Abstract
Anterior cruciate ligament (ACL) injuries are common, catastrophic events that incur large expense and lead to degradation of the knee. As such, various motion capture techniques have been applied to identify athletes who are at increased risk for suffering ACL injuries. The objective of this clinical commentary was to synthesize information related to how motion capture analyses contribute to the identification of risk factors that may predict relative injury risk within a population. Individuals employ both active and passive mechanisms to constrain knee joint articulation during motion. There is strong evidence to indicate that athletes who consistently classify as high-risk loaders during landing suffer from combined joint stability deficits in both the active and passive knee restraints. Implementation of prophylactic neuromuscular interventions and biofeedback can effectively compensate for some of the deficiencies that result from poor control of the active knee stabilizers and reduce the incidence of ACL injuries.
Keywords: Anterior cruciate ligament injury, Injury risk classification, Motion capture, Motion analysis, Neuromuscular training
INTRODUCTION
Upwards of 55% of all athletic injuries are incurred on the lower extremity,1–4 while damage specific to the knee accounts for approximately 15% of all athletic injuries.5 43% of these knee injuries are classified as strains or sprains, which makes them the third most prevalent form of lower extremity injury with a rate of 102 incidents per 100,000 athletes per year.6 Of these knee injuries, it is estimated that 45% involve internal knee trauma and 49% of those entail anterior cruciate ligament (ACL) rupture,7 as 1 in 3000 persons are likely to suffer an ACL disruption each year.8 However, ACL injury is a sex-specific event, as females are 2–10 times more likely to suffer ACL disruption than their male counterparts,9–14 which produces an incidence rate of 1 ACL tear in every 50 to 70 female athletes per year.15 These high incidence rates of ACL rupture lead to an estimated 250,000 ACL tears and 127,000 ACL reconstructions (ACLR) annually in the United States.16,17 With conservative repair estimates ranging from $5,000 - $44,000 per ACLR depending on the type of repair and severity of injury,13,18–20 the annual medical expense of ACL injury treatments in the United States alone may exceed $2 billion. Worldwide, it is estimated that the annual incidence of ACL tears could reach as high as 2 million patients,21 which would exponentially increase these costs. Unfortunately, despite the expense associated with ACLR, surgical repair has not been found to significantly reduce the long-term outlook of knee osteoarthritis compared to non-operative rehabilitation.22,23 As many as 86% of patients demonstrate early onset osteoarthritis following ACLR and 75% report degradation in knee quality of life within 20 years post-surgery.23–25 For these reasons the focus on treating ACL injuries may be best served through identification and treatment of modifiable injury risk factors that may prevent ruptures before they happen.
Two- and three-dimensional (2D & 3D) motion analysis systems have been used in vivo to identify, classify, and associate biomechanical risk factors with the likelihood of future ACL injury within athletic populations (Figure 1). Specifically, in a cohort of 205 female athletes, 3D motion analysis prospectively determined that those who went on to ACL injury expressed larger knee abduction moments when landing from a drop vertical jump task than did healthy controls.26 This association between frontal plane knee torque and increased ligament loading, which was initially defined through motion analysis, has since been affirmed in a multitude of in vitro, in situ, and in sim models.27–29 In addition, 3D motion analysis has identified that decreased knee flexion,30 increased hip adduction,31–33 and greater trunk instability34–37 when landing from a jump are all related to abnormal loading at the knee and potentially increased ACL injury-risk. Many of these specific factors identified in 3D models can be generalized in 2D motion analyses that are more cost effective to the clinical environment. Relative presence of trunk instability,38,39 knee valgus angle,40 and knee flexion angle,40 and knee excursion in the frontal plane41 can be assessed in 2D capture. While not as precise as 3D analyses, these 2D generalizations have effectively been used to adapt biomechanical nomograms that identify athletes within an athletic cohort who are predisposed to ACL injury risk.40–44
Figure 1:
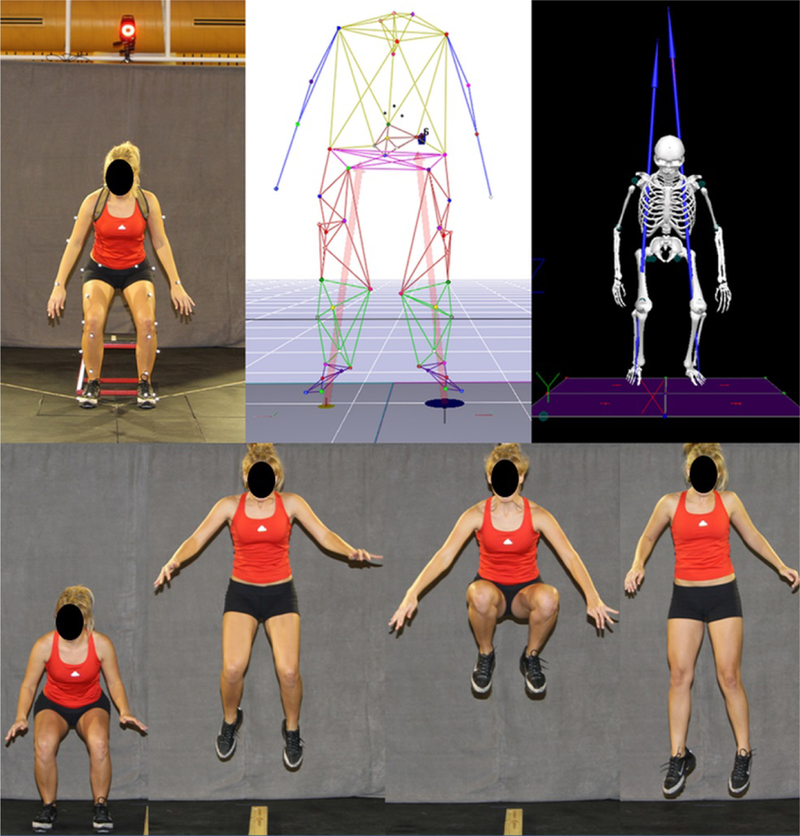
(Top Row) From left to right depicts 3D motion capture models at the minimum center of gravity for a drop vertical jump performed in vivo within a 3D motion analysis laboratory, within the tracking software used to process 3D positional data from the in vivo markers, and within the musculoskeletal modeling software used to process 3D kinematic and kinetic biomechanics from the positional data and recorded ground reaction forces. Knee abduction torque from a drop vertical jump is commonly used to assess ACL injury risk in 3D motion analyses.26,75 (Bottom Row) Progression of a tuck jump task during 2D motion analysis. The tuck jump task has been demonstrated as a clinician-friendly, 2D assessment that can be used to identify high-risk mechanics and provide direction for neuromuscular intervention.44
