Abstract
Background:
The relationship between sleep disruption, independent of obstructive sleep apnea (OSA), and atrial fibrillation (AF) is unknown.
Objective:
To determine whether poor sleep itself is a risk factor for AF.
Methods:
We first performed an analysis of participants in the Health eHeart Study and validated those findings in the longitudinal Cardiovascular Health Study, including a subset undergoing polysomnography. To determine if the observed relationships readily translated to medical practice, we examined 2005-2009 data from the California Healthcare Cost and Utilization Project.
Results:
Among 4,553 Health eHeart participants, the 526 with AF exhibited more frequent nighttime awakening (OR 1.47, 95% CI 1.14 – 1.89, p=0.003). In 5,703 Cardiovascular Health Study participants followed for a median 11.6 years, frequent nighttime awakening predicted a 33% greater risk of AF (HR 1.33, 95% CI 1.17 – 1.51, p<0.001). In those with polysomnography (N=1,127), every standard deviation percentage decrease in REM sleep was associated with a 18% higher risk of developing AF (HR 1.18, 95% CI 1.00 – 1.38, p=0.047). Among 14,330,651 California residents followed for a median 3.9 years, an insomnia diagnosis predicted a 36% increased risk of new AF (HR 1.36, 95% CI 1.30 – 1.42, p<0.001).
Conclusions:
Sleep disruption consistently predicted AF before and after adjustment for OSA and other potential confounders across several different populations. Sleep quality itself may be important in the pathogenesis of AF, potentially representing a novel target for prevention.
Keywords: atrial fibrillation, sleep, insomnia, obstructive sleep apnea, REM
INTRODUCTION
There are several known risk factors for atrial fibrillation (AF),1 but it remains difficult to predict onset and identify strategies for primary prevention.2 Obstructive sleep apnea (OSA) has been established as a risk factor for AF,3 but the mechanism remains unclear. While episodes of hypopnea and apnea may cause cardiopulmonary stress, induce inflammation, and contribute to cardiovascular disease, OSA also causes poor sleep.4 Aspects of poor sleep such as altered sleep duration, efficiency, and architecture have been linked to other cardiovascular diseases.5 Sleep disturbance in general is more common than OSA,6 and since strategies to enhance sleep quality are different than those that focus on relieving airway obstruction,7 it is important to investigate the relationship between sleep itself and AF.
One cross-sectional analysis demonstrated that those with prevalent AF had reduced sleep efficiency and reduced slow-wave-sleep (also known as stage N3).8 Furthermore, AF episodes follow a circadian variation,9 and patients sometimes report that poor sleep can trigger an episode.10 However, these studies were limited to patients that already had an AF diagnosis, so “effect-cause” remains a possibility — AF itself may impair sleep. Before considering methods to enhance sleep quality as a broadly relevant approach to prevent AF, the influence of sleep disturbance prior to disease onset must be determined. Therefore, we sought to determine if poor sleep would predict an increased risk of developing AF independent of OSA.
METHODS
We evaluated the sleep-AF relationship in three distinct datasets. First, we identified the characteristics of sleep that were associated with prevalent AF in the Health eHeart Study. Next, we used the Cardiovascular Health Study (CHS) to test whether the patterns of poor sleep identified in the Health eHeart Study would predict incident AF in a longitudinal cohort. We leveraged a subset of CHS with polysomnography (PSG) data to further validate our findings using objective measurements. Finally, to test for a sleep-AF association in clinical practice, we utilized the California Healthcare Cost and Utilization Project (HCUP) to assess a physician-coded diagnosis of insomnia as a predictor of incident AF. We adjusted for OSA using markers available in each dataset. Approval for the Health eHeart Study and permission to use de-identified data from CHS and HCUP was obtained from the University of California, San Francisco Institutional Review Board.
Health eHeart Study
To identify aspects of sleep associated with prevalent AF, we utilized the Health eHeart Study (www.health-eheartstudy.org), an internet-based prospective cohort study that began enrolling interested adults age ≥ 18 years with an active email address on March 8th, 2013 (Supplemental Methods). Participants provided baseline demographic and medical information via online surveys during their initial “eVisit”. Those who enrolled through February 21st, 2016 and provided age, sex, AF status, and completed a sleep survey were included in this study.
Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI).11 Demographics and medical conditions, including prevalent AF and OSA, were determined by participant self-report. Self-reported AF in the Health eHeart Study has been previously validated by review of medical records of a subset (N = 42) of participants, and was found to be 100% sensitive (exact 95% CI, 86 to 100) and 100% specific (exact 95% CI, 80 to 100).12
The PSQI score, standard interpretation (“poor” sleep if score ≥ 5), component subscores, and individual questions were compared between participants with and without prevalent AF (Supplemental Methods). Sleep measures associated with prevalent AF with p-values < 0.05 for both the overall ordinal variable and test for trend were included together in a multivariable model.
Cardiovascular Health Study (CHS)
CHS is a population-based prospective cohort study that has been described in detail elsewhere.13 Briefly, 5,201 adults ≥ 65 years were recruited in 1989-90 from Medicare eligibility lists of 4 U.S. counties. An additional 687 African Americans were recruited during 1992-93. Participants underwent a comprehensive baseline examination including surveys, vital sign measurements, and an electrocardiogram (ECG). Participants were followed by alternating semiannual clinic visits and phone calls until 1999 and phone calls every 6 months thereafter. Participants with prevalent AF were excluded, and those remaining were censored at the time of incident AF, death, or administratively censored at the end of follow-up.
Sleep quality was ascertained during the baseline examination by five yes or no questions. OSA status was determined by the affirmative answer to either of two questions regarding snoring and apneic episodes (Supplemental Methods). Objective measures of sleep quality and OSA were available in a subset of CHS participants co-enrolled in the Sleep Heart Health Study that underwent at-home PSG during 1995-98.14 For this subset, the apnea hypopnea index (AHI), a continuous measure of OSA severity, was used to adjust for OSA. In a sensitivity analysis we adjusted for the dichotomous presence of OSA, defined as AHI ≥ 5, a standard clinical cutoff.4
Prevalent AF was identified from baseline ECG or self-report of a physician’s diagnosis.15 Incident AF was identified by ECGs at follow-up study visits or by hospital discharge diagnosis codes supplemented with Medicare inpatient claims data.16 The analysis in the PSG subset excluded any AF detected prior to the PSG study. Other covariates were by standardized questionnaire and study personnel (Supplemental Methods).
California Healthcare Cost and Utilization Project (HCUP)
The California HCUP is a set of medical records databases that has been described in detail previously.17 Briefly, all California residents ≥ 21 years old who received care in a California ambulatory surgery unit, emergency department, or inpatient hospital unit between January 1, 2005 and December 31, 2009 were identified using the HCUP databases (Supplemental Methods). Participants entered the cohort at their first encounter and were censored upon incident diagnosis of AF, at the time of inpatient death, or were administratively censored at the end of follow-up. Patients with prevalent AF (defined as carrying the diagnosis at the first recorded encounter) were excluded.
Demographics and medical diagnoses were accumulated at each encounter and carried forward over time. Up to 25 International Classification of Diseases–9th Edition (ICD-9) codes were provided for each encounter. The specific codes used for insomnia, AF, and other covariates are described in Supplemental Table 1. Because postoperative AF after cardiothoracic surgery may have a different underlying mechanism, AF was not recorded if a patient had undergone cardiothoracic surgery during the same hospitalization or within the previous 30 days.
Statistical Analyses
Normally distributed continuous variables are presented as means ± SD, and others are presented as medians (interquartile ranges). For cross-sectional analyses in Health eHeart, logistic regression models were used to obtain crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). The likelihood-ratio test was used to check for model misspecification, “dfbeta” influence statistics were used to assess for influential points, and the Hosmer-Lemeshow goodness-of-fit test was used to assess model fit. All logistic regression models were satisfactory. For longitudinal analyses in CHS and HCUP, Cox proportional hazards models were used to obtain crude and adjusted hazard ratios (HRs) and 95% CIs. The proportional hazards assumption was checked using the Schoenfeld test and graphical assessment of scaled Schoenfeld residuals. All Cox models satisfied the proportional hazards assumption. Covariates in all adjusted analyses included baseline age, sex, race, hypertension (HTN), diabetes, coronary artery disease (CAD), congestive heart failure (CHF), smoking history, alcohol use, income, and available markers of OSA. Body mass index (BMI) was available in CHS only and BMI was included as a covariate in all adjusted models using CHS data. All analyses were repeated after exclusion of all participants with OSA (according to available markers). No consistent interactions by sex were detected (Supplemental Table 2). There were no interactions by race (Supplemental Table 3).
