | 0 | N/A |
mRS: modified Rankin scale; sICH: symptomatic intracerebral haemorrhage; aICH: asymptomatic intracerebral haemorrhage.
Discussion
We have three findings in this study. First, endovascular treatment was safe and effective in acute anterior stroke patients with tandem occlusion. Second, a retrograde approach with distal-to-proximal recanalisation may be superior to an anterograde approach with proximal-to-distal recanalisation. Third, the endovascular retrograde approach was associated with favourable outcomes in tandem stroke patients.
In recent trials, the MR CLEAN7 trial included 146 patients with an additional extracranial internal-carotid-artery occlusion undergoing intra-arterial therapy, and the OR for a good outcome was 1.43 (95% CI 0.78–2.64). In the ESCAPE6 and REVASCAT9 trials which recruited 45 and 23 tandem occlusions stroke patients within interventional therapy, respectively, 7.8% and 12.9% of patients achieved a favourable independent outcome at 90 days. These results indicated that tandem patients would benefit from endovascular therapy, but the efficacy among these trials was significantly different. Several other studies also suggested angioplasty and/or stenting in extracranial ICA and mechanical thrombectomy in intracranial MCA for tandem lesions reasonable and acceptable, with results of recanalisation 62.5–91%, sICH 0–22.0%, mRS 0–2 at 90 days 29.2–76%, and mortality 8–39%12,13,20–24. (Table 4). Our results were in line with these series. In this study, successful recanalisation was high, sICH and mortality rate were acceptable, and nearly a half of patients benefited from interventional treatment.
Table 4.
Summary of present study and other studies of tandem stroke endovascular treatment.
| Study | Methods | Sample | Treatment strategy | Recanalisation | sICH | mRS 0–2 at 90 days | Mortality at 90 days |
|---|---|---|---|---|---|---|---|
| Rangel-Castilla et al.12 | Retrospective single-centre | 45 | Anterograde/retrograde | 87% TICI ≥ 2b | 4.4% | 73.3% | 11%a |
| Lockau et al.13 | Retrospective single-centre | 37 | Anterograde/retrograde | 73% TICI ≥ 2b | 10.8%b | 45.9% | 18.9% |
| Lescher et al.20 | Retrospective single-centre | 39 | Anterograde/retrograde | 64% TICI ≥ 2b | 10% | 36% | 10% |
| Heck and Brown21 | Retrospective single-centre | 23 | Anterograde | 91% TICI ≥ 2b | 22% | 52% | 39% |
| Stampf et al.22 | Retrospective single-centre | 24 | Anterograde/retrograde | 62.5% TICI ≥ 2b | 16.6% | 29.2% | 16.6% |
| Cohen et al.23 | Retrospective single-centre | 24 | Anterograde | 79% TIMI 3, TICI ≥ 2b | 0 | 76% | 8% |
| Maurer et al.24 | Retrospective single-centre | 43 | Anterograde | 76.7% TICI ≥ 2b | 11.6%b | 32.6% | 32.6%c |
| Our study | Retrospective multi-centre | 60 | Anterograde/retrograde | 78.3% mTICI ≥ 2b | 11.7% | 50% | 20% |
Mortality in hospital.
Symptomatic parenchymal haemorrhage.
Mortality at discharge.
TIMI: thrombolysis in myocardial infarction; TICI: thrombolysis in cerebral ischaemia.
We described two different endovascular therapy approaches in this study, and aimed to investigate the optimal therapy strategy. The results suggested a distinctly better clinical outcome in patients who received MCA thrombectomy prior to ICA recanalisation. The reasons may be several. Above all, distal artery recanalisation was more important than proximal occlusion treatment. Rangel-Castilla et al.12 found that, for tandem ICA/MCA occlusions, when distal recanalisation of an occluded intracranial vessel was performed, the duration of ischaemia would decrease, which may result in an improvement of circulation collateral. Another study demonstrated in tandem stroke patients that clinical improvement was determined by resumption of flow in the MCA, therefore outcomes would be better once MCA was recanalised on time even though ICA remained occluded.25 Rahme et al.10 also considered that if both extracranial and intracranial occlusions were coexistent, treatment of the latter would dictate the final outcome. Besides that, primary distal thrombectomy before recanalisation of an occluded cervical carotid would reduce time. Cohen et al.11 found that the retrograde technique reduced cerebral ischaemia time by postponing ICA reconstruction until after successful intracranial revascularisation. Lockau et al.13 deemed that thrombectomy prior to proximal stenting was associated with shorter reperfusion times. In our study, the procedure time showed no difference between the approaches, but time to distal occlusion recanalisation was shorter in patients undergoing the retrograde method. Therefore, compared with anterograde therapy, retrograde treatment may avoid the time delay to intracranial recanalisation. In addition, it was reported that the anterograde strategy could lead to some potential complications, such as distal embolisation into intracranial vessels, dissection or perforation of the extracranial carotid, and cerebral reperfusion haemorrhage.10 In this series, two patients undergoing anterograde treatment presented with artery dissection during the procedure. Simultaneously, more intracerebral haemorrhage, especially sICH, occurred in patients who received the anterograde approach than patients undergoing the retrograde method. Due to the small sample size, there was no statistically significant difference between the groups. However, this tendency may indicate a higher technical complication rate of the proximal-to-distal strategy.