As previously stated, the intent behind the identification of high-risk biomechanical behaviors and the athletes that display them is to treat and prevent ACL injuries before they occur. It has been repeatedly demonstrated that prophylactic neuromuscular interventions can have a positive influence on the reduction of ACL injuries within an athletic population.18,45–50 neuromuscular training (NMT) is effective in reducing the magnitude of knee abduction moments generated by athletes during the performance of athletic tasks.51–54 Since these frontal plane torques are directly associated with ACL injury,26 it is likely that decreasing their magnitudes is in part responsible for the overall reduction in injury incidence following NMT. Further, NMT has been demonstrated to have a greater biomechanical effect on high-injury-risk athletic populations than medium- or low-risk cohorts.52 Accordingly, the classification of athletes into injury-risk levels and definition of the underlying mechanisms that lead to these levels of risk may be vital to maximize the future efficacy of ACL injury prevention.
In this clinical prediction commentary we synthesize information related to how motion capture analyses contribute to the identification of risk factors that may predict relative injury risk within a population. We argue that an athlete’s relative ACL injury risk is dependent on which systems of control an athlete can effectively employ to restrain the knee joint during athletic tasks. In the first section of this commentary, we define the systems of control available at the knee and identify differences in mechanical outcomes between effective and less effective systems. In the second section we identify the divisions of relative ACL injury risk based on knee abduction moment and justify our stated arguments. In subsequent sections we address how robust the classifications of injury risk may be and examine the effectiveness of incorporation of biofeedback techniques with motion capture analysis to reduce relative injury risk within an athlete and an athletic population.
KNEE JOINT RESTRAINTS
Motion at the knee is constrained by a series of active and passive restraints that work in conjunction to stabilize the articulating structures when forces and perturbations are applied during an athletic maneuver. Active restraints reference the musculature surrounding the joint and the neuromuscular control mechanisms used to activate this musculature, specifically the proprioceptive, kinesthetic, visual, vestibular, and motor command systems.55 With respect to the ACL, it has been hypothesized that ligament injury risk is related to measureable and modifiable deficits within these neuromuscular control mechanisms that influence muscle strength, power, and activation and, ultimately, knee joint and ACL loads.26,56–58 Relative to muscle mechanics, quadriceps activation has traditionally been seen as antagonist to the ACL, adding strain, whereas hamstrings activation has been agonist, providing protection.59 Accordingly, a poor quadriceps-hamstrings activation ratio can lead to increased knee extension during landing and excess anterior tibial translation, both of which increase ACL strain.60–62 This preferential activation of knee extensors over knee flexors in order to stabilize the knee during motion tasks is termed quad dominance.63 Quadriceps-dominant traits can lead to lower flexion angles upon landing that indicate less time and movement to absorb the impulse forces generated from landing and lead to greater joint force generation as a result of this more extended position.55,62,64 A second deficit within active knee restraints is leg dominance, where the imbalance between muscular strength and recruitment on opposing limbs leads to contralateral asymmetries.63 Kinetic and kinematic analyses have shown this type of deficit to be especially prevalent in female athletes, as they demonstrate greater limb asymmetries, especially in regard to frontal plane kinematics, than their male counterparts.57,65 Further, motion analysis investigations have revealed that contralateral asymmetries are greater following ACL reconstruction; and therefore, may be related to the increased likelihood of secondary or contralateral ACL rupture that exists within the ACL-injured population following return to sport.66 The common outcome brought on by the variety of neuromuscular control deficits documented here is that the active knee stabilizers are unable to restrain the joint from articulating into dynamic valgus rapid deceleration tasks, a trait that is able predict ACL injury risk with 78% sensitivity and 73% specificity.26
Passive knee restraints reference the ligaments, bony structures, and generalized laxity within the joint that contribute to the mechanical constraint of motion in the absence of a neuromuscular response. Excessive employment of the passive restraints in the knee can be related to insufficient control of the active restraints that results in a ligament dominant condition.63,67 Ligament dominance refers to decreased medial/lateral muscle control that leads to high valgus torque and vertical ground reaction forces.63,68,66,69 In this condition, the ground reaction forces dictate joint movement direction rather than the musculature and allow for substantial loading of the ligamentous structures within the knee.63,67 In addition, increased knee laxity, especially in the antero-posterior degree of freedom, leads to increased tibial translation, which leads to additional loading of the knee ligaments as they mechanically work to resist this excess motion. Morphologic changes that can relate to ACL injury risk include tibial plateau slope70 and femoral notch width.71 As posterior tibial slope increases, the femur is more likely to slide posteriorly on the tibial plateau, which places mechanical demand on the ACL as the ligament resists up to 85% of anterior tibial translational force in the knee.70,72,73 As the femoral notch width decreases, the likelihood of ACL impingement increases, which adds strain to the ligament.74 Unlike active restraints, these passive knee restraints cannot be altered by intervention training and are thus referred to as non-modifiable risk factors as changes require invasive surgical intervention.