Analyses for Health eHeart and CHS were performed using Stata 13 (StataCorp LP, College Station, Texas) and those for HCUP were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina). A 2-tailed p<0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the participants in each study are shown in Table 1. Participants in CHS tended to be older compared to Health eHeart and HCUP. Participants in Health eHeart and CHS were predominantly White, while HCUP had a substantial proportion of Hispanic patients. All analyses were repeated after exclusion of participants with OSA, and no meaningful differences were observed (Supplemental Figures 1 – 4).
Table 1.
Baseline participant characteristics by study
| Characteristic | HeH (N = 4,553) | CHS (N = 5,703) | HCUP (N = 14,330,651) |
|---|---|---|---|
| Follow-up, median (IQR), years | Cross-sectional | 11.6 (6.2 to 16.4) | 3.9 (2.3 to 4.6) |
| Age, mean ± SD, years | 51 ± 15 | 73 ± 6 | 49 ± 18 |
| Female, n (%) | 2,499 (55%) | 3,310 (58%) | 8,159,023 (57%) |
| BMI, mean ± SD, kg/m2 | – | 26.7 ± 4.7 | – |
| Race/Ethnicity, n (%) | |||
| White | 3,704 (81%) | 4,760 (83%) | 7,753,344 (54%) |
| Black | 92 (2%) | 907 (16%) | 1,048,804 (7%) |
| Hispanic | 249 (5%) | – | 3,596,611 (25%) |
| Asian | 305 (7%) | 4 (0.07%) | 1,931,892 (13%) |
| Other | 203 (4%) | 32 (0.6%) | – |
| Annual Income category, n (%) | |||
| 1 Lowest | 783 (21%) | 1,420 (27%) | 3,152,668 (22%) |
| 2 | 1,017 (27%) | 1,872 (35%) | 3,504,131 (24%) |
| 3 | 773 (20%) | 1,353 (25%) | 3,792,557 (26%) |
| 4 Highest | 1,209 (32%) | 688 (13%) | 3,881,295 (27%) |
| CAD, n (%) | 629 (14%) | 1,110 (19%) | 1,085,791 (8%) |
| CHF, n (%) | 191 (4%) | 232 (4%) | 677,180 (5%) |
| Diabetes mellitus, n (%) | 298 (7%) | 910 (16%) | 1,834,375 (13%) |
| HTN, n (%) | 1,584 (35%) | 3,338 (59%) | 3,664,816 (26%) |
| OSA, n (%) | 546 (13%) | 1,455 (30%) | 230,358 (2%) |
| Ever smoker, n (%) | 1,599 (35%) | 2,708 (54%) | 1,191,747 (8%) |
| Alcohol user, n (%) | 2,484 (83%) | 2,826 (50%) | 254,394 (3%) |
HeH = Health eHeart Study; CHS = Cardiovascular Health Study; HCUP = California Healthcare Cost and Utilization Project; IQR = interquartile range; SD = standard deviation; BMI = body mass index; CAD = coronary heart disease; CHF = congestive heart failure; HTN = hypertension; OSA = obstructive sleep apnea.
Identification of sleep measures associated with prevalent AF in Health eHeart
Among 4,553 Health eHeart participants, 526 (12%) had prevalent AF. In initial analyses of all components of the PSQI, there were six sleep measures that remained statistically significantly associated with prevalent AF after adjustment (Supplemental Figure 5). After combining these sleep measures along with the other pre-specified covariates into a single model, only longer (worse) sleep onset latency and frequent nighttime awakening remained statistically significantly associated with prevalent AF (Figure 1).
