Abstract
Purpose:
Romidepsin is a histone deacetylase inhibitor (HDI) approved for the treatment of both cutaneous and peripheral T-cell lymphoma (CTCL and PTCL). During development, a thorough assessment of cardiac toxicity was conducted.
Experimental Design:
A phase II single-agent nonrandomized study of romidepsin was conducted in patients with CTCL or PTCL who had progressed after at least 1 prior systemic therapy.
Results:
Results for the first 42 patients enrolled on the NCI 1312 phase II study of romidepsin in CTCL or PTCL showed no cardiac toxicity based on serial electrocardiograms (ECG), troponins, and MUGA scans/echocardiograms. The cardiac assessments reported herein confirm the safety of romidepsin among 131 enrolled patients, while supporting a role for electrolyte replacement. Heart rate increased an average 11 bpm following romidepsin infusion; there was no evidence of increased arrhythmia. Criteria for potassium/magnesium replacement were met before 55% of 1365 romidepsin doses; an association with hypoalbuminemia was confirmed. We propose a mechanism for ST segment flattening and depression, the most common ECG abnormalities observed: HDI-induced alteration of the activity or expression of KATP) channels. In addition, examination of the variants of the active transporter of romidepsin, ABCB 1, showed a trend toward smaller heart rate changes in the peri-infusion period among wild-type than variant diplotypes.
Conclusions:
We conclude that in the context of appropriate attention to electrolyte levels, the data support the cardiac safety of romidepsin.
Introduction
Histone deacetylase inhibitors (HDI) are a novel class of epigenetic agents, among which 2 were approved by the U .S. Food and Drug Administration (FDA) for patients with progressive or recurrent cutaneous T-cell lymphoma (CTCL): vorinostat (Zolinza, Merck) and romidepsin (Istodax, Celegene Corporation). Romidepsin is also approved for the treatment of progressive or recurrent peripheral T-cell lymphoma (PTCL). The HDIs promote acetylation of lysine residues of histone proteins present as an octomer surrounded by DNA in the nucleosome chromatin complex. They have been shown to modulate gene expression and to provoke cell-cycle arrest, cell differentiation, and cell death.
Clinical trials with romidepsin, vorinostat, and other HDIs including belinostat, panobinostat, entinostat, and mocentinostat are ongoing, with the hope of extending the activity of HDIs into solid tumors. During the development of this class of agents, concerns were raised about cardiac toxicity due to observed electrocardiograph (ECG) changes, although some preclinical data have suggested that in the setting of cardiac hypertrophy, HDAC inhibition may reduce atrial arrhythmia inducibility (1). Phase I and II clinical trials of HDIs have shown nondiagnostic ECG changes including T-wave flattening and ST segment depression as well as QT interval prolongation and tachyarrhythmias (2–10). No clear mechanistic explanation has been identified. Several sudden deaths occurring early in the development of romidepsin led to amendment of the protocol to exclude patients with significant underlying cardiac disease at risk for sudden death and also to avoid concomitant medications that prolong the QT interval or inhibit CYP3A4. A strict electrolyte replacement regimen was also instituted during conduct of the protocol, coupled with continued ECG monitoring, and development continued without further incident.
Piekarz and colleagues previously reported cardiac studies for 42 of the first 43 patients enrolled on the NCI 1312 phase II trial of romidepsin in TCL (2). Serial ECGs, serial troponin I levels, and MUGA or echocardiograms were reviewed, with no evidence of cardiotoxicity detected. T-wave flattening (grade I) and ST segment depression (grade II) was observed in more than half of the electrocardiograms and was of short duration and reversible (2).
We now provide new cardiac assessment studies for NCI 1312. We observed a consistent increase in heart rate following romidepsin treatment, an observation not previously reported. We report the remarkable frequency with which potassium and magnesium supplementation was required and provide the cardiac adverse events for the complete 131 patients on the trial. The effect of variants of the active transporter of romdepsin, ABCB1, on heart rate following romidepsin is assessed. These data support the overall cardiac safety of romidepsin and other HDIs but they also support continued vigilance regarding the patient population to be treated, and potassium and magnesium supplementation.
Patients and Methods
Patients with relapsed or refractory CTCL or PTCL were enrolled in a phase II trial evaluating the safety and efficacy of romidepsin, [NCI protocol 1312]. The patients and methods and results for the CTCL and the PTCL cohorts (11, 12) have been reported in detail. The trial was registered at ClinicalTrials.gov, NCT00007345, and approved by the NCI Institutional Review Board. All participants gave written informed consent. All toxicities were graded according to the NCI Common Toxicity Criteria, version 2.0. At the NIH site, ECGs were obtained before commencement of therapy, before the infusion of each dose, within 1 hour after completion of the infusion, and on the day following treatment. An additional ECG was obtained on day 2 of the first cycle. ECGs were recorded using a HP Pagewriter XLi or a GE Marquette MAC 1200 and recorded at 25 mm/s, with an amplitude of 10 mm/mV and with 60 Hz filtering. ECGs were analyzed using Pagewriter A.O.4.01 electrocardiogram analysis software (Philips Medical Systems). In this program, the QT interval measurement is obtained by averaging the 5 longest QT intervals with a T- or T’-wave amplitude of >0.15 mV. T-wave and ST segment abnormalities were assessed by either R.L. Piekarz or S.E. Bates and graded using NCI Common Toxicity Criteria version 2.0.
Cardiac exclusion criteria and electrolyte replacement
Following protocol amendment to refine the cardiac eligibility criteria, patients with the following cardiac risk factors were excluded from the study: congenital long QT syndrome, QTc interval > 480 milliseconds; myocardial infarction within 12 months of study entry; active coronary artery disease or screening ECG suggestive of cardiac ischemia (ST depression ≥ 2 mm); NYHA class II-IV congestive heart failure or ejection fraction <45% by MUGA scan or <50% by echocardiogram and/or cardiac MRI; history of dilated, hypertrophic, or restrictive cardiomyopathy; uncontrolled hypertension [systolic blood pressure (SBP) > 160 mmHg, diastolic blood pressure (DBP) > 95 mmHg]; cardiac arrhythmia requiring anti-arrhythmic other than β-blocker or calcium channel blocker; Mobitz type II second-degree heart block not controlled by a pacemaker.