Our results revealed that retrograde treatment was superior to anterograde therapy, which was consistent with previous investigators.10,13,14 We also found the retrograde approach was associated with favourable independent outcomes. Nevertheless, other factors such as age, presenting NIHSS, recanalisation and ASPECTS were not found to be predictive of the clinical outcome in our series, which was reported in some studies.14,26 In our study, age, NIHSS and ASPECTS were balanced in both groups so that these variables were not taken into account in multivariable regression analysis. Besides, successful recanalisation was not found to be a predictor of independent functional outcomes in this study, which may be related to its overall high frequency.
In the retrograde group, 25 patients achieved successful recanalisation (mTICI 2b/3), and in these recanalised patients, collateral circulation was not observed in 92% (23/25) patients after forward blood flow was restored. It has been reported that the pial collateral formation during acute ischaemic stroke would influence clinical outcomes.27,28 Collateral circulation could be classified as ‘good’ collateral (scores 1 or 2) and ‘bad’ collateral (scors 3–5) according to pial collaterals based on angiograms.29 In our study, 28 patients achieved independent outcomes at 90 days, of which 64.7% (22/28) of patients presented with good collateral circulation, while 28.6% (6/28) presented with poor collateral circulation. It appeared that endovascular treatment may have a greater clinical impact in patients with better pial collateral formation.
Distal occlusion thrombectomy using stent retrieval was acceptable, safe and effective in acute tandem patients on the basis of confirmed analysis.30 In accord with this, the majority of patients in our study were treated with retrievable stents. Furthermore, more various treatment methods including intra-arterial thrombolysis, angioplasty and aspiration were also performed in intracranial thrombectomy procedures. Vessel recanalisation rate was achieved to a high level, and clinical outcome was also fine in this series, suggesting this multimodal therapy for distal recanalisation to be a feasible and effective treatment modality in acute tandem lesion stroke.
Whether the extracranial carotid lesion should be stented or not is controversial. Some authors considered that stenting in carotid stenosis in the situation of tandem lesions in ischaemic stroke may be potentially hazardous,30 while others tended to perform carotid artery stenting immediately after successful thrombectomy.31 Twenty-five (41.7%) patients were treated with ICA stenting in the acute setting in our study, and the choice of stenting or not was left to the judgement of physicians based on an individual case basis. To stent or not to stent, there has been no consensus to date. We did not discuss stent placement in the extracranial occlusion there, and the question needs to be investigated in a further study. Beyond that, the endovascular approach for dissection remains poorly studied. In our study, 11 (18.3%) cases were carotid artery dissection, and it was possible that the aetiology of the stroke influenced the approaches and effects of endovascular therapy.
This study has certain limitations. First, as a retrospective study, it may have selection bias. In our study, the anterograde group included more cardioembolism occlusion patients. In addition, multiple thrombectomy techniques and devices were used to treat tandem occlusions, and this may lead to a lack of standardisation of therapy. Furthermore, the retrograde approach also included some risks and potential complications such as vessel dissections, vessel perforation or even distal embolisation. These complications were not reported obviously because of the small size of the sample, which limited the power of analysis. However, the data presented a multicentre study,15 and our results draw the conclusion that distal recanalisation before addressing proximal occlusion was a priority selection for tandem lesion stroke, whereas a randomised clinical trial is warranted in the future.
Conclusions
For tandem stroke patients undergoing endovascular therapy in this study, successful recanalisation was achieved in the majority of patients, and nearly half of the patients obtained favourable functional outcomes. The retrograde treatment strategy has a shorter distal reperfusion time and better good functional outcomes at 90 days than the anterograde treatment strategy.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the National Natural Science Foundation of China (nos. 81530038, 81220108008 and 81671172) and the Jiangsu Provincial Special Program of Medical Science (no. BL2013025).