Figure 1. Associations between selected self-reported sleep characteristics and prevalent AF in the Health eHeart Study.
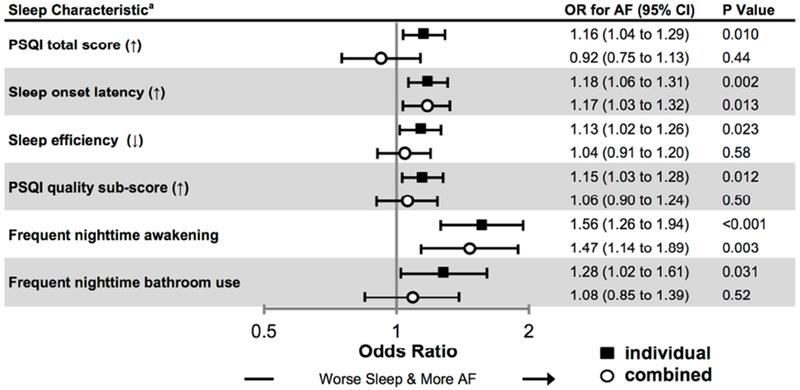
Odds ratios (ORs) for atrial fibrillation (AF) from multivariable logistic regression models examining individual sleep characteristics (black squares) and all sleep characteristics (white circles). Both types of analysis were adjusted for potential confounders: age, sex, race, hypertension, diabetes, obstructive sleep apnea, coronary artery disease, congestive heart failure, smoking history, alcohol use, and income. Bars denote 95% confidence intervals (CIs).
a Continuous characteristics are scaled per SD increase (↑) or decrease (↓) in accordance with the direction of worse sleep.
Associations between sleep measures and incident AF in CHS
Among 5,703 participants in CHS, 1,588 (28%) developed incident AF over a median of 11.6 years (IQR 6.2 to 16.4). As shown in Figure 2, and consistent with findings from Health eHeart, a report of nighttime awakening at baseline was associated with a statistically significant 33% increased risk of AF after adjusting for potential confounders and evidence of OSA. No other measure of sleep recorded at baseline in CHS revealed a statistically significant relationship with incident AF.
Figure 2. Associations between self-reported sleep measures and incident AF in the Cardiovascular Health Study (CHS).
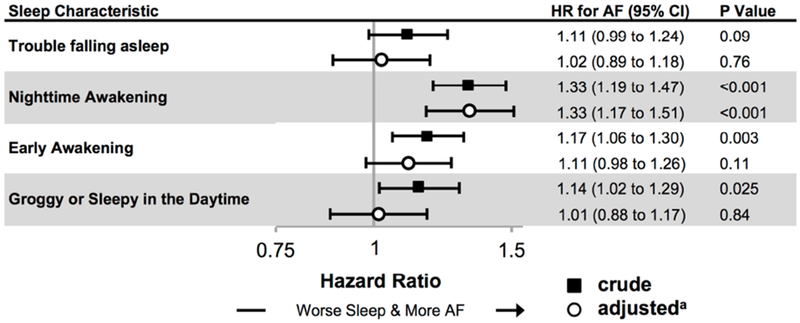
Crude (black squares) and adjusted (white circles) hazard ratios (HR) from Cox proportional hazards models for incident atrial fibrillation (AF). Bars denote 95% confidence intervals (CIs).
a All adjusted models include: age, sex, race, BMI, hypertension, diabetes, obstructive sleep apnea, coronary artery disease, congestive heart failure, smoking history, alcohol use, and income.
In the subset of 1,127 CHS participants who underwent at-home PSG (characteristics available in Supplemental Table 4), 259 (23%) developed AF over a median follow-up of 9.8 years (IQR 5.5 to 11.7). Less REM sleep was associated with increased risk of AF after adjusting :for confounders, including PSG-based measures of OSA (Figure 3). These results were not meaningfully different in a sensitivity analysis controlling for the dichotomous presence of OSA by AHI ≥ 5.
Figure 3. Associations between polysomnographic (PSG) sleep measures and incident AF in a subset (N = 1,127) of the Cardiovascular Health Study (CHS).