After amendment, potassium and magnesium were monitored. For patients with a serum potassium level <3.5 mmol/L, a total of 80 mEq of potassium were administered given as 40 mEq intravenously and 40 mEq orally. For serum potassium ≥3.5 mmol/L but <4.0 mmol/l, 40 mEq was administered either intravenously or orally. For patients with a serum magnesium level <0.85 mmol/L, 1 g MgSO4 (8.12 mEq) was administered intravenously for every 0.05 mmol/L below 0.85 mmol/L to a maximum of 4 g MgSO4 (32.48 mEq).
Statistical methods
The Wilcoxon rank-sum test, Fisher exact test, and Jonckheere–Terpstra trend test were used to test the relationship between low albumin and low potassium and magnesium levels. ANOVA was used to test the differences in albumin and potassium and magnesium levels by histology and by cycle and day of treatment.
Diplotype arrangements of the ABCB1 single-nucleotide polymorphisms (SNP) were calculated using Haploview (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview) [See Supplementary Table S1 (ref. 13)] Heart rates and romidepsin-induced heart rate changes versus genotype or β-blocker therapy were evaluated using Kruskal–Wallis, ANOVA, Wilcoxon rank-sum test, and Jonckheere–Terpstra trend test. As multiple tests were conducted, comprising 3 individual genotypes and 3 diplotype organizations (accounting for the coinheritance of ABCB1 alleles), tests that were P < 0.05 were considered marginally significant. The a priori level of nominal significance was set at P < 0.01 rather than accounting for a strict Bonferroni adjustment as repeated measures of heart rates were correlated and ABCB1 alleles were coinherited (i.e., correlated). Data are reported as mean ± SD unless otherwise indicated.
Results
Patient characteristics
Table 1 illustrates the patient characteristics for all 131 patients treated in the study and for the 63 patients treated at the NIH Clinical Center only. The CTCL cohort was almost twice as large as the PTCL cohort. The majority of patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 1. Thirty-three percent of patients received 3 to 5 cycles and 40% received 6 or more cycles of romidepsin. Fifty-one percent of all patients in the trial had received prior doxorubicin (52% of the NIH cohort). Excluding the differences in ECOG performance status, the NIH cohort is mostly representative of all patients in the trial. In all, the 131 patients received 3,358 doses of romidepsin in 1, 198 cycles; the 63 patients enrolled at the NIH received 1,292 doses in 475 cycles.
Table 1.
Baseline characteristics and cycle and dose data for all 131 patients treated on NCI 1312 or only for the NIH cohort
| Data for all 131 patients on NCI 1312 | |||
|---|---|---|---|
| Characteristic | Number of patients | ||
| Gender | Total | CTCL | PTCL |
| Male | 82 | 57 | 25 |
| Female | 49 | 27 | 22 |
| Diagnosis | 131 | 84 | 47 |
| Prior doxorubicin | |||
| Yes | 66 | 22 | 44 |
| No | 65 | 62 | 3 |
| ECOG | |||
| 0 | 44 | 26 | 18 |
| 1 | 71 | 47 | 24 |
| 2 | 16 | 11 | 5 |
| Cycles administered | |||
| 1–2 | 36 | 14 | 22 |
| 3–5 | 43 | 34 | 9 |
| ≥6 | 52 | 36 | 16 |
|
Median (range) |
|||
| Age | 59.4 (27.6–84.8) | 59.2 (28.2–84.8) | 59.6 (27.6–84.3) |
| Cycles | 4 (1–82) | 4.5 (1–79) | 3 (1–82) |
| Doses | 12.0 (1–244) | 12.5 (2–158) | 9 (1–244) |
|
Doses |
|||
| Total doses | 3359 | 2067 | 1292 |
| Data for NIH cohort | |||
| Characteristic | Number of patients | ||
| Gender | Total | CTCL | PTCL |
| Male | 40 | 29 | 11 |
| Female | 23 | 13 | 10 |
| Diagnosis | 63 | 42 | 21 |
| Prior doxorubicin | |||
| Yes | 33 | 12 | 21 |
| No | 30 | 30 | 0 |
| ECOG | |||
| 0 | 9 | 4 | 5 |
| 1 | 46 | 32 | 14 |
| 2 | 8 | 6 | 2 |
| Cycles administered | |||
| 1–2 | 19 | 8 | 11 |
| 3–5 | 19 | 16 | 3 |
| ≥6 | 25 | 18 | 7 |
|
Median (range) |
|||
| Age | 53.3 (27.6–80.3) | 55.5 (28.2–80.3) | 57.6 (27.6–79.4) |
| Cycles | 4 (1–79) | 4 (1–79) | 2 (1–29) |
| Doses | 12 (1–158) | 12 (2–158) | 6 (1–81) |
|
Doses |
|||
| Total doses | 1403 | 1005 | 398 |
ECG changes
ECGs for the 63 patients treated at the NIH site were analyzed; in total, 3,633 electrocardiograms were reviewed. Table 2 summarizes the commonly observed T-wave (grade I) or ST segment (grade II) abnormalities noted on ECGs following romidepsin treatment. On examining ECGs from all doses given (Table 2), 42% had grade I and 1% had grade II abnormalities immediately after completion of romidepsin infusion and on the day following infusion 66% had grade I and 4% had grade II abnormalities. ECG abnormalities observed with the administration of the first dose of romidepsin are detailed in the middle panel of Table 2. Last, considering the worst ECG grade obtained at any time during the study for each of the 63 patients, 73% had grade I and 25% had grade II abnormalities at some point during the treatment protocol (Table 2). These results were comparable to the findings in the original analysis of 42 patients (2).