RELATIVE INJURY RISK
Traditionally, the relative level of ACL injury-risk displayed by an athlete has been determined by the magnitude of knee abduction moment he or she generates during drop landing (Figure 2).26,40 In a cohort of 205 female athletes that underwent 3D motion analysis and were prospectively monitored for subsequent ACL injury, it was discovered that those athletes who went on to injury exhibited greater knee abduction moments than did healthy controls.26 The mean peak knee abduction torque for injury patients was 21.74 N*m. Within this population, it was later assessed that peak knee abduction moments above 25.3 N*m increased risk for subsequent ACL injury from 0.4% to 6.8%; thus, this was established as the cutoff threshold for the classification of high-injury-risk athletes.75 Similarly, during 3D motion analysis of the same drop vertical jump task, a separate patient population with patellofemoral pain was found to generate greater peak knee abduction moments on their uninvolved limb than did healthy controls.76 Within these two population cohorts that were examined separately for ACL injury and patellofemoral pain, the incidence of patellofemoral pain was found to be 2.2 times greater than ACL injury when normalized for athlete seasons.75 This discrepancy in incidence indicated that a tertiary level of injury risk may be present. Indeed, a second investigation based on the same 3D motion analysis techniques that defined high-injury-risk athletes found that probability of patellofemoral pain occurrence increased dramatically in athletes with peak knee abduction moments greater than 15.4 N*m.75,77 This threshold predicted knee abduction load associated with increased patellofemoral pain risk with 92% sensitivity and 74% specificity and, as such, was determined to represent a medium-injury-risk classification among athletes.75,77
Figure 2:
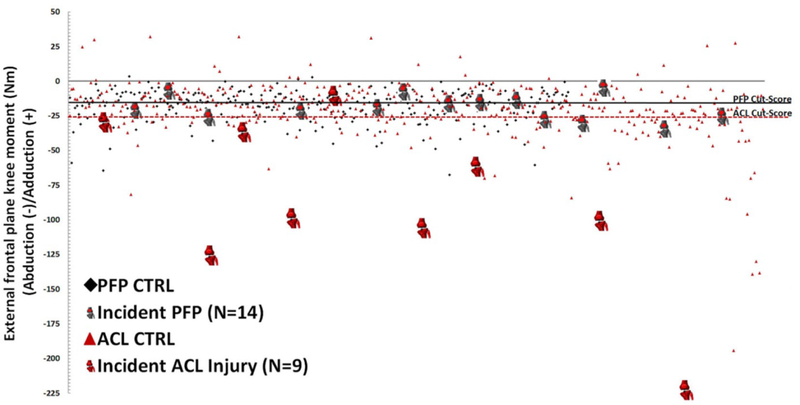
Peak knee abduction moments generated by two prospectively analyzed cohorts of adolescent female athletes during a DVJ task. The first cohort (red) was used to determine the magnitude of knee abduction torque that would predict ACL injury risk,26 while the second cohort (black) was used to determine the magnitude of knee abduction torque that would predict patellofemoral pain risk.75,77,78 The horizontal lines designate relative injury risk divisions for the medium (solid line) and high (dashed line) risk groups. (Reprinted with permission from Myer GD, Ford KR, Di Stasi SL, Barber-Foss KD, Micheli LJ, Hewett TE. Br J Sports Med 2015;49:120.)
As noted previously, the data from these 3D motion analyses were disseminated into algorithms that could predict both ACL injury40–42,78 and patellofemoral pain.77 The purpose of these 2D injury-risk nomograms was to create clinically-relevant tools that could quickly, accurately, and cost-efficiently categorize athletes into relative injury-risk groups based on these peak knee abduction moment divisions. While 3D motion analysis is an effective laboratory tool, these systems are cost prohibitive and involve intensive data analysis, which makes them inaccessible to most clinical environments.40,79 To this effect, the selected clinically-based nomograms involve minimal investment (two video cameras and laptop),40,79 can be quickly and reliably assessed by a trained clinician,80 and exhibit high sensitivity and moderate specificity in the classification of relative risk for ACL injury (84% and 67%, respectively) or patellofemoral pain (92% and 46%, respectively).77,81
Despite having equivalent fall heights, 3D motion capture analysis of the first and second landings of a DVJ has revealed several differences in kinetic and kinematic performance.82 Because of these differences, the first landing may be more predisposed to exhibit larger frontal plane moments than the second. As such the two landing phases exhibit a potential shift in injury risk classification. A population of 239 adolescent female athletes underwent 3D motion analysis as they completed both the first and second landings of a DVJ, as was described previously in the literature.82–84 Of these athletes, both landings were successfully captured on 206 subjects. Peak knee abduction moments calculated from the motion capture model were used to classify these athletes into relative injury risk groups as specified above. It was found that during the first landing 57.2% were classified as low ACL injury-risk, 21.8% were medium-risk, and 20.9% were high-risk; while during the second landing 66.5% were low-risk, 21.4% were medium-risk, and 12.1% were high-risk (Figure 3). This cohort of athletes was previously shown to generate a slightly larger mean peak frontal plane knee torque during the first landing of a drop vertical jump than during the second.82 The frequency of high-risk classifications between landings presented here corresponded with that finding; however, the relative infrequency of high-risk kinetics during the second landing may indicate that it is more selective in diagnosis. Of the athletes who expressed high-risk knee abduction torque during the first landing, 37.2% continued to demonstrate torque magnitudes indicative of high-risk biomechanics during the second landing, while 30.2% and 32.6% exhibited medium and low risk, respectively (Figure 4). Conversely, 64.0% of athletes who exhibited “high-risk” torques in the second landing were also classified as high-risk in the first landing. Kinematic and kinetic variability within the performance of an athletic task, as captured by motion analysis systems, has been previously documented.80,85 For peak knee abduction moments within session, the interclass correlation coefficient was 0.931, which represents excellent reliability and consistent results.85 Accordingly, it would be expected that subjects would steadily demonstrate the same level of ACL injury-risk when landing from a jump. However, the classification consistency of this data did not match the reported reliability. It is possible to generate high-risk knee abduction torques with poor control over either the dynamic or passive knee restraints, but perhaps athletes who consistently exhibit high-risk frontal plane torques between landings represent subjects who express poor control over both mechanisms of knee restraint.