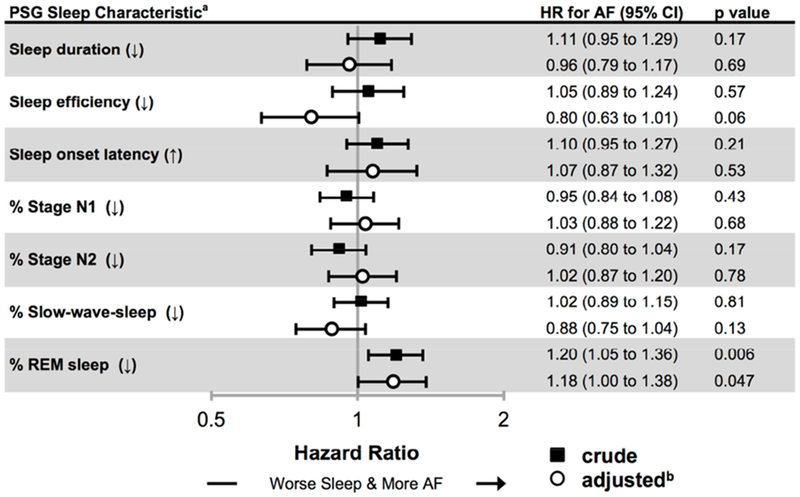
Crude (black squares) and adjusted (white circles) hazard ratios (HR) for incident atrial fibrillation (AF). Bars denote 95% confidence intervals (CIs).
a Sleep onset latency is scaled per SD increase (↑) while all other predictors are scaled per SD decrease (↓).
b Covariates in the adjusted models include: age, sex, race, BMI, hypertension, diabetes, obstructive sleep apnea, coronary artery disease, congestive heart failure, smoking history, alcohol use, and income.
Assessment of sleep-AF relationship in the clinical setting in HCUP
Of 14,330,651 patients in HCUP, 258,716 (1.8%) developed AF over a median follow-up of 3.9 years (IQR 2.3 to 4.6). A diagnosis of insomnia was associated with an approximate 36% (HR 1.36, 95% CI 1.30 to 1.42, p<0.001) increased risk of incident AF after adjusting for potential confounders including OSA (Figure 4). By comparison, in the same model, a diagnosis of OSA was independently associated with an approximately 76% increased risk of AF (HR 1.76, 95% CI 1.72 to 1.80, p<0.001) and smoking with a 32% increased risk of AF (HR 1.32, 95% CI 1.30 to 1.34, p<0.001).
Figure 4. Cumulative incidence of AF by insomnia diagnosis in the California Healthcare Cost and Utilization Project (HCUP).
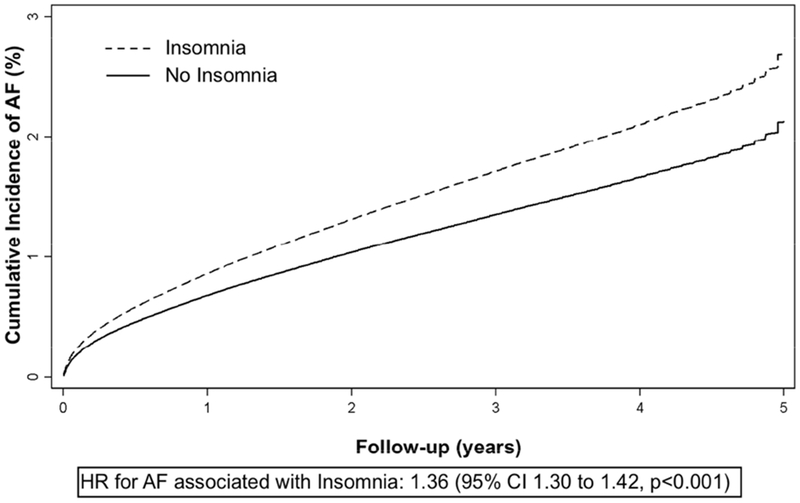
Adjusted cumulative incidence of atrial fibrillation (AF) in patients with (dashed line) and without (solid line) a diagnosis of insomnia. Covariates include age, sex, race, hypertension, diabetes, obstructive sleep apnea, coronary artery disease, congestive heart failure, smoking history, alcohol use, and income. HR = hazard ratio.