Table 2.
T-wave and ST-segment changes at baseline and following romidepsin administration for patients in the NIH cohort (n = 63)
| T-wave and ST-segment abnormalities by time after administration of romidepsin | ||||
|---|---|---|---|---|
| No. of ECGs | Grade 0 [n, (%)] | Grade I [n, (%)] | Grade II [n, (%)] | |
| Pretreatment | 1,101 | 898 (82) | 192 (17) | 11 (1) |
| Immediate posttreatment | 1,061 | 604 (57) | 446 (42) | 11 (1) |
| Day after treatment | 1,024 | 309 (30) | 672 (66) | 43 (4) |
| Unscheduled | 447 | 246 (55) | 181 (40) | 20 (5) |
| T-wave and ST-segment abnormalities associated with the first dose of the first cycle | ||||
| No. of ECGs | Grade 0 [n, (%)] | Grade I [n, (%)] | Grade II [n, (%)] | |
| Pretreatment | 63 | 59 (94) | 3 (5) | 1 (<2) |
| Immediate posttreatment | 61 | 48 (79) | 12 (20) | 1 (<2) |
| Day 2 | 62 | 27 (44) | 33 (53) | 2 (3) |
| Day 3 | 49 | 26 (53) | 22 (45) | 1 (2) |
| T-wave and ST-segment abnormalities observed at any time point | ||||
| No. of patients | Grade 0 [n, (%)] | Grade I [n, (%)] | Grade II [n, (%)] | |
| Cycle 1 only | 63 | 8 (13) | 49 (78) | 6 (<10) |
| All cycles | 63 | 1 (<2) | 46 (73) | 16 (25) |
Heart rate changes following romidepsin
A new observation was that the heart rate modestly but consistently increased in patients receiving romidepsin. Supplementary Figure S1A–S1C show heart rate increases following days 1, 8, and 15 dosing in cycle 1. To determine whether the heart rate increase varied by baseline heart rate, the change in heart rate was also analyzed in sets of 10, commencing with patients whose baseline heart rates were in the 40 seconds. Notably, the magnitude of heart rate increase did not vary with baseline (Supplementary Fig. S2A-S2C). Median baseline heart rate was 85 ± 16 bpm, increasing to 96 ± 15 bpm on cycle 1 day 1. Cycle 2 day 1 values showed a similar increase (81 ± 15 bpm to 93 ± 15 bpm postinfusion). Supplementary Figures S3-S8 show similar patterns for cycle 1 and 2. Analysis was conducted both including and excluding the patients who received β-blockers.
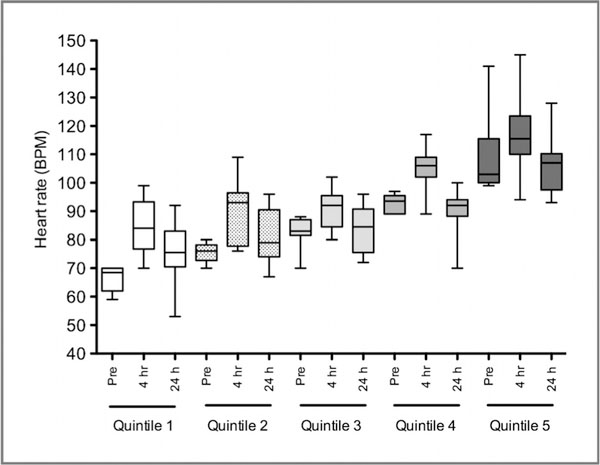
Fourteen patients were treated with β-blockers. Nine patients were noted to be on β-blockers on cycle 1 day 1 before receiving romidepsin: 8 for hypertension and 1 for long QT and atrial flutter. Five patients were commenced on β-blockers between cycle 1 day 2 and cycle 3 day 15 for tachycardia or arrhythmias observed during monitoring; these patients had pre-existing tachyarrhythmias on preenrollment Holter monitoring. Patients receiving β-blockers before romidepsin start had lower heart rates (74 ± 15 bpm vs. 85 ± 16 bpm; P = 0.019). β-Blockers did not prevent the heart rate increase following romidepsin. Values after infusion on cycle 1 day 1 were 86 ± 16 bpm versus 96 ± 15 bpm; P = 0.053, respectively. Figure 1 shows the heart rate changes examined in quintiles for cycle 1 day 1 excluding patients on β-blockers. Cycle 2 day 1 values were similar, with and without β-blockers showing similar increases in magnitude (pre-infusion, 64 ± 10 bpm vs. 81 ± 15 bpm; P = 0.0008, respectively, and after infusion, 75 12 bpm vs. 93 ± 15 bpm; P = 0.0011, respectively). We therefore accounted for β-blocker therapy in subsequent analyses of heart rate.
Figure 1.
Analysis of heart rate changes by quintile for cycle 1 day 1 of romidepsin, excluding patients on β-blockers. Heart rate increases were noted at the end of the 4-hour infusion of romidepsin. The majority returned to baseline heart rate level 24 hours after romidepsin infusion.