Figure 3:
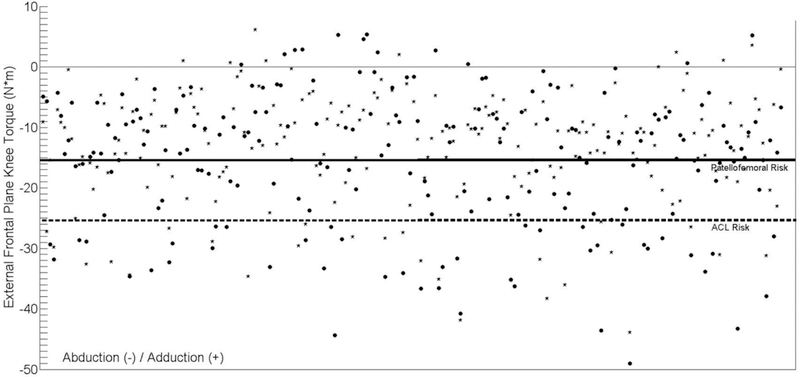
Depiction of risk category divisions during the first (circles) and second (stars) landing of a DVJ within the 206 adolescent female athletes examined in the present manuscript. Peak abduction torques below the patellofemoral risk line designate the medium injury-risk classification, while values below the ACL risk line designate the high injury-risk classification.
Figure 4:
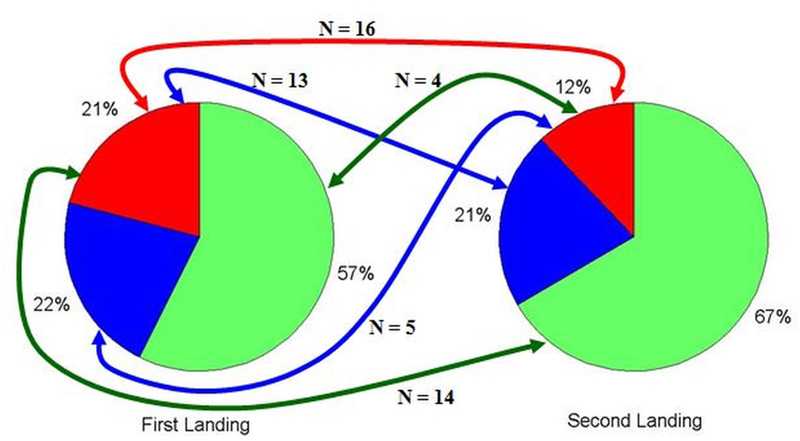
Pie chart depiction of ACL injury risk classifications for 206 adolescent female athletes during the first and second landing of a DVJ. Lines demonstrate the number of high-risk athletes that changed injury risk classifications between landings. Only 16 athletes (7.8%) were classified as high-risk in both landings based on their peak knee abduction moment.
The authors propose that athletes who continually express relatively high levels of injury risk during rapid deceleration tasks have poor control over both their active and passive mechanisms of knee restraint. Those athletes who express medium injury risk, or fluctuate between groups, may only express poor control over one set of knee restraints, either the active or passive systems. During the performance of athletic tasks, athletes generate large impulse forces at the knee that are primarily absorbed over time as the musculature flexes the joint through a range of motion.30,55 When muscular strength or activation proves insufficient to properly flex the joint and restrain these impulse forces, the knee is forced into frontal and transverse plane rotations.57,86 As the musculature at the knee primarily functions in the sagittal plane, such perturbations may require passive structures to restrain the joint from collapse.57,86–88 Therefore, as relative injury risk levels are classified by peak knee abduction torque generated during landing,26 athletes who exhibit high knee abduction torques during drop vertical jumps have, by definition, identified themselves to have poor neuromuscular control. This is because their musculature failed to constrain the resultant ground reaction forces within the sagittal plane of the knee.57,86 However, not all athletes who express weak musculature or poor hamstrings to quadriceps strength ratios generate high knee abduction moments during landing.
The previously described 206 athletes who were successfully evaluated via 3D motion analysis during the first and second landing of a drop vertical jump were also assessed for hamstrings and quadriceps strength on a dynamometer using previously published methods.89 117 of 206 athletes were found to have poor hamstrings-to-quadriceps strength ratios (below 0.60 hamstrings-to-quadriceps peak strength at 300 °/sec);90 yet, within this subset, only 29 and 15 of these athletes generated knee abduction torques greater than 25.3 N*m in the first and second landing, respectively. Further, as landing from a drop vertical jump generates ground reaction force magnitudes up to 4.5*bodyweight,84 if active knee restraints were the sole contributor to injury risk classification, athletes with the lowest peak strength to body mass ratio would be expected to almost entirely comprise the high-injury-risk group. However, of the 43 athletes classified as high-risk in the first landing, only 17 came from athletes who were in the bottom half of strength-to-body-mass ratios. This behavior indicated that well-developed passive restraints within the knee may be able to compensate for active restraint deficiencies. Furthermore, this implicated that consistent high-risk torque generation may require poor control in both active and passive knee restraint mechanisms. In addition to motion capture and strength measures, the same cohort of adolescent female athletes underwent arthrometry evaluations for joint laxity using previously published methods.91 Again, increased knee laxity alone was not sufficient to predict knee injury risk as only 21 of 43 high-risk subjects exhibited relatively high knee laxity values (greater than 10.22 mm anterior translation under 134 N of anterior force as this was the mean laxity value reported in the contralateral limbs of ACL injury patients).87,88 Of the 206 subjects, 43 exhibited each poor hamstrings-to-quadriceps ratio, lower peak-strength-to-body-mass ratio, and high joint laxity.91,92 Between both landings, 11 of 43 (25.6%) subjects in this subset also exhibited high knee abduction moments during landing. While, far from precise in their indication of risk, the presence of high peak knee abduction torques in these athletes with poor active and passive knee restraints was greater than it was in the overall cohort. These data support that underdeveloped passive restraints contribute to overall injury-risk and that a consistent high-injury-risk classification may be indicative of poor coordination over both systems of knee stabilization.
