Abstract
A fetal posterior cerebral artery (FPCA) is an anatomic variant in which the posterior cerebral artery is an embryological derivative of the internal carotid artery. Although most cases of ischemic strokes in patients with FPCAs involve embolic infarcts, emergent large vessel occlusion of a FPCA is extremely rare. We present two cases of successful endovascular intervention for emergent occlusion of a FPCA, one of which is only the second reported case of a mechanical thrombectomy of a FPCA. We review the embryology of FPCA, the controversy regarding its association with cerebral infarcts, and various approaches used in the treatment of such occlusive lesions.
Keywords: Endovascular, fetal posterior cerebral artery, ischemic stroke, posterior cerebral artery, stent retriever, stroke, thrombectomy
Introduction
Most adult humans possess a classic vascular anatomy in which the posterior cerebral artery (PCA) originates from the basilar artery as part of the vertebrobasilar system. In humans with a fetal-type or fetal posterior cerebral artery (FPCA), the posterior communicating artery (PcomA) continues as the P2 segment of the PCA, along with an absent or hypoplastic ipsilateral P1 segment.1 Although FPCA is considered a normal anatomic variant, its presence may modify the distribution and severity of cerebral thromboembolic events.2,3
Five recent major randomized trials, showing the benefit of mechanical thrombectomy in patients with acute ischemic stroke (AIS) due to emergent large-vessel occlusions (ELVOs), all involved anterior circulation occlusions.4 Although a FPCA is an embryologic derivative of the anterior circulation, ELVO of a FPCA is extremely rare. We herein present two cases of AIS due to emergent occlusion of a FPCA, both of which were treated with endovascular intervention, and one of which is only the second report of a successful FPCA mechanical thrombectomy. We review the embryology of FPCA, the controversy regarding the association of cerebral infarcts and FPCA, and various approaches used in the treatment of such occlusive lesions.
Methods
The two patients’ medical records were retrospective reviewed, including the clinical presentation, initial workup, and medical and interventional management. Clinical and radiographic follow-up was also reviewed. This study received Institutional Review Board approval. In addition, patients provided written consent to be included in all research-related activities.
Results
Case 1
A 56-year-old male with hypertension, diabetes, coronary artery disease and ischemic cardiomyopathy presented to the emergency room 2 h after acute onset of left sided hemiplegia and a left-sided visual field cut, with a National Institutes of Health Stroke Scale (NIHSS) of 17. A non-contrast computed tomography (CT) scan and CT angiogram (CTA) of the head demonstrated an Alberta Stroke Program Early CT Score (ASPECTS) of 9 and intraluminal thrombus in the communicating segment of the right internal carotid artery (ICA) and extending into a prominent PcomA (Figure 1(a)). Due to significant thrombocytopenia, the patient was not a candidate for intravenous alteplase (tPA). The decision was made to proceed with emergent angiography given the patient’s severity of symptoms, the advantageous timing of presentation, and favorable imaging characteristics, similarly to a patient with a traditional anterior circulation ELVO.
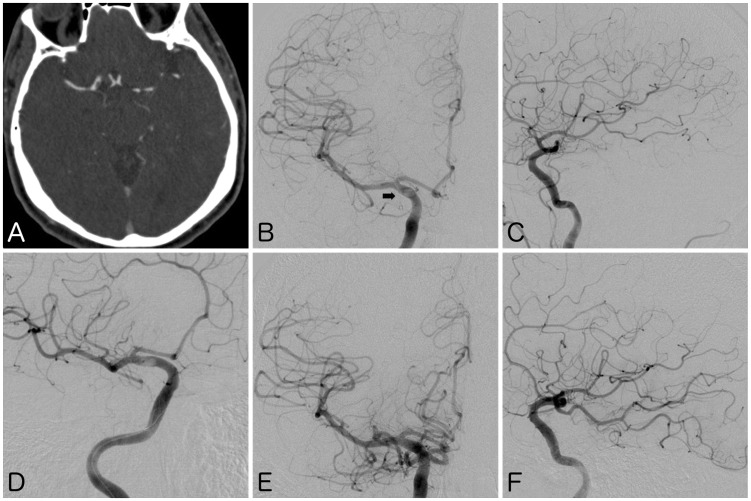
Figure 1.