Holter monitor and telemetry results in NIH cohort
Baseline 24-hour holter data were available in 56 of the 63 patients. Cardiac rhythm monitoring with telemetry totaling 24 hours during and after romidepsin infusion was available for 59 patients; 19 of these patients also had concurrent holter monitoring. Table 3 summarizes the holter and telemetry data for baseline and after cycle 1 day 1. Frequent atrial premature complexes (APC) were defined as >200 APCs on 24-hour holter based on 2 studies showing increased incidence of arrhythmia and stroke with >200 APCs/24 h (14, 15). Frequent premature ventricular complexes (PVC) were defined as >1,000/24 h (16). On the baseline holter monitor recorded before commencing romidepsin infusion, 23 patients had grade I supraclavicular tachycardia (SVE), 5 patients had grade I ventricular tachycardia (W), and 3 patients had accelerated idioventricular rhythm (AIVR). In addition, one patient had grade II atrial flutter and was cardioverted before commencing on protocol. A11 events reported on telemetry and holter monitoring during and for 24 hours after the first romidepsin infusion were grade I, apart from one patient considered to have grade III arrhythmia—frequent runs of SVT, VT, and AIVR after romidepsin infusion in a patient who also had frequent APCs, frequent PVCs, and AIVR on baseline holter before romidepsin. This patient had electrophysiologic testing within 24 hours of receiving his third dose of romidepsin, which showed no evidence of increased susceptibility to arrhythmia. One patient with grade I trigeminy was found to have concomitant hypomagnesemia (magnesium 0.59 mmol/L) and hypokalemia (potassium 3.4 mmol/L).
Table 3.
Holter monitor and telemetry results in NIH cohort
| N | Frequent APCs >200/24 h [n, (%)] (14,15) | Frequent PVCs >1,000/24 h [n, (%)] (16) | SVT [n, (%)] | VT [n, (%)] | AIVR [n, (%)] | |
|---|---|---|---|---|---|---|
| Baseline Holter pre-romidepsin | 56 | 10 (18) | 5 (9) | 23 (41) | 5 (9)a | 3 (5) |
| Telemetry during and for 24 h after romidepsin infusionb | 59 | 0 (0)c | 0 (0)c | 6 (10)c | 6 (10)c | 0 (0)c |
| Cycle 1 day 1 Holter during and for 24 h after romidepsin infusionb,d | 19 | 5 (8)c | 0 (0)c | 6 (10)c | 3 (5)c | 0 (0)c |
One event was due to line misplacement.
Some patients had both SVT and VT.
Percentages calculated using denominator of 59.
This is a subset of patients who had both telemetry and holter monitoring during cycle 1 day 1, often triggered by ectopy observed on the baseline holter.
Cardiac adverse events in the entire population
Supplementary Table S2 outlines the cardiovascular adverse events reported for all 131 patients on the trial. The majority of events were grade I. Two deaths were observed before institution of electrolyte monitoring; both were unexpected and considered episodes of sudden cardiac death, occurring in one patient with multivalvular cardiac disease and in a second patient with severe atherosclerotic disease. Fourteen episodes of SVT occurring in 13 patients were reported during or after the romidepsin infusion; 6 of these were atrial fibrillation: 2 of which were grade I, 2 grade II, and 2 grade III. Among the patients with atrial fibrillation, grade I hypomagnesemia was seen in one patient and magnesium below the replacement level was seen in another. Potassium levels below the replacement level were seen in 3 patients. One episode of grade III atrial flutter was reported in a patient whose potassium and magnesium were both above the replacement levels. Troponin levels were routinely measured in the NIH patients within 48 hours before romidepsin administration, on day 1, and before, and 1 day after each romidepsin dose (i.e., on days 1, 2, 8, 9, 15, and 16) of each treatment cycle; 2,353 were collected over all cycles. Sixteen events of elevated troponin I were observed in 1 1 patients. Eleven of these were normal on repeat assay or subsequent measurement within 24 hours and were thought to be lab errors, except for 1 patient with a lymphomatous myocardial wall mass (17) who had repeatedly elevated troponin levels.
Potassium and magnesium monitoring
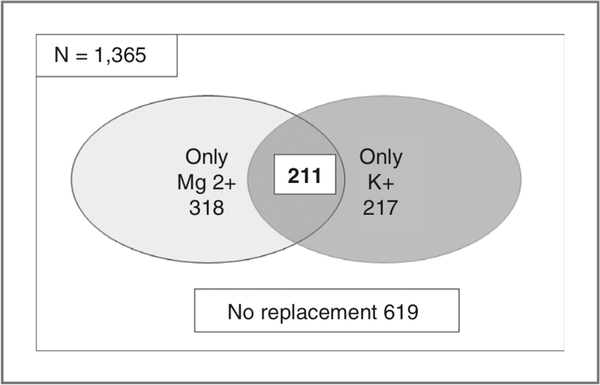
Given that electrolyte monitoring and supplementation was required in patients enrolled on the clinical trial, we quantified the incidence of hypomagnesemia and hypokalemia requiring intervention as defined per protocol. Serum electrolyte measurements for 1,365 doses administered to 128 of 131 patients (between cycles 1 and 6 assessed) were analyzed. Only 10 patients (7.6%) on the trial never required electrolyte replacement as defined in the protocol during their course of therapy. Figure 2 shows the number of doses requiring magnesium and potassium replacement. Fifty-five percent (746 of 1,365) of doses of romidepsin administered between cycles 1 and 6 were associated with pretreatment levels meeting criteria for protocol-mandated supplementation. Magnesium repletion was required more often than potassium repletion [529 doses (39%) vs. 428 doses (31%)].
Figure 2.
Number of doses of romidepsin during which replacement of magnesium or potassium or both electrolytes was required per protocol. Only 619 doses (45%) did not require potassium or magnesium replacement.