NEUROMUSCULAR INTERVENTIONS
Prophylactic neuromuscular interventions have been shown to effectively reduce ACL injury incidence over time through successful alteration of negative biomechanical tendencies that contribute to injury-risk.18,45–50 Specifically, a review of interventions discovered that NMT produces a relative risk reduction of 73.4% for non-contact ACL injury in female athletes and that these interventions prevent approximately one injury per every 108 individuals that participate in training.47 It was also found that those neuromuscular interventions that incorporated multiple types of training, each strengthening, plyometric, and balance exercises, were more effective in the reduction of injury rates than interventions focused on a single training type.47 An additional mechanism investigators have recently begun to incorporate into intervention training is biofeedback. Biofeedback utilizes motion analysis techniques to assess deficiencies in dynamic task performance and immediately reinforces them with visual or audible cues that indicate directly to the patient how he or she might optimize movement patterns. In clinical settings, biofeedback has primarily been used to treat gait abnormalities in both pediatric and adult populations.93 Further, investigation has found that biofeedback can instantly reduce relative injury risk for those athletes that exhibit high-risk mechanics.52 Motion analysis investigations have demonstrated that feedback will correct for pain and deficits in gait with moderate to large treatment effect on adult patients.93 During jump landing, feedback has been shown to effectively reduce jump landing forces,94 reduce trunk sway,95 and reduce knee hyperextension.96 Furthermore, real-time kinetic-focused biofeedback implemented in conjunction with 3D motion analysis during a squat activity has been found to immediately reduce peak knee abduction moment during a drop vertical jump, the primary indicator of ACL injury-risk, by 32.8% in adolescent female athletes.97 As previously noted, increased magnitudes of each of these factors has been associated with ACL injury risk; therefore, reduction through biofeedback exemplifies the potential of this tool to further limit the incidence of ACL rupture. Future implementations of biofeedback could be applied in conjunction with the previously documented injury-risk nomograms based on 2D motion analysis as a clinically-relevant mechanism for the diagnosis and immediate initiation of corrective treatment for athletes who exhibit high-risk tendencies.
SUMMARY
Motion-capture analyses have made significant contributions to the identification of factors that contribute to ACL injury risk. The classification of relative injury-risk via 2D and 3D motion analysis techniques plays an essential role for both researchers and clinicians who wish to intervene and prevent ACL in athletic populations. There is strong evidence to indicate that athletes who consistently classify as high-risk loaders during landing suffer from combined joint stability deficits in both their active and passive restraints at the knee joint. Implementation of prophylactic neuromuscular interventions has been shown to effectively compensate for some of the deficiencies that results from poor control of the active knee stabilizers and, in turn, reduce the incidence of ACL injuries. With continued development of these interventions, such as through the incorporation of motion-analysis-based biofeedback, researchers and clinicians can continue to improve the efficacy of ACL injury prevention initiatives.
ABBREVIATIONS
- 2D
Two dimensional
- 3D
Three dimensional
- ACL
Anterior cruciate ligament
- ACLR
Anterior cruciate ligament reconstruction
- NMT
Neuromuscular training
REFERENCES
- 1.Agel J, Olson DE, Dick R, Arendt EA, Marshall SW, Sikka RS. Descriptive epidemiology of collegiate women’s basketball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2003–2004. J. Athl. Train. Apr-Jun 2007;42(2):202–210 [PMC free article] [PubMed] [Google Scholar]
- 2.Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2003–2004. J. Athl. Train. Apr-Jun 2007;42(2):221–233 [PMC free article] [PubMed] [Google Scholar]
- 3.Dick R, Hertel J, Agel J, Grossman J, Marshall SW. Descriptive epidemiology of collegiate men’s basketball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2003–2004. J. Athl. Train. Apr-Jun 2007;42(2):194–201 [PMC free article] [PubMed] [Google Scholar]
- 4.Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women’s soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2002–2003. J. Athl. Train. Apr-Jun 2007;42(2):278–285 [PMC free article] [PubMed] [Google Scholar]
- 5.Yard E, Comstock D. Injury patterns by body mass index in US high school athletes. J Phys Act Health. February 2011;8(2):182–191 [DOI] [PubMed] [Google Scholar]
- 6.Lambers K, Ootes D, Ring D. Incidence of Patients with Lower Extremity Injuries Presenting to US Emergency Departments by Anatomic Region, Disease Category, and Age. Clin Orthop Relat Res. July 2011;470(1):284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. June 2006;13(3):184–188 [DOI] [PubMed] [Google Scholar]
- 8.Miyasaka KC, Daniel DM, Stone ML, Hirshman P. The incidence of knee ligament injuries in the general population. Am. J. Knee Surg. 1991;4(1):3–8 [Google Scholar]
- 9.Arendt EA, Agel J, Dick R. Anterior Cruciate Ligament Injury Patterns Among Collegiate Men and Women. J. Athl. Train. 1999;34:86–92 [PMC free article] [PubMed] [Google Scholar]
- 10.Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578 [DOI] [PubMed] [Google Scholar]