DISCUSSION
Across three independent data sources, we found that sleep disruption was consistently associated with prevalent and incident AF. Examining sleep characteristics in a hypothesis-generating manner, we found that taking longer to fall asleep (greater sleep onset latency) and frequent nighttime awakening were independently associated with prevalent AF among a national internet-based cohort (Health eHeart). Recognizing that effect-cause could not be excluded in such a cross-sectional analysis, we examined a longitudinal cohort study where sleep assessments were performed on average many years prior to incident AF. In CHS, frequent nighttime awakening was associated with greater risk for incident AF, validating our findings from Health eHeart. Analyses of objective sleep quality measures from PSG pointed to decreased REM sleep as potentially important. Finally, in considering next steps and whether the sleep-AF association ascertained in formal studies might already be evident in clinical practice, we utilized HCUP, a large administrative dataset of healthcare encounters in California. Indeed, a diagnosis of insomnia predicted a subsequent diagnosis of AF. All of these positive findings persisted after adjustment for conventional AF risk factors and available measures of OSA. Taken together, these findings suggest that something inherent to sleep quality itself may be important in the pathogenesis of AF.
Our findings suggest that sleep quality is as important as previously described risk factors. In HCUP, the adjusted HR for insomnia (1.36), was of a similar magnitude as the HRs for smoking (1.32) and OSA (1.76). This is consistent with the notion that sleep is an important but often under-recognized determinant of cardiovascular health.18
We found no evidence that sleep duration per se was a risk factor for AF. Indeed, the previous literature on this subject has been conflicting.19 Instead, we consistently found sleep disruption to be an important risk factor. The underlying mechanisms remain unknown, but these findings may motivate novel ways to think about, and hence future research into, factors that influence AF risk.
The influence of sleep on autonomic tone may explain the increased risk of AF. Enhanced vagal tone may be important in AF: stimulation of parasympathetic ganglia innervating the left atria results in AF,20 and activities associated with more vagal tone can trigger AF episodes.21 Sympathetic tone is high during REM sleep,22 suggesting that those with less REM sleep may on average experience greater vagal tone. Kwon et al. reported a lower odds of AF among those with a longer duration of slow-wave sleep.8 As patients in that study already had a diagnosis of AF, it is possible the differences in sleep architecture were an effect (rather than a cause) of AF. Conversely, enhanced sympathetic tone may be the culprit given evidence that post-operative AF can occur following greater sympathetic activity.23 Transitions from sleep to wakefulness (normal waking and sleep disruptions) are associated with increased sympathetic tone,24 which may promote atrial changes via corresponding hemodynamic stress,10 or other pathways yet to be recognized. However, the relationships between sleep, AF, and autonomic tone are likely more complex than a simple increase or decrease of one branch of the autonomic nervous system.
While consideration of mechanisms may inspire new research, one of the primary motivators for this study was to identify a common and modifiable risk factor for AF. Ultimately, the utility of the current findings would be realized if strategies to enhance sleep quality (e.g. optimization of sleep hygiene,7 avoidance of night-time smartphone use,25 and cognitive behavioral therapy)26 demonstrably reduced the risk of AF, or the burden of AF episodes, in a randomized trial. If sleep quality itself is indeed a causal factor for AF, it may represent an important and common modifiable risk factor for AF. Our data from HCUP also suggests this problem may be easily recognizable and therefore ripe for meaningful interventions in clinical practice.
Limitations
Our analyses bring several strengths: our multi-layered assessment provided reproducible and robust results; each study utilized different populations; as HCUP alone includes several million individuals, this is to our knowledge the largest study addressing sleep and AF. However, several limitations must be considered.