Because both our data and previous reports suggested that hypokalemia and hypomagnesemia were common in CTCL (18) and because we observed frequent hypoalbuminemia, we tested the association of these findings. using the NIH lower limit of normal for albumin, 3.7 mg/dL, we asked whether potassium or magnesium below or equal to the lower limit of normal (potassium ≤3.5 mmol/L or magnesium ≤0.75 mmol/L) or below the protocol-defined replacement levels (potassium <4.0 mmol/L or magnesium <0.85 mmol/L) was associated with low albumin. This was analyzed initially by the Wilcoxon-rank sum test. Doses from cycles 1–3 and days 1, 8, and 15 were analyzed. Results, as shown in Supplementary Table S3, showed that 28 of 98 analyses revealed a P < 0.05. Similar results were obtained with Fisher’s exact test and the Jonckheere–Terpstra trend test (Supplementary Table S3), indicating that higher albumin levels were associated with higher potassium or magnesium levels. However, many of the P values were rather large, indicating a weak association. To further examine the association, a set of repeated measures of ANOVA was conducted on the albumin data by histology (CTCL or PTCL or both), with data restricted to the first 3 cycles. Starting with the initial model, a backward selection process was conducted on the model in a hierarchical manner. The results are summarized in Supplementary Table S4. Again, association of albumin with potassium and magnesium was found. Variation with cycle and day was also noted in some of the models. Together, the results of these 4 statistical analyses show that hypoalbuminemia, aggravated by the disease process in TCL, although not the only factor, is associated with low potassium and magnesium.
ABCB1 genotyping
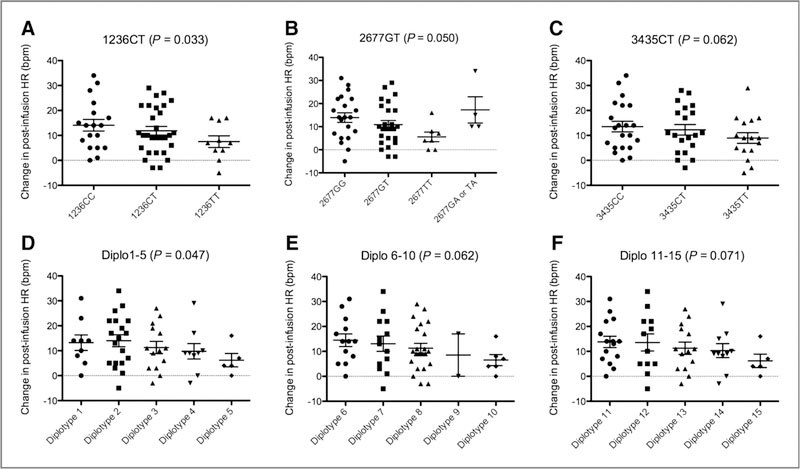
Genotype data for the ABCB1 (P-glycoprotein) transporter was available for 61 patients (ref. 13; Supplementary Table S4). We previously noted that several ABCB1 polymorphisms and diplotypes conferred a reduced impact on the QT interval on cycle 1 day 1 following romidepsin administration (13). As observed in Fig. 3, the data (excluding patients who were on β-blockers) point to a similar trend with romidepsin-induced heart rate changes (C1D1 post-baseline), although most trends are not statistically significant (Ptrend ≤ 0.18; Fig. 3). Nonetheless, those carrying genotypes and diplotypes with increasing numbers of variant alleles have smaller heart rate changes than their counterparts carrying more wild-type alleles. Similarity between heart rate changes versus the various genotypes was expected given that all 3 ABCB1 loci are in significant linkage disequilibrium. No apparent relationship was observed when the same SNPs and diplotypes were compared with heart rate data obtained on cycle 2 day 1 of romidepsin treatment (P > 0.05).
Figure 3.
Trends were observed in romidepsin-induced heart rate (HR) changes for patients carrying (A) ABCB1 1236C>T (15.5, 12.3, and 6.4 msec, respectively; P = 0.033); (B) ABCB1 2677G>T/A (14.4, 11.3, 5.6, 19.7, and 10.0 msec, respectively; P = 0.050 when GA and AA alleles were not accounted for); C, ABCB1 3435C>T (15.2, 12.5, and 8.4 msec, respectively; P = 0.062), or combinations of these genotypes, including (D) diplotypes 1–5 (15.0, 14.8, 1 1.2, 9.8, and 6.2 msec, respectively; P = 0.047), (E) diplotypes 6–10 (15.8, 13.5, 11.6, 8.5, and 6.5 msec, respectively; P = 0.062), and (F) diplotypes 11–15 (14.6, 15.1, 1 1.2, 10.3, and 6.2 msec, respectively; P = 0.071). All P values are derived from the Jonckheere–Terpstra test for trend, and patients who received β-blockers were not included in this analysis.
Discussion
Romidepsin is an HDI approved by the FDA for treatment of CTCL and PTCL. While the drug functions as an inhibitor of deacetylase activity, the downstream events that emerge are pleiotropic. The HDIs as a class cause cell-cycle arrest, gene induction, reactive oxygen species production, and apoptosis. The relative importance of these various activities likely depends on the context of pre-existing genetic aberrations. Clinically, the HDIs have similar efficacy and toxicity profiles; activity in TCL; fatigue, nausea, and vomiting the most common adverse events; transient thrombocytopenia and neutropenia; and ECG effects including minor QT prolongation and ST and T wave changes. The mechanisms underlying these cardiac effects have not been worked out although there was speculation that acetylation of the hERG channel might cause QT prolongation (19, 20). In recently reported data, intensive investigation of romidepsin has suggested minimal impact on the QT interval, no evidence of myocardial damage, and no cumulative effects (21, 22). We have theorized that the more interesting cardiac effect may be the ST and T wave change observed in as many as 75% of patients receiving romidepsin. This report summarizes our studies of the cardiac effects of romidepsin on heart rate and ST and T wave changes, including cardiac rhythm monitoring, which did not suggest an increase in the incidence of arrhythmias following romidepsin. However, the monitoring did suggest that the patient population with TCL is one with frequent ectopy at baseline. Finally, our data call attention to the preponderance of patients whose potassium and magnesium levels met protocol criteria for electrolyte replacement.