- 11.Toth AP, Cordasco FA. Anterior cruciate ligament injuries in the female athlete. J. Gend. Specif. Med. 2001;4(4):25–34 [PubMed] [Google Scholar]
- 12.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J. Athl. Train. Apr-Jun 2007;42(2):311–319 [PMC free article] [PubMed] [Google Scholar]
- 13.Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J. Sci. Med. Sport. November 2009;12(6):622–627 [DOI] [PubMed] [Google Scholar]
- 14.Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J. Am. Acad. Orthop. Surg. May-Jun 2000;8(3):141–150 [DOI] [PubMed] [Google Scholar]
- 15.Myer GD, Ford KR, Hewett TE. Preventing ACL injuries in Women. J Musculoskel Med. 2006;23(1):12–38 [Google Scholar]
- 16.Johnson DL, Warner JJP. Diagnosis for anterior cruciate ligament surgery. Clin. Sports Med. 1993;12(4):671–684 [PubMed] [Google Scholar]
- 17.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J. Bone Joint Surg. Am. June 1 2011;93(11):994–1000 [DOI] [PubMed] [Google Scholar]
- 18.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am. J. Sports Med. Nov-Dec 1999;27(6):699–706 [DOI] [PubMed] [Google Scholar]
- 19.Bates NA, McPherson AL, Rao MB, Myer GD, Hewett TE. Characteristics of inpatient anterior cruciate ligament reconstructions and concomitant injuries. Knee Surg. Sports Traumatol. Arthrosc. December 16 2015;in-press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagda SH, Altobelli GG, Bowdry KA, Brewster CE, Lombardo SJ. Cost analysis of outpatient anterior cruciate ligament reconstruction: autograft versus allograft. Clin Orthop Relat Res. May 2010;468(5):1418–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renstrom PA. Eight clinical conundrums relating to anterior cruciate ligament (ACL) injury in sport: recent evidence and a personal reflection. Br. J. Sports Med. April 2013;47(6):367–372 [DOI] [PubMed] [Google Scholar]
- 22.Neuman P, Englund M, Kostogiannis I, Friden T, Roos H, Dahlberg LE. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am. J. Sports Med. September 2008;36(9):1717–1725 [DOI] [PubMed] [Google Scholar]
- 23.Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br. J. Sports Med. March 2005;39(3):127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann. Rheum. Dis. March 2004;63(3):269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. October 2004;50(10):3145–3152 [DOI] [PubMed] [Google Scholar]
- 26.Hewett TE, Myer GD, Ford KR, et al. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. Am. J. Sports Med. February 8 2005;33(4):492–501 [DOI] [PubMed] [Google Scholar]
- 27.Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am. J. Sports Med. February 2013;41(2):385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech. November 2006;21(9):977–983 [DOI] [PubMed] [Google Scholar]
- 29.Shin CS, Chaudhari AM, Andriacchi TP. The effect of isolated valgus moments on ACL strain during single-leg landing: A simulation study. J. Biomech. December 17 2008;42(3):280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufek JS, Bates BT. Biomechanical factors associated with injury during landing in jumping sports. Sports Med. 1991;12(5):326–337 [DOI] [PubMed] [Google Scholar]
- 31.Myer GD, Imwalle LE, Ford KR, Hewett TE. Hip Adduction, Not Hip Internal Rotation, Dictates Knee Motion Related to ACL Injury During Cutting Maneuvers. Med & Sci in Sports & Exerc. 2006;38(5) [Google Scholar]
- 32.Imwalle LE, Myer GD, Ford KR, Hewett TE. Relationship Between Hip and Knee Kinematics in Athletic Women During Cutting Maneuvers: A Possible Link to Noncontact Anterior Cruciate Ligament Injury and Prevention. J Strength Cond Res. November 2009;23(8):2223–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewett TE, Ford KR, Myer GD, Wanstrath K, Scheper M. Gender Differences in Hip Adduction Motion and Torque During a Single Leg Agility Maneuver. J. Orthop. Res. 2006;24(3):416–421 [DOI] [PubMed] [Google Scholar]
- 34.Hewett TE, Myer GD. The Mechanistic Connection between the Trunk, Knee, and ACL Injury. Exerc. Sport Sci. Rev. July 21 2011;39(4):161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter DA. Biomechanics and Motor Control of Human Movement. 3rd ed. ed. New York: John Wiley & Sons, Inc.; 2005 [Google Scholar]
- 36.Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br. J. Sports Med. June 2009;43(6):417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiological study. Am. J. Sports Med. March 2007;35(3):368–373 [DOI] [PubMed] [Google Scholar]
- 38.DiCesare CA, Bates NA, Myer GD, Hewett TE. The validity of 2-dimensional measurement of trunk angle during dynamic tasks. International Journal of Sports Physical Therapy. 2014;9(4):421–427 [PMC free article] [PubMed] [Google Scholar]
- 39.Dingenen B, Malfait B, Vanrenterghem J, Robinson MA, Verschueren SM, Staes FF. Can two-dimensional measured peak sagittal plane excursions during drop vertical jumps help identify three-dimensional measured joint moments? Knee. December 2014;22(2):73–79 [DOI] [PubMed] [Google Scholar]
- 40.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Development and Validation of a Clinic-Based Prediction Tool to Identify Female Athletes at High Risk for Anterior Cruciate Ligament Injury. Am. J. Sports Med. October 2010;38(10):2025–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Clinical correlates to laboratory measures for use in non-contact anterior cruciate ligament injury risk prediction algorithm. Clin Biomech. August 2010;25(7):693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myer GD, Ford KR, Khoury J, Hewett TE. Three-dimensional motion analysis validation of a clinic-based nomogram designed to identify high ACL injury risk in female athletes. Phys Sportsmed. February 2011;39(1):19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zebis MK, Andersen LL, Bencke J, Kjaer M, Aagaard P. Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am. J. Sports Med. October 2009;37(10):1967–1973 [DOI] [PubMed] [Google Scholar]