(a) Computed tomography angiogram showing mural thrombus in right internal carotid artery (ICA), extending into fetal posterior cerebral artery (FPCA). (b), (c) Frontal and lateral views of digital subtraction angiogram (DSA) confirming the intracranial occlusion. Note the thrombus lucency at the expected location of the double density ostium of FPCA (arrow). (d) DSA showing deployed stent retriever within the ICA, across the ostium of the FPCA. (e), (f) Follow-up DSA after thrombectomy showing recanalization of FPCA with restoration of anterograde flow into parieto-occipital and inferior lateral temporal branches.
Digital subtraction angiography (DSA) confirmed a mural thrombus in the communicating segment of the right ICA extending into a FPCA, with patency of the right anterior cerebral artery (ACA) and middle cerebral artery (MCA) (Figure 1(b) and (c)). The right PCA territory demonstrated delayed retrograde opacification via leptomeningeal collaterals from the right MCA. Left vertebral artery DSA confirmed an aplastic P1 segment of the right PCA with delayed anterograde flow. Through an 8-French Merci Concentric balloon guide catheter (Concentric Medical, Mountain View, CA, USA), a 6 mm × 25 mm Trevo stent retriever (Stryker Neurovascular, Fremont, CA, USA) was deployed within the communicating segment of the right ICA, across the FPCA ostium (Figure 1(d)). Using proximal balloon flow-arrest, the stent retriever was recovered under continuous aspiration, 230 min after the patient’s onset of symptoms. Follow-up DSA demonstrated restoration of anterograde flow through the FPCA, with TICI 3 recanalization (Figure 1(e) and (f)).
On immediate post-procedure exam, the patient’s NIHSS improved to 9. The patient continued to improve and was discharged on hospital day #4 with a mild left arm pronator drift, and a Modified Rankin Scale (mRS) of 0. The etiology of the stroke was cryptogenic, and the patient was treated with aspirin for secondary stroke prevention.
Case 2
A 70-year-old male with diabetes and coronary artery disease presented 50 min after acute onset of left-sided visual field cut, left-sided weakness, left facial droop and anosognosia, with a NIHSS of 19. Non-contrast CT of the head showed an ASPECTS of 9. Initial review of the CT angiogram of the head and neck showed calcific atherosclerotic disease of the ipsilateral cervical ICA as well as apparent patency of all traditional anterior circulation cerebral vessels. However, CT perfusion images showed a large perfusion deficit involving the posterior right frontal, right parietal and right occipital lobes. Upon closer review of the CTA, an intraluminal thrombus was subsequently identified in a FPCA (Figure 2(a) and (b)). At 81 min from the onset of the patient’s symptoms, intravenous tPA was administered at a dosage of 0.9 mg/kg and the patient was taken emergently to the angiography suite. DSA demonstrated severe calcific atherosclerotic disease of the cervical right ICA causing 80% stenosis with concomitant intraluminal thrombus, the severity of which was not fully appreciated on the initial CTA (Figure 2(c)). The tandem intracranial occlusion of the right FPCA was confirmed, without significant anterograde flow into the right PCA territory. The ACA and MCA were patent, although leptomeningeal collateralization to the PCA territory was not present (Figure 2(d) to (f)). Given the concern of traversing the unstable cervical right ICA intraluminal thrombus with a guide catheter or a microcatheter, as well as the immediate lack of evidence on emergent thrombectomy of a FPCA, the decision was made to forgo aggressive interventional maneuvers. Subsequently, at 141 min after the onset of the patient’s symptoms, 7 mg of intra-arterial tPA was administered through the guide catheter into the ICA, and the procedure was terminated.