References
- 1.Adams HP, Jr, Bendixen BH, Leira E, et al. Antithrombotic treatment of ischemic stroke among patients with occlusion or severe stenosis of the internal carotid artery: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999; 53: 122–125. [DOI] [PubMed] [Google Scholar]
- 2.Rubiera M, Ribo M, Delgado-Mederos R, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke; a journal of cerebral circulation 2006; 37: 2301–2305. [DOI] [PubMed] [Google Scholar]
- 3.Rubiera M, Alvarez-Sabin J, Ribo M, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke; a journal of cerebral circulation 2005; 36: 1452–1456. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 5.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 7.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 9.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 10.Rahme R, Abruzzo TA, Ringer AJ. Acute ischemic stroke in the setting of cervical carotid occlusion: a proposed management strategy. World Neurosurg 2011; 76: S60–S65. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JE, Gomori M, Rajz G, et al. Emergent stent-assisted angioplasty of extracranial internal carotid artery and intracranial stent-based thrombectomy in acute tandem occlusive disease: technical considerations. J Neurointerv Surg 2013; 5: 440–446. [DOI] [PubMed] [Google Scholar]
- 12.Rangel-Castilla L, Rajah GB, Shakir HJ, et al. Management of acute ischemic stroke due to tandem occlusion: should endovascular recanalization of the extracranial or intracranial occlusive lesion be done first? Neurosurgl Focus 2017; 42: E16. [DOI] [PubMed] [Google Scholar]
- 13.Lockau H, Liebig T, Henning T, et al. Mechanical thrombectomy in tandem occlusion: procedural considerations and clinical results. Neuroradiology 2015; 57: 589–598. [DOI] [PubMed] [Google Scholar]
- 14.Mbabuike N, Gassie K, Brown B, et al. Revascularization of tandem occlusions in acute ischemic stroke: review of the literature and illustrative case. Neurosurg Focus 2017; 42: E15. [DOI] [PubMed] [Google Scholar]
- 15.Zi W, Wang H, Yang D, et al. Clinical effectiveness and safety outcomes of endovascular treatment for acute anterior circulation ischemic stroke in China. Cerebrovasc Dis 2017; 44: 248–258. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Hao Y, Zi W, et al. Effect of retrievable stent size on endovascular treatment of acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol 2017; 38: 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong KS, Saver JL. Quantifying the value of stroke disability outcomes: WHO global burden of disease project disability weights for each level of the modified Rankin Scale. Stroke; a journal of cerebral circulation 2009; 40: 3828–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008; 29: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke; a journal of cerebral circulation 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 20.Lescher S, Czeppan K, Porto L, et al. Acute stroke and obstruction of the extracranial carotid artery combined with intracranial tandem occlusion: results of interventional revascularization. Cardiovasc Intervent Radiol 2015; 38: 304–313. [DOI] [PubMed] [Google Scholar]
- 21.Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointervent Surg 2015; 7: 170–175. [DOI] [PubMed] [Google Scholar]
- 22.Stampfl S, Ringleb PA, Mohlenbruch M, et al. Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. Am J Neuroradiol 2013; 35: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen JE, Gomori JM, Rajz G, et al. Extracranial carotid artery stenting followed by intracranial stent-based thrombectomy for acute tandem occlusive disease. J Neurointervent Surg 2015; 7: 412–417. [DOI] [PubMed] [Google Scholar]
- 24.Maurer CJ, Joachimski F, Berlis A. Two in one: endovascular treatment of acute tandem occlusions in the anterior circulation. Clin Neuroradiol 2014; 25: 397–402. [DOI] [PubMed] [Google Scholar]
- 25.Christou I, Felberg RA, Demchuk AM, et al. Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimag: Official journal of the American Society of Neuroimaging 2002; 12: 119–123. [DOI] [PubMed] [Google Scholar]
- 26.Grigoryan M, Haussen DC, Hassan AE, et al. Endovascular treatment of acute ischemic stroke due to tandem occlusions: large multicenter series and systematic review. Cerebrovasc Dis 2016; 41: 306–312. [DOI] [PubMed] [Google Scholar]
- 27.Christoforidis GA, Karakasis C, Mohammad Y, et al. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. AJNR Am J Neuroradiol 2009; 30: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christoforidis GA, Vakil P, Ansari SA, et al. Impact of pial collaterals on infarct growth rate in experimental acute ischemic stroke. AJNR Am J Neuroradiol 2017; 38: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christoforidis G, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005; 26: 1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 30.Sivan-Hoffmann R, Gory B, Armoiry X, et al. Stent-retriever thrombectomy for acute anterior ischemic stroke with tandem occlusion: a systematic review and meta-analysis. Eur Radiol 2017; 27: 247–254. [DOI] [PubMed] [Google Scholar]
- 31.Behme D, Molina CA, Selim MH, et al. Emergent carotid stenting after thrombectomy in patients with tandem lesions. Stroke; a journal of cerebral circulation 2017; 48: 1126–1128. [DOI] [PubMed] [Google Scholar]