- 44.Myer GD, Ford KR, Hewett TE. Tuck Jump Assessment for Reducing Anterior Cruciate Ligament Injury Risk. Athl Ther Today. September 1 2008;13(5):39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myklebust G, Engebretsen L, Braekken IH, Skjolberg A, Olsen OE, Bahr R. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin. J. Sport Med. March 2003;13(2):71–78 [DOI] [PubMed] [Google Scholar]
- 46.Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am. J. Sports Med. July 2005;33(7):1003–1010 [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto D, Myer GD, McKeon JM, Hewett TE. Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a critical review of relative risk reduction and numbers-needed-to-treat analyses. Br. J. Sports Med. November 2012;46(14):979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chappell JD, Limpisvasti O. Effect of a neuromuscular training program on the kinetics and kinematics of jumping tasks. Am. J. Sports Med. June 2008;36(6):1081–1086 [DOI] [PubMed] [Google Scholar]
- 49.Hewett TE, Myer GD, Ford KR. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. J. Knee Surg. January 2005;18(1):82–88 [DOI] [PubMed] [Google Scholar]
- 50.Hewett TE, Ford KR, Myer GD. Anterior Cruciate Ligament Injuries in Female Athletes: Part 2, A Meta-analysis of Neuromuscular Interventions Aimed at Injury Prevention. Am. J. Sports Med. December 28 2006;34(3):490–498 [DOI] [PubMed] [Google Scholar]
- 51.Myer GD, Ford KR, McLean SG, Hewett TE. The Effects of Plyometric Versus Dynamic Stabilization and Balance Training on Lower Extremity Biomechanics. Am. J. Sports Med. 2006;34(3):490–498 [DOI] [PubMed] [Google Scholar]
- 52.Myer GD, Ford KR, Brent JL, Hewett TE. Differential neuromuscular training effects on ACL injury risk factors in “high-risk” versus “low-risk” athletes. BMC Musculoskelet Disord. 2007;8(39):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myer GD, Ford KR, Brent JL, Hewett TE. The Effects of Plyometric versus Dynamic Balance Training on Power, Balance and Landing Force in Female Athletes. J Strength Cond Res. 2006;20(2):345–353 [DOI] [PubMed] [Google Scholar]
- 54.Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. February 2005;19(1):51–60 [DOI] [PubMed] [Google Scholar]
- 55.Lephart SM, Abt JP, Ferris CM. Neuromuscular contributions to anterior cruciate ligament injuries in females. Curr. Opin. Rheumatol. March 2002;14(2):168–173 [DOI] [PubMed] [Google Scholar]
- 56.Myer GD, Brent JL, Ford KR, Hewett TE. Real-time assessment and neuromuscular training feedback techniques to prevent ACL injury in female athletes. Strength and Conditioning Journal. 2011;33(3):21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med. Sci. Sports Exerc. October 2003;35(10):1745–1750 [DOI] [PubMed] [Google Scholar]
- 58.Myer GD, Ford KR, Hewett TE. Rationale and Clinical Techniques for Anterior Cruciate Ligament Injury Prevention Among Female Athletes. J. Athl. Train. Dec 2004;39(4):352–364 [PMC free article] [PubMed] [Google Scholar]
- 59.Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am. J. Sports Med. Jan-Feb 1986;14(1):83–87 [DOI] [PubMed] [Google Scholar]
- 60.Podraza JT, White SC. Effect of knee flexion angle on ground reaction forces, knee moments and muscle co-contraction during an impact-like deceleration landing: implications for the non-contact mechanism of ACL injury. Knee. August 2010;17(4):291–295 [DOI] [PubMed] [Google Scholar]
- 61.Bates NA, Myer GD, Shearn JT, Hewett TE. Anterior cruciate ligament biomechanics during robotic and mechanical simulations of physiologic and clinical motion tasks: A systematic review and meta-analysis. Clin Biomech. 2015;30(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech. June 2001;16(5):438–445 [DOI] [PubMed] [Google Scholar]
- 63.Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clin. Orthop. 2002;402:76–94 [DOI] [PubMed] [Google Scholar]
- 64.Colby SM, Francisco A, Yu B, Kirkendall DT, Finch M, Garrett W. Electromyographic and Kinematic Analysis of Cutting Maneuvers. Implications for Anterior Cruciate Ligament Injury. Am J Sport Med. 2000;28(2):234–240 [DOI] [PubMed] [Google Scholar]
- 65.Pappas E, Carpes FP. Lower extremity kinematic asymmetry in male and female athletes performing jump-landing tasks. J. Sci. Med. Sport. January 2012;15(1):87–92 [DOI] [PubMed] [Google Scholar]
- 66.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am. J. Sports Med. October 2010;38(10):1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hewett TE, Myer GD, Ford KR. The influence of growth and pubertal maturation on neuromuscular performance in high-risk female athletes. Med. Sci. Sports Exerc. 2002. 2002;34(5) [Google Scholar]
- 68.Pappas E, Zampeli F, Xergia SA, Georgoulis AD. Lessons learned from the last 20 years of ACL-related in vivo-biomechanics research of the knee joint. Knee Surg. Sports Traumatol. Arthrosc. April 2013;21(4):755–766 [DOI] [PubMed] [Google Scholar]
- 69.Hewett TE, Ford KR, Hoogenboom BJ, Myer GD. Understanding and preventing ACL injuries: Current biomechanical and epidemilogic considerations - update 2010. North American Journal of Sports Physical Therapy. 2010;5(4):234–251 [PMC free article] [PubMed] [Google Scholar]