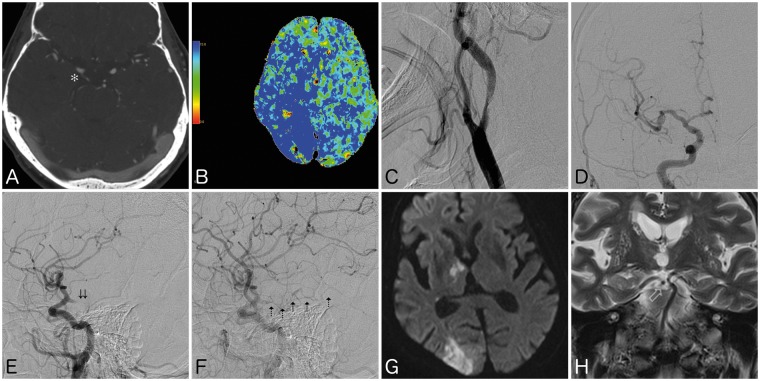
Figure 2.
(a) Computed tomography (CT) angiogram showing intraluminal thrombus located completely within a right fetal posterior cerebral artery (FPCA) (asterisk). (b) CT perfusion scan showing elevated mean transit time involving the posterior right frontal, right temporal, right parietal, and right occipital lobes. (c) Digital subtraction angiogram (DSA) of cervical right internal carotid artery showing severe stenosis with concomitant intraluminal thrombus. (d), (e) DSA in frontal and lateral views showing patency of traditional anterior circulation, with acute occlusion of FPCA (double arrows). (f) Lateral view in late arterial phase showing intralumal thrombus in FPCA with delayed opacification of P3 segments (dashed arrows). (g) Follow-up magnetic resonance image showing moderate infarct of the right thalamus and occipital lobe, with (h) coronal T2 sequence showing patent recanalization of the FPCA. Note the absence of ipsilateral P1 segment.
Twenty-four hours after tPA administration, the patient was treated with dual antiplatelet therapy with aspirin and Plavix (Bristol-Meyers Squibb, New York, NY, USA). A follow-up magnetic resonance angiogram showed recanalization of the FPCA. Magnetic resonance imaging (MRI) of the brain showed infarct in the right occipital lobe, inferior right temporal lobe, and right thalamus, without hemorrhage (Figure 2(g) and (h)). Areas showing prior CT perfusion deficits, including the posterior right frontal lobe and right parietal lobe, were spared of infarct. The etiology of the FPCA occlusion was determined to be secondary to artery-to-artery embolus from an unstable right ICA stenosis. Thus, on hospital day #10, the patient was treated with uneventful right carotid endarterectomy. The patient’s neurological exam moderately improved, and on three-month follow-up, the patient demonstrated a NIHSS of 10, with a mRS of 2.
Discussion
The prevalence of a FPCA has been reported to be between 3% and 26%, based on anatomic and angiographic studies.5,6 Although the presence of a FPCA has been implicated in multi-territory and paradoxical cerebral infarcts, AIS due to emergent occlusion of a FPCA is extremely rare.2,6 Despite the fact that a FPCA is an embryologic derivative of the anterior circulation, five recent major randomized trials showing the benefit of mechanical thrombectomy in anterior circulation occlusions did not include ELVOs of FPCAs.4 The rarity of an ELVO of a FPCA is likely due to a combination of the low anatomic incidence, a low ICA-FPCA pressure gradient, and the fluid dynamics of embolus trajectory, which strongly favors a terminal embolus position within the MCA rather than isolated to a FPCA.2,7
Guidelines do not currently exist for treatment of AIS due to FPCA occlusion. The 2018 American Heart Association/American Stroke Association guidelines for the management of AIS provide Level IIb evidence for the use of stent retriever thrombectomy in patients with either basilar artery or PCA occlusion, stating uncertain benefits in patients treated within 6 h of symptom onset.8 Although a complete homonymous hemianopsia due to acute PCA infarction can severely affect a patient’s quality of life, the NIHSS underestimates the severity of such symptoms (only two NIHSS points). Because of this relatively small contribution, intravenous thrombolysis and/or mechanical thrombectomy in the setting of acute PCA infarction is often not indicated.9 In our cases, in addition to the homonymous hemianopsia, both patients demonstrated severe symptoms typically encountered in AIS due to traditional anterior circulation ELVO. Thus, although specific guidelines do not exist for AIS due to FPCA occlusion, we decided to intervene by applying analogous guidelines of standard care for ELVO of anterior circulation cerebral vessels.