First, as all three studies were observational, we could not determine causality or exclude residual confounding. It remains possible that OSA was underdiagnosed. However, there is no association between survey-reported sleep disruption (as was used in the current study) and PSG-diagnosed OSA,27 we adjusted for all available markers of OSA, and an analysis excluding all patients with sleep apnea revealed no meaningfully different results; perhaps most compelling, our positive findings persisted after adjustment for the gold-standard assessment of OSA in the PSG subset. Although we were able to adjust for alcohol use and smoking in all analyses, it remains possible that these were underreported, and that poor sleep was a bystander. Alcohol and nicotine are known risk factors for AF,21,28 can cause sleep disruption,7 and have variable effects on REM sleep.29 On the other hand, impaired sleep could be a mechanism by which alcohol and smoking promote AF. Nocturia is another example of a factor that disrupts sleep, could impact REM sleep, and has been associated with increased cardiovascular morbidity.30
Misclassification of variables, particularly AF as an outcome, is commonly a challenge in clinical research. While the Health eHeart dataset is limited by self-report, this method has previously been validated.12 CHS relied on well-established methods to ascertain AF, including serial ECGs, hospital discharge records and death certificates, and the cohort has previously served as the foundation of several seminal papers on predictors of AF.16,31 HCUP relied on ICD-9 coding; notably, a prior study revealed administrative ICD-9 coding at a large health maintenance organization exhibited 95% sensitivity and 99% specificity for the diagnosis of AF when compared to record review by trained abstractors.32 In addition, research using these methods and particularly the HCUP database has proven to provide a powerful accepted tool for large population studies.17 Although frequent awakening in the Health eHeart Study and CHS predicted AF, we were limited to a general diagnosis of insomnia as our primary predictor in HCUP. While we believe that these findings provide compelling evidence of a relationship between poor sleep, in a general sense, and incident AF, we cannot exclude the possibility that the insomnia diagnoses captured some other element of sleep other than frequent nighttime awakening.
Lastly, as with many studies of AF, there is likely under-ascertainment of AF. However, for sleep measures and AF, it is likely that ascertainment of each was limited primarily by poor sensitivity rather than reduced specificity, which would decrease our power to detect associations rather than result in spurious false positive results.
CONCLUSIONS
Sleep disruption independent of OSA appears to be an important risk factor for AF. This effect may be explained by a reduction in REM sleep. Given the high prevalence of sleep problems and the substantial negative impacts of AF, research examining interventions to improve sleep quality may prove valuable in preventing AF.
Supplementary Material
Acknowledgments
FUNDING:
Dr. Christensen was a research fellow supported by the Sarnoff Cardiovascular Research Foundation. The Health eHeart Study is supported by the National Institute of Health [1U2CEB021881] and the Patient Centered Outcomes Research Institute [PPRN-1306-04709]. The Cardiovascular Health Study was supported by the National Heart, Lung, and Blood Institute [contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114] and National Institute of Neurological Disorders and Stroke. Additional support was provided by National Institute on Aging [R01AG023629]. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org
Disclosures: Dr. Dewland has received education-related travel support from Medtronic and Boston Scientific; Dr. Psaty serves on the data safety monitoring board of a clinical trial funded by Zoll LifeCor and on the Yale Open Data Access Project funded by Johnson & Johnson; Dr. Marcus has received research support from Medtronic, Rhythm Diagnostic Systems, and Cardiogram and is a consultant and equity holder in InCarda; none of the other authors have any potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benjamin EJ, Chen P-S, Bild DE, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation 2009; 119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus GM. Predicting Incident Atrial Fibrillation. Arch Intern Med United States, 2010; 170:1874–1875. [DOI] [PubMed] [Google Scholar]
- 3.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive Sleep Apnea, Obesity, and the Risk of Incident Atrial Fibrillation. J Am Coll Cardiol 2007; 49:565–571. [DOI] [PubMed] [Google Scholar]