- 70.Wordeman SC, Quatman CE, Kaeding CC, Hewett TE. In vivo evidence for tibial plateau slope as a risk factor for anterior cruciate ligament injury: a systematic review and meta-analysis. Am. J. Sports Med. Jul 2012;40(7):1673–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alentorn-Geli E, Mendiguchia J, Samuelsson K, et al. Prevention of anterior cruciate ligament injuries in sports. Part I: systematic review of risk factors in male athletes. Knee Surg. Sports Traumatol. Arthrosc. January 2014;22(1):3–15 [DOI] [PubMed] [Google Scholar]
- 72.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J. Bone Joint Surg. Am. Mar 1980;62(2):259–270 [PubMed] [Google Scholar]
- 73.Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J. Bone Joint Surg. Am. 1990;72(4):557–567 [PubMed] [Google Scholar]
- 74.Souryal TO, Freeman TR. Intercondylar notch size and anterior cruciate ligament injuries in athletes. A prospective study. Am. J. Sports Med. Jul-Aug 1993;21(4):535–539 [DOI] [PubMed] [Google Scholar]
- 75.Myer GD, Ford KR, Di Stasi SL, Foss KD, Micheli LJ, Hewett TE. High knee abduction moments are common risk factors for patellofemoral pain (PFP) and anterior cruciate ligament (ACL) injury in girls: is PFP itself a predictor for subsequent ACL injury? Br. J. Sports Med. January 2015;49(2):118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Myer GD, Ford KR, Barber Foss KD, et al. The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clin Biomech. Aug 2010;25(7):700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Myer GD, Ford KR, Foss KD, Rauh MJ, Paterno MV, Hewett TE. A predictive model to estimate knee-abduction moment: implications for development of a clinically applicable patellofemoral pain screening tool in female athletes. J. Athl. Train. May-Jun 2014;49(3):389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br. J. Sports Med. June 17 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Myer GD, Ford KR, Hewett TE. New method to identify athletes at high risk of ACL injury using clinic-based measurements and freeware computer analysis. Br. J. Sports Med. November 16 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myer GD, Wordeman SC, Sugimoto D, et al. Consistency of clinical biomechanical measures between three different institutions: Implications for multi-center biomechanical and epidemiological research. International Journal of Sports Physical Therapy. 2014;9(3):289–301 [PMC free article] [PubMed] [Google Scholar]
- 81.Ford KR, Myer GD, Hewett TE. Longitudinal effects of maturation on lower extremity joint stiffness in adolescent athletes. Am. J. Sports Med. September 2010;38(9):1829–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bates NA, Ford KR, Myer GD, Hewett TE. Kinetic and kinematic differences between first and second landings of a drop vertical jump task: Implications for injury risk assessments. Clin Biomech. April 4 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bates NA, Ford K, Myer GD, Hewett TE. Timing differences in the generation of ground reaction forces between the initial and secondary landing phases of the drop vertical jump. Clin Biomech. 2013;In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bates NA, Ford KR, Myer GD, Hewett TE. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J. Biomech. April 26 2013;46(7):1237–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ford KR, Myer GD, Hewett TE. Reliability of landing 3D motion analysis: implications for longitudinal analyses. Med. Sci. Sports Exerc. November 2007;39(11):2021–2028 [DOI] [PubMed] [Google Scholar]
- 86.Andrews JR, Axe MJ. The classification of knee ligament instability. Orthop. Clin. North Am. 1985;16(1):69–82 [PubMed] [Google Scholar]
- 87.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J. Biomech. 2003;36:765–776 [DOI] [PubMed] [Google Scholar]
- 88.McLean SG, Huang X, Su A, van den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech. 2004;19:828–838 [DOI] [PubMed] [Google Scholar]
- 89.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J. Bone Joint Surg. Am. 2004;86-A(8):1601–1608 [DOI] [PubMed] [Google Scholar]
- 90.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am. J. Sports Med. 1996;24(6):765–773 [DOI] [PubMed] [Google Scholar]
- 91.Kiapour AM, Wordeman SC, Paterno MV, et al. Diagnostic value of knee arthrometry in the prediction of anterior cruciate ligament strain during landing. Am. J. Sports Med. February 2014;42(2):312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wordeman SC, Paterno MV, Quatman CE, Bates NA, Hewett TE. Arthrometric curve-shape variables to assess anterior cruciate ligament deficiency. Clin Biomech. October 2012;27(8):830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tate JJ, Milner CE. Real-time kinematic, temporospatial, and kinetic biofeedback during gait retraining in patients: a systematic review. Phys. Ther. Aug 2010;90(8):1123–1134 [DOI] [PubMed] [Google Scholar]
- 94.Onate JA, Guskiewicz KM, Sullivan RJ. Augmented feedback reduces jump landing forces. J. Orthop. Sports Phys. Ther. September 2001;31(9):511–517 [DOI] [PubMed] [Google Scholar]
- 95.Verhoeff LL, Horlings CG, Janssen LJ, Bridenbaugh SA, Allum JH. Effects of biofeedback on trunk sway during dual tasking in the healthy young and elderly. Gait Posture. July 2009;30(1):76–81. [DOI] [PubMed] [Google Scholar]
- 96.Teran-Yengle P, Birkhofer R, Weber MA, Patton K, Thatcher E, Yack HJ. Efficacy of gait training with real-time biofeedback in correcting knee hyperextension patterns in young women. J. Orthop. Sports Phys. Ther. 2011;41(12):948–952 [DOI] [PubMed] [Google Scholar]
- 97.Ford KR, DiCesare CA, Myer GD, Hewett TE. Real-Time Biofeedback to Target Risk of Anterior Cruciate Ligament Injury: A Technical Report for Injury Prevention and Rehabilitation. Journal of Sports Rehabilitation. 2014;In-Press [DOI] [PubMed] [Google Scholar]