Notably, atrial fibrillation has been observed in the development of almost every HDI. It was observed as a dose limiting toxicity in the romidepsin phase I, occurring at 24.9 mg/m2 on the day 1 and 5 schedule (a dose well above the approved 14 mg/m2 dose; ref. 8). Atrial fibrillation or flutter was observed during the Phase I trials of panobinostat (LBH589), LAQ824, belinostat, plitidepsin, and CHR-3996; often occurring in more than one patient for any given agent, and occasionally below doses that were defined as the maximum tolerated dose (7, 23–27). This is in contrast to the QT interval–associated arrhythmia torsades de pointes, which was observed with only one agent, LAQ824, a cinnamic hydroxamate no longer in clinical development (28). Recent evaluation of romidepsin data suggests a minimal effect on the QT interval using the Friderica (QTcF) correction, considered a better assessor of the QT interval than the Bazett (QTcB) correction at faster heart rates (2, 22).
Romidepsin is the most potent HDI currently in development largely targeting class I HDACs. Extensive cardiac safety data have been gathered, in part because it was the first potent and effective HDI in development and in part because of the early observation of ECG changes. During early development, 4 unexpected deaths were observed in other trials in addition to 2 in the NCI 1312 study that were suggestive of sudden death. upon review, it was recognized that each of the deaths occurred in patients with risk factors for cardiac events including severe valvular pathology, severe atherosclerotic heart disease, sarcoidosis, and uncontrolled hypertension in a patient with neuroendocrine tumor (2, 11, 24, 29). Notably, sudden death has also been reported in patients receiving other HDIs (30–32). After these observations, the protocol was amended to exclude patients with significant cardiac disease and to require potassium and magnesium supplementation to maintain levels in a high normal range, as electrolyte deficiencies increase arrhythmia risk (33–36). Hypomagnesemia is a known risk factor for cardiac arrhythmia and sudden cardiac death (SCD; refs. 34, 35). Data from the Nurses’ Health Study reported in 2011 showed an inverse linear relationship between plasma magnesium level and SCD in which each 0.25 mg/dL increment in plasma magnesium was associated with a 41% lower risk (35). Hypokalemia is also a well-documented risk factor for cardiac arrhythmia and SCD (36, 37). Consistent with a previous report of hypomagnesemia in patients with advanced-stage CTCLs (18), we observed that half of doses required supplementation with either magnesium or potassium.
The most reproducible electrocardiographic effect of the HDIs appears to be ST segment flattening and depression and not QT prolongation. ST shift is an unusual drug effect, and in cardiology practice, this ECG finding is most frequently associated with ischemia, particularly occurring in the subendocardial region of the left ventricle. Certain drugs can induce cardiac ischemia and ST depression by inducing vasospasm of the coronary arteries, with cocaine and amphetamines being the most notorious culprits. These drugs may also result in myocardial necrosis. In addition, some anticancer drugs, such as 5-fluorouracil (5FU), are known to cause coronary vasospasm (38). Importantly, objective evidence of ischemia was sought and excluded in the case of romidepsin (2). Subjects with ST depression did not manifest mechanical dysfunction or enzyme release by troponin assay, a particularly sensitive measure. Instead, the evidence is clear that ST depression occurs in the absence of significant ischemia.
The mechanism underlying ST segment shifts induced in ischemia is somewhat controversial but likely involves activation of the ATP-sensitive potassium conductance (KATP)) in the ischemic region due to energy deprivation (39). Often considered to be cardioprotective, opening of KATP) channels can also promote arrhythmias. In response to a decrease in the ATP:MgADP ratio, opening of just 1% of the KATP) channels shortens the action potential by 50% (40, 41). When channel opening occurs in one region of the heart but not another, a voltage gradient can develop between the de-energized subendocardium with shortened action potentials and adjacent tissue with normal action potentials, resulting in arrhythmogenic dispersion in repolarization. In addition, the shortened action potential duration and refractory period can promote phase II re-entry (42).
The dual capability of KATP) channels to promote reentry (e.g., atrial fibrillation) and ST segment shifts may suggest that HDIs affect the activity or expression of KATP) channels. In cardiac myocytes, KATP) channels are composed of 2 different subunits—a pore-forming Kir6.2 and a regulatory member of the ATP-binding cassette family, either SUR1 or SUR2A (43). HDIs are known to affect the expression of ATP-binding cassette (ABC) proteins, and in recently reported data, Flagg and colleagues found that HDIs downregulate SUR1 but upregulate SUR2 in the transformed HL-1 atrial cell line (44). Furthermore, we recently reported romidepsin-induced increased trafficking to the cell surface of an ABC protein ABCG2 due to changes in both expression and protein processing (45). In mice, KATP) channel structure is chamber-specific (atrial SUR1+Kir6.2 vs. ventricular SUR2A+Kir6.2; refs. 46, 47); however, both combinations appear to exist in both chambers in the human heart (48). Increased activity of the KATP channel in the atrium has been associated with an increased incidence of atrial fibrillation, an arrhythmia that is rarely seen with QT prolonging drugs (48). Although functional data are needed to determine whether the effects of HDI on SUR2 expression results in increased channel activity, these data support a hypothetical explanation for the reported observations, that is, differential downregulation of SUR protein in the ventricle (resulting in ST depression) and upregulation in the atria (resulting in atrial fibrillation).
Subjects carrying variant alleles in the gene encoding an active transporter of romidepsin, ABCB1 (P-glycoprotein, MDR-I), are thought to express higher levels of ABCB1 in the cardiac endothelium (49). Consistent with these data, we recently showed that ABCB1 variant carriers have a modestly lower risk of developing QT prolongation following romidepsin than similar subjects carrying wild-type alleles (13). We previously reported that ABCB1 expression directly limits intracardiac exposure in a murine model and that mice lacking Abcb1-type P-glycoprotein are consequently more susceptible to romidepsin-induced ECG changes. In our current data, there were no clinically significant differences in heart rate changes between genotypes, but a trend to lower heart rate change in the variants did appear to be consistent with the QT observations.