- 4.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009; 373:82–93. [DOI] [PubMed] [Google Scholar]
- 5.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis 2009; 51:285–293. [DOI] [PubMed] [Google Scholar]
- 6.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health 2015; 36:417–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin CM, Benca R. Chronic insomnia. Lancet 2012; 379:1129–1141. [DOI] [PubMed] [Google Scholar]
- 8.Kwon Y, Gharib S a., Biggs ML, Jacobs DR, Alonso A, Duprez D, Lima J, Lin G-M, Soliman EZ, Mehra R, Redline S, Heckbert SR. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax 2015; 70:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita T, Murakawa Y, Sezaki K, Inoue M, Hayami N, Shuzui Y, Omata M. Circadian variation of paroxysmal atrial fibrillation. Circulation 1997; 96:1537–1541. [DOI] [PubMed] [Google Scholar]
- 10.Verrier RL, Josephson ME. Impact of sleep on arrhythmogenesis. Circ Arrhythm Electrophysiol 2009; 2:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28:193–213. [DOI] [PubMed] [Google Scholar]
- 12.Dixit S, Pletcher MJ, Vittinghoff E, Imburgia K, Maguire C, Whitman IR, Glantz SA, Olgin JE, Marcus GM. Secondhand smoke and atrial fibrillation: data from the Health eHeart Study. Hear Rhythm 2016; 13:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276. [DOI] [PubMed] [Google Scholar]
- 14.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep 1997; 20:1077–1085. [PubMed] [Google Scholar]
- 15.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol 1994; 74:236–241. [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997; 96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 17.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, Blacks, and Whites. Circulation 2013; 128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra A, Loscalzo J. Sleep and Cardiovascular Disease: An Overview. Prog Cardiovasc Dis Elsevier Inc, 2009; 51:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khawaja O, Sarwar A, Albert CM, Gaziano JM, Djoussé L. Sleep duration and risk of atrial fibrillation (from the Physicians’ Health Study). Am J Cardiol United States, 2013;111:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen P-S, Chen LS, Fishbein MC, Lin S-F, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 2014; 114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandyam MC, Vedantham V, Scheinman MM, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Gerstenfeld EP, Olgin JE, Marcus GM. Alcohol and vagal tone as triggers for paroxysmal atrial fibrillation. Am J Cardiol 2012; 110:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993; 328:303–307. [DOI] [PubMed] [Google Scholar]
- 23.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological Mechanisms of Atrial Fibrillation: A Translational Appraisal. Physiol Rev 2011; 91:265–325. [DOI] [PubMed] [Google Scholar]
- 24.Lanfranchi PA, Somers VK. Cardiovascular Physiology: Autonomic Control in Health and in Sleep Disorders In Kyrger MH, Roth T, Dement WC, eds: Principles and Practice of Sleep Medicine. Fifth Edition St. Louis, Missouri: Elsevier Saunders, 2010, pp. 226–236. [Google Scholar]
- 25.Christensen MA, Bettencourt L, Kaye L, Moturu ST, Nguyen KT, Olgin JE, Pletcher MJ, Marcus GM. Direct Measurements of Smartphone Screen-Time: Relationships with Demographics and Sleep. Romigi A, ed: PLoS One 2016; 11:e0165331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 2016; 165:125. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama T, Mizuno T, Kojima M, Suzuki S, Kitajima T, Ando KB, Kuriyama S, Nakayama M. Criterion validity of the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale for the diagnosis of sleep disorders. Sleep Med Elsevier B.V., 2014;15:422–429. [DOI] [PubMed] [Google Scholar]
- 28.Andrade J, Khairy P, Dobrev D, Nattel S. The Clinical Profile and Pathophysiology of Atrial Fibrillation: Relationships Among Clinical Features, Epidemiology, and Mechanisms. Circ Res United States, 2014; 114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 29.Garcia AN, Salloum IM. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: A focused review. Am J Addict 2015; 24:590–598. [DOI] [PubMed] [Google Scholar]
- 30.Parthasarathy S, Fitzgerald MP, Goodwin JL, Unruh M, Guerra S, Quan SF. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS One 2012; 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med 2013/December/04 2013; 159:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glazer NL, Dublin S, Smith NL, French B, Jackson LA, Hrachovec JB, Siscovick DS, Psaty BM, Heckbert SR. Newly Detected Atrial Fibrillation and Compliance With Antithrombotic Guidelines. Arch Intern Med American Medical Association, 2007; 167:246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