Several studies have examined the association of FPCA with ischemic stroke; however, the clinical significance of its presence remains controversial. Although dated postmortem studies showed more FPCAs in brains with infarcts than in those without, more recent studies yield conflicting results.3,6,10–13 Several individual reports describe embolic cerebral infarcts in patients with FPCA, while larger series by Yang et al. and Arjal et al. show an increased frequency of a “causative” FPCA ipsilateral to cerebral infarctions, as well as a greater predisposition to ischemic stroke in patients with FPCA.2,14,15–20 However, in another series of patients with PCA infarcts, de Monyé et al. show no increased risk in patients with a FPCA.3 In the largest series of patients with ischemic stroke examined for the presence of FPCA, Shaban et al. conclude that although a FPCA may predispose to stroke mechanism, it is not associated with vascular distribution, stroke severity, or early outcome.1
Variation in leptomeningeal collateralization has been postulated to be a factor in the association of FPCA and PCA infarcts.2,6 Extensive ischemic stroke literature has shown that pathophysiological recruitment of cerebral collateral circulation independently predicts reperfusion, final infarct size, and clinical outcome.21 In patients with FPCA, leptomeningeal collaterals fail to develop between the anterior and ipsilateral posterior circulations since the MCA/ACA and PCA are all derivatives of the fetal ICA. Further, the tentorium prevents the development of cerebellar collaterals with the PCA territory.1,16 Our two cases demonstrate the inherent variability of leptomeningeal collaterals, although a conclusion on significance is difficult to ascertain. Whereas our first patient demonstrated robust retrograde filling of the right PCA territory via MCA leptomeningeal collaterals, the large perfusion deficit in the second patient illustrates the failure of such collaterals. Although current literature does not exist examining the presence of anterior–posterior circulation leptomeningeal collaterals in the presence of FPCAs, this remains a topic of future research.
Patients with PCA ischemia typically present with homonymous hemianopsia, although studies have shown that 17% of patients with pure cortical PCA strokes have face–arm–leg motor deficits and 23% have sensory deficits in the same distribution.22 In our second case, the patient’s clinical presentation did not immediately correlate with the patency of the typical anterior circulation vessels shown by the CTA imaging. Only after recognition of the CT perfusion deficit did we closely re-examine the vascular imaging for the presence of anatomic variants. Interestingly, in the only other report of a FPCA emergent thrombectomy, Otsuji et al. experience a similar clinical-imaging discrepancy, and postulate that occlusion of the tuberothalamic artery and other perforating thalamic vessels, which originate from the middle third of the PcomA, may explain a presentation of diminished consciousness along with a MCA-syndrome.23 Occlusions of these perforating vessels may cause acute perseverative behavior with abnormal cognition and speech, and inability to perform memory-related and executive tasks.24 If a combination of hemianopsia, hemiparesis and neuropsychological deficits occur, clinicians should suspect an insult to the PCA territory, as well as to the thalamus and posterior limb of the internal capsule.17 In our first patient, the presence of hemianopsia and hemiplegia suggests PCA territory ischemia with failure of leptomeningeal collaterals, as evidenced on DSA. In our second patient, however, given the neuropsychological disorder and the large CT perfusion deficit, perhaps the clinical presentation was due to both PCA-territory and thalamic ischemia. This theory is further supported by the subsequent thalamic infarct on follow-up MRI. Furthermore, since the thrombus was positioned completely within the FPCA in our second case, there may have been a greater probability of occluding perforating thalamic vessels compared with our first case, in which the thrombus occluded the ostium and proximal-third of the PcomA, where fewer thalamic perforators exist.