Taken together, these studies again support the cardiac safety of romidepsin in the treatment of CTCL and PTCL and support its continued development in solid tumors. The studies also highlight the frequency with which low potassium and magnesium levels are observed in the TCL patient population. Mechanistically, it is not a simple matter to tie together the ST-T wave changes, the heart rate increase, and the observation of atrial fibrillation occurring as a dose-limiting toxicity. We have here hypothesized that some effects are linked to the variable penetration of romidepsin (and equally the other HDIs) through the myocardium from the perfusing vessels. A gradient could result that altered repolarization and thereby affected the ST and T wave, as well as the potential for ectopy. An increase in heart rate due to increased catecholamines, a decrease in electrolytes due to disease, and an alteration in repolarization due to increased activity of SUR in one part of the myocardium relative to another could create a perfect storm that in a patient with a hypertrophic myocardium or a poorly perfused myocardium could induce arrhythmia. This is a hypothesis that argues for, as recommended in the FDA-approved package insert, careful monitoring of potassium and magnesium and close observation of patients with known underlying cardiac disease (50).
Supplementary Material
Translational Relevance.
Romidepsin is a histone deacetylase (HDAC) inhibitor U.S. Food and Drug Administration (FDA)–approved for the treatment of cutaneous and peripheral T-cell lymphoma and continues in development. Cardiac safety has been intensively investigated for romidepsin and other HDAC inhibitors, generating confidence in the safety of the class. However, ST and T-wave changes in the electrocardiograph (ECG) of unclear etiology are observed in a majority of patients. This report documents a consistent increase in heart rate in all patients treated without evidence of a pro-arrhythmic effect. A high fraction of patients with T-cell lymphoma require potassium and magnesium supplementation. We present a mechanistic hypothesis for the ST segment and T-wave changes observed on serial ECGs, explaining why electrolyte replacement would be important. The report affirms the safety of romidepsin in the context of attention to potassium and magnesium supplementation; the data are important for both the academic and clinical communities, among them physicians prescribing romidepsin
Acknowledgments
Grant Support
S.E. Bates received research funding from Celgene Pharmaceuticals through a Cooperative Research and Development Agreement with the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
S.E. Bates received research funding from Celgene Pharmaceuticals through a Cooperative Research and Development Agreement with the National Cancer Institute. No potential conflicts of interest were disclosed.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Liu F, Levin MD, Petrenko NB, Lu MM, Wang T, Yuan LJ, et al. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol 2008;45:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piekarz RL, Frye AR, Wright JJ, Steinberg SM, Liewehr DJ, Rosing DR, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res 2006; 12:3762–73. [DOI] [PubMed] [Google Scholar]
- 3.Niesvizky R, Ely S, Mark T, Aggarwal S, Gabrilove JL, Wright JJ, et al. Phase 2 trial of the histone deacetylase inhibitor romidepsin for the treatment of refractory multiple myeloma. Cancer 2011;117: 336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molife LR, Attard G, Fong PC, Karavasilis V, Reid AHM, Patterson S, et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann Oncol 2009;21:109–13. [DOI] [PubMed] [Google Scholar]
- 5.Molife R, Fong P, Scurr M, Judson I, Kaye S, de Bono J. HDAC inhibitors and cardiac safety. Clin Cancer Res 2007;13:1068. [DOI] [PubMed] [Google Scholar]
- 6.Lassen U, Molife LR, Sorensen M, Engelholm SA, Vidal L, Sinha R, et al. A phase I study of the safety and pharmacokinetics of the histone deacetylase inhibitor belinostat administered in combination with carboplatin and/or paclitaxel in patients with solid tumours. Br J Cancer 2010;103:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steele NL, Plumb JA, Vidal L, Tjornelund J, Knoblauch P, Rasmussen A, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res 2008; 14:804–10. [DOI] [PubMed] [Google Scholar]
- 8.Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res 2002;8:718–28. [PubMed] [Google Scholar]
- 9.Marshall JL, Rizvi N, Kauh J, Dahut W, Figuera M, Kang MH, et al. A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J Exp Ther Oncol 2002;2:325–32. [DOI] [PubMed] [Google Scholar]
- 10.Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 2012;30:631–7. [DOI] [PubMed] [Google Scholar]
- 11.Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 2009;27:5410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, et al. Phase II trial of romidepsin in patients with peripheral T-cell lymphoma. Blood 2011;117:5827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sissung TM, Gardner ER, Piekarz RL, Howden R, Chen X, Woo S, et al. Impact of ABCB1 allelic variants on QTc interval prolongation. Clin Cancer Res 2011;17:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engstrom G, Hedblad B, Juul-Moller S, Tyden P, Janzon L. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke 2000;31:2925–9. [DOI] [PubMed] [Google Scholar]
- 15.Todo K, Moriwaki H, Saito K, Naritomi H. Frequent premature atrial contractions in stroke of undetermined etiology. Eur Neurol 2009;61: 285–8. [DOI] [PubMed] [Google Scholar]
- 16.Kwa AT, Li Z, Amsterdam EA, Srivatsa UN. Ventricular ectopy and long-term cardiac function. Crit Pathw Cardiol 2011;10:52–4. [DOI] [PubMed] [Google Scholar]
- 17.CYMahony D, Peikarz RL, Bandettini WP, Arai AE, Wilson WH, Bates SE. Cardiac involvement with lymphoma: a review of the literature. Clin Lymphoma Myeloma 2008;8:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan M, Maloney D, Duvic M. Hypomagnesemia and hypocalcemia in mycosis fungoides: a retrospective case series. Leuk Lymphoma 2002;43:1297–302. [DOI] [PubMed] [Google Scholar]
