Abstract
Nerve growth factor (NGF) is reportedly involved in the changes in C-fiber bladder afferent pathways that induce detrusor overactivity (DO) following spinal cord injury (SCI). This study examined the roles of NGF in TRP channel expression in bladder afferent neurons in mice with SCI using laser-capture microdissection (LCM) methods. Spinal intact (SI) and SCI mice were divided into 3 groups: (1) SI with vehicle treatment; (2) SCI with vehicle treatment; and (3) SCI with anti-NGF antibody. Two weeks after SCI, an osmotic pump was placed subcutaneously into the back of the mice and vehicle or anti-NGF antibody was administered at a rate of 10 μg/kg per hour for two weeks. Four weeks after SCI, the L6 dorsal root ganglia (DRG) were removed. Expression of the TRPV1, TRPC1, TRPC3, and TRPC6 genes was analyzed using real-time polymerase chain reaction (PCR) following LCM of the bladder afferent neurons, which were labeled by Fast Blue injected into the bladder wall 1 week prior to tissue removal. The mRNA expression of TRPV1 was found to be higher in vehicle-treated SCI mice than in SI mice. The expression level of TRPC3 and TRPC6 in vehicle-treated SCI mice was lower than in SI mice. However, in SCI mice treated with anti-NGF antibody, the mRNA expression of TRPV1 was lower, and the mRNA levels of TRPC3 and TRPC6 were higher than in vehicle-SCI mice. These results suggest that the NGF-dependent changes in specific TRP channel genes, such as TRPV1, TRPC3, and TRPC6, could be involved in SCI-induced afferent hyperexcitability and DO.
Keywords: Spinal cord injury, mouse, dorsal root ganglia, laser-capture microdissection, TRP channel, nerve growth factor, TRPC channels
Introduction
NGF is reportedly involved in changes in C-fiber bladder afferent pathways and induces detrusor overactivity (DO) following spinal cord injury (SCI) [1, 8, 13] Research in rats and mice indicates that NGF is overexpressed in the bladder and spinal cord following SCI and is involved in the emergence of neurogenic lower urinary tract dysfunction (LUTD) [8, 9, 14]. It has been proposed that afferent nerves in the bladder take up NGF and transport it to the dorsal root ganglia (DRG), where it alters the expression of ion channels and receptors and induces hyperexcitability of C-fiber bladder afferent pathways, which in turn initiate neurogenic LUTD [1, 12, 15].
The expression of TRP channels such as TRPV1 is known to be involved in the sensitization of C-fiber afferent pathways. Additionally, TRPC channels such as TRPC1, TRPC3, and TRPC6 are expressed in DRG neurons [2], though their role in the control of bladder afferent function is unclear. Therefore, we investigated the effects of anti-NGF antibody treatment on TRP channel expression in laser-captured bladder afferent neurons using SCI mice.
Materials and methods
Ethical approval
All animal experiments were conducted in accordance with the ARRIVE and National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committees (IACUC) (Protocol approval #15086776).
Animal preparation
A total of 25 9- to 10-week-old female C57BL/6N mice (Envigo, Frederick, MD) weighing between 18 and 22 g were used. SCI mice underwent complete transection at the thoracic 8/9 level of the spinal cord after induction of anesthesia by intraperitoneal (i.p.) injection of pentobarbital (50 mg/kg) according to methods described previously [3, 6, 7]. SCI animals were treated with ampicillin (100 mg/kg, subcutaneously) once daily for 5 days post-SCI followed by twice per week injections until final experiments to prevent urinary tract infection. The bladders of SCI animals were emptied by manual bladder compression and perineal stimulation daily for 4 weeks post-SCI. Spinal intact (SI) mice underwent a sham operation without spinal cord transection, and received the antibiotic treatment with ampicillin once daily for 5 days after surgery. SI and SCI mice were then divided into 3 groups: (1) spinal intact group (SI, n = 8); (2) spinal cord injury group (SCI, n = 7); and (3) SCI group treated with anti-NGF antibody (NGF-Ab) (SCI+NGF-Ab, n = 10).
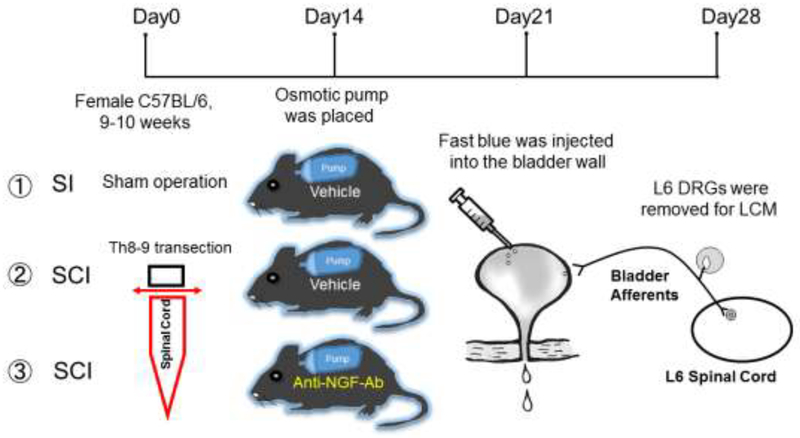
Two weeks after SCI, an osmotic pump (#1002, Alzet Osmotic Pumps, Cupertino, CA) was placed subcutaneously under the skin on the back of the animal to continuously administer vehicle to the SI and the SCI groups and 10 μg/kg per hour of anti-NGF antibody (#L148M, Exalpha Biologicals Inc., Shirley, MA) to the SCI+NGF-Ab group for two weeks. The dosage of the antibody was determined according to previous studies [8, 11, 14] as well as our own preliminary experiments. Four weeks after SCI, the L6 DRG were removed fresh and stored at −80°C until use. Gene expression of TRPV1, TRPC1, TRPC3, and TRPC6 in bladder afferent neurons was analyzed by real-time PCR. The bladder afferent neurons were labeled with Fast Blue (1.8% w/w, Poly Sciences Inc., Warrington, PA), which was injected into the bladder wall using a 31-gauge Hamilton syringe 1 week prior to tissue removal. (Fig. 1).
Figure 1. Schema showing the study design.
SI: spinal intact, SCI: spinal cord injury, LCM: laser-capture microdissection
LCM and Real-Time PCR