The patient reported by Otsuji et al.23 presented with a mural thrombus in the ICA extending into the FPCA ostium, which was similar to our first patient. In both cases, the thrombus was likely initially located at the ICA-FPCA bifurcation and then migrated into the larger-diameter FPCA. Deployment of the stent retriever within the ICA, across the FPCA ostium, was successful in both instances despite the fact that the majority of the thrombus was located within the FPCA. In the event that these retrieval attempts had failed, the next maneuver may have been to directly micro-catheterize the FPCA, and subsequently deploy the stent retriever completely within the FPCA. In our second case, this maneuver was considered since the thrombus was completely within the FPCA, but was ultimately avoided due to reluctance to traverse the unstable cervical ICA and reluctance to directly micro-catheterize the FPCA. Operators should be aware that direct micro-catheterization of a FPCA for thrombectomy has never been reported, and the risks include injury to the PcomA/FPCA, and/or displacing the thrombus and causing iatrogenic large-vessel occlusion (LVO) of the ICA or MCA. Additional theoretical risks of direct catheterization of a FPCA may include hemodynamic ischemia of tuberothalamic perforators with inadequate collateral reserves.24,25 Thus the decision was made to proceed with intra-arterial thrombolysis alone, based on our interpretation of limited data. Intra-arterial lytic therapy played a limited role in the recent large endovascular trials, and was used as rescue therapy, not initial treatment. In MR CLEAN, 24 of 233 (10.3%) patients had treatment with a second modality, such as intra-arterial thrombolysis.4 In THRACE, an intra-arterial lytic was used to a maximum dose of 0.3 mg/kg, with a mean dose of 8.8 mg administered in 15 of 141 patients (11%), and allowed to establish goal reperfusion. In our second case, we implemented a similar dosage as rescue therapy in lieu of primary treatment with mechanical thrombectomy.26
Conclusion
Acute stroke due to emergent occlusion of a FPCA is extremely rare. Current consensus does not exist on whether FPCA increases the overall risk of stroke independent of other factors. In patients presenting with a visual field-cut, a MCA syndrome, and patency of the traditional anterior circulation, the imaging should be closely re-examined for LVO of anatomic variants such as FPCA. Stent retriever thrombectomy of an ICA mural thrombus extending into FPCA may be effective in select cases. If this maneuver fails, operators should give cautious consideration to direct micro-catheterization of, and deployment of a stent retriever within, the FPCA.
Acknowledgement
All authors contributed equally to this work.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Shaban A, Albright KC, Boehme AK, et al. Circle of Willis variants: Fetal PCA. Stroke Res Treat 2013; 2013: 105937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert SL, Williams FJ, Oganisyan ZZ, et al. Fetal-type variants of the posterior cerebral artery and concurrent infarction in the major arterial territories of the cerebral hemisphere. J Investig Med High Impact Case Rep 2016; 4: 2324709616665409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Monyé C, Dippel DW, Siepman TA, et al. Is a fetal origin of the posterior cerebral artery a risk factor for TIA or ischemic stroke? A study with 16-multidetector-row CT angiography. J Neurol 2008; 255: 239–245. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 5.Lochner P, Golaszewski S, Caleri F, et al. Posterior circulation ischemia in patients with fetal-type circle of Willis and hypoplastic vertebrobasilar system. Neurol Sci 2011; 32: 1143–1146. [DOI] [PubMed] [Google Scholar]
- 6.Van Raamt AF, Mali WP, van Laar PJ, et al. The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovasc Dis 2006; 22: 217–224. [DOI] [PubMed] [Google Scholar]