- 19.Cho YS, Whitehead L, Li J, Chen CH, Jiang L, Vogtle M, et al. Conformational refinement of hydroxamate-based histone deacetylase inhibitors and exploration of 3-piperidin-3-ylindole analogues of dacinostat (LAQ824). J Med Chem 2010;53:2952–63. [DOI] [PubMed] [Google Scholar]
- 20.Shultz MD, Cao X, Chen CH, Cho YS, Davis NR, Eckman J, et al. Optimization of the in vitro cardiac safety of hydroxamate-based histone deacetylase inhibitors. J Med Chem 2011;54:4752–72. [DOI] [PubMed] [Google Scholar]
- 21.Cabell C, Bates S, Piekarz R, Whittaker S, Kim Youn H, Currie M, et al. Systematic assessment of potential cardiac effects of the novel histone deacetylase (HDAC) inhibitor romidepsin. American Society of Hematology Annual Meeting Blood 2009; 114 (suppl; abstr 3709). [Google Scholar]
- 22.Godfrey CJ, Cabell C, Balser B, Wolfson J, Nichols J, Burris HA. Exposure-QTc response analysis of class 1 selective histone deacetylase inhibitor. American Society of Hematology Annual Meeting. Blood 2011;1 18 (suppl; abstr 2680). [Google Scholar]
- 23.Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res 2006;12: 4628–35. [DOI] [PubMed] [Google Scholar]
- 24.Stadler WM, Margolin K, Ferber S, McCulloch W, Thompson JA. A phase II study of depsipeptide in refractory metastatic renal cell cancer. Clin Genitourin Cancer 2006;5:57–60. [DOI] [PubMed] [Google Scholar]
- 25.de Bono JS, Kristeleit R, Tolcher A, Fong P, Pacey S, Karavasilis V, et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin Cancer Res 2008;14:6663–73. [DOI] [PubMed] [Google Scholar]
- 26.Ramalingam SS, Kummar S, Sarantopoulos J, Shibata S, LoRusso P, Yerk M, et al. Phase I study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: a National Cancer Institute Organ Dysfunction Working Group study. J Clin Oncol 2010;28:4507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerji U, van Doorn L, Papadatos-Pastos D, Kristeleit R, Debnam P, Tall M, et al. A phase I pharmacokinetic and pharmacodynamic study of CHR-3996, an oral class I selective histone deacetylase inhibitor in refractory solid tumors. Clin Cancer Res 2012;18:2687–94. [DOI] [PubMed] [Google Scholar]
- 28.Rowinsky EK, de Bono J, Deangelo DJ, van Oosterom A, Morganroth J, Laird GH, et al. Cardiac monitoring in phase I trials of a novel histone deacetylase (HDAC) inhibitor LAQ824 in patients with advanced solid tumors and hematologic malignancies. J Clin Oncol (Meeting Abstracts) 23(16), 2005. (suppl; abstr 3131). [Google Scholar]
- 29.Shah MH, Binkley P, Chan K, Xiao J, Arbogast D, Collamore M, et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res 2006; 12: 3997–4003. [DOI] [PubMed] [Google Scholar]
- 30.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, et al. Phase 11b multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 2007;25:3109–15. [DOI] [PubMed] [Google Scholar]
- 31.Gojo l, Jiemjit A, Trepel JB, Sparreboom A, Figg WD, Rollins S, et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood 2007;109:2781–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli M, Salmoiraghi S, Golay J, Gozzini A, Crippa C, Pescosta N, et al. A phase Il multiple dose clinical trial of histone deacetylase inhibitor ITF2357 in patients with relapsed or progressive multiple myeloma. Ann Hematol 2010;89:185–90. [DOI] [PubMed] [Google Scholar]
- 33.Kjeldsen K. Hypokalemia and sudden cardiac death. Exp Clin Cardiol 2010;15:e96–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2010; 160:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiuve SE, Korngold EC, Januzzi JL Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr 2011;93:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoro A, Mancini E, London G, Mercadal L, Fessy H, Perrone B, et al. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant 2008;23:1415–21. [DOI] [PubMed] [Google Scholar]
- 37.Osadchii OE. Mechanisms of hypokalemia-induced ventricular arrhythmogenicity. Fundam Clin Pharmacol 2010;24:547–59. [DOI] [PubMed] [Google Scholar]
- 38.Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res 1993;53: 3028–33. [PubMed] [Google Scholar]
- 39.Li RA, Leppo M, Miki T, Seino S, Marban E. Molecular basis of electrocardiographic ST-segment elevation. Circ Res 2000;87: 837–9. [DOI] [PubMed] [Google Scholar]
- 40.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Cardiovasc Res 1997;35:256–72. [DOI] [PubMed] [Google Scholar]
- 41.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res 1991;68:280–7. [DOI] [PubMed] [Google Scholar]
- 42.Di Diego JM, Antzelevitch C. Cellular basis for ST-segment changes observed during ischemia. J Electrocardiol 2003;36 Suppl:1–5. [DOI] [PubMed] [Google Scholar]
- 43.Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev 2010;90:799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fatima N SJ, Flagg TP. Evidence for epigenetic regulation of chamber-specific structure of cardiac ATP-sensitive potassium (KATP) channels. Circulation 2011;124:A13506. [Google Scholar]
- 45.Basseville A, Tamaki A, lerano C, Trostel S, Ward Y, Robey RW, et al. Histone deacetylase inhibitors influence chemotherapy transport by modulating expression and trafficking of a common polymorphic variant of the ABCG2 efflux transporter. Cancer Res 2012;72: 3642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, et al. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res 2008;103:1458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol 2010;48:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, et al. Effects of KAT P channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol 2011;51: 215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meissner K, Jedlitschky G, Meyer zu Schwabedissen H, Dazert P, Eckel L, Vogelgesang S, et al. Modulation of multidrug resistance P-glycoprotein 1 (ABCB1) expression in human heart by hereditary polymorphisms. Pharmacogenetics 2004;14:381–5. [DOI] [PubMed] [Google Scholar]
- 50.Grant C, Rahman F, Piekarz R, Peer C, Frye R, Robey RW, et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther 2010; 10:997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.