- 7.Chung EM, Hague JP, Chanrion MA, et al. Embolus trajectory through a physical replica of the major cerebral arteries. Stroke 2010; 41: 647–652. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 9.Park MG, Yoon CH, Baik SK, et al. Susceptibility vessel sign for intra-arterial thrombus in acute posterior cerebral artery infarction. J Stroke Cerebrovasc Dis 2015; 24: 1229–1234. [DOI] [PubMed] [Google Scholar]
- 10.Jongen JC, Franke CL, Ramos LM, et al. Direction of flow in posterior communicating artery on magnetic resonance angiography in patients with occipital lobe infarcts. Stroke 2004; 35: 104–108. [DOI] [PubMed] [Google Scholar]
- 11.Schomer DF, Marks MP, Steinberg GK, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 1994; 330: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 12.Kameyama M, Okinaka SH. Collateral circulation of the brain with special reference to atherosclerosis of the major cervial and cerebral arteries. Neurology 1963; 13: 279–286. [DOI] [PubMed] [Google Scholar]
- 13.Battacharji SK, Hutchinson EC, McCall AJ. The Circle of Willis – the incidence of developmental abnormalities in normal and infarcted brains. Brain 1967; 90: 747–58. [DOI] [PubMed] [Google Scholar]
- 14.Masoud H, Nguyen TN, Thatcher J, et al. Duplication of the posterior cerebral artery and the ‘true fetal’ variant. Interv Neurol 2015; 4: 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JH, Choi HY, Nam HS, et al. Mechanism of infarction involving ipsilateral carotid and posterior cerebral artery territories. Cerebrovasc Dis 2007; 24: 445–451. [DOI] [PubMed] [Google Scholar]
- 16.Arjal RK, Zhu T, Zhou Y. The study of fetal-type posterior cerebral circulation on multislice CT angiography and its influence on cerebral ischemic strokes. Clin Imaging 2014; 38: 221–225. [DOI] [PubMed] [Google Scholar]
- 17.Kuker W, Mull M, Block F, et al. Carotid artery dissections presenting as isolated posterior cerebral artery infarctions. J Neurol 1997; 244: 324–327. [DOI] [PubMed] [Google Scholar]
- 18.Hunter JM, Tehrani SK, Wood T, et al. Internal carotid artery stenosis presenting as ipsilateral posterior cerebral artery ischaemic stroke: A lesson to be learnt. BMJ Case Rep. Epub 22 April 2013. DOI: 10.1136/bcr-2013-008848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoque R, Gonzalez-Toledo E, Minagar A, et al. Circuitous embolic hemorrhagic stroke: Carotid pseudoaneurysm to fetal posterior cerebral artery conduit: A case report. J Med Case Rep 2008; 2: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolukisa M, Gursoy AE, Kocaman G, et al. Carotid endarterectomy in a patient with posterior cerebral artery infarction: Influence of fetal type PCA on atypical clinical course. Case Rep Neurol Med 2015; 2015: 191202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer OC, Berkefeld J, Nolte CH, et al. Collateral vessels in proximal middle cerebral artery occlusion: The ENDOSTROKE study. Radiology 2015; 274: 851–858. [DOI] [PubMed] [Google Scholar]
- 22.Kumral E, Bayulkem G, Atac C, et al. Spectrum of superficial posterior cerebral artery territory infarcts. Eur J Neurol 2004; 11: 237–246. [DOI] [PubMed] [Google Scholar]
- 23.Otsuji R, Kameda K, Uno J, et al. Pure posterior communicating artery occlusion treated with mechanical thrombectomy. BMJ Case Rep. Epub 27 May 2017. DOI: 10.1136/bcr-2017-219589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghika-Schmid F, Bogousslavsky J. The acute behavioral syndrome of anterior thalamic infarction: A prospective study of 12 cases. Ann Neurol 2000; 48: 220–227. [PubMed] [Google Scholar]
- 25.Endo H, Sato K, Kondo R, et al. Tuberothalamic artery infarctions following coil embolization of ruptured posterior communicating artery aneurysms with posterior communicating artery sacrifice. AJNR Am J Neuroradiol 2012; 33: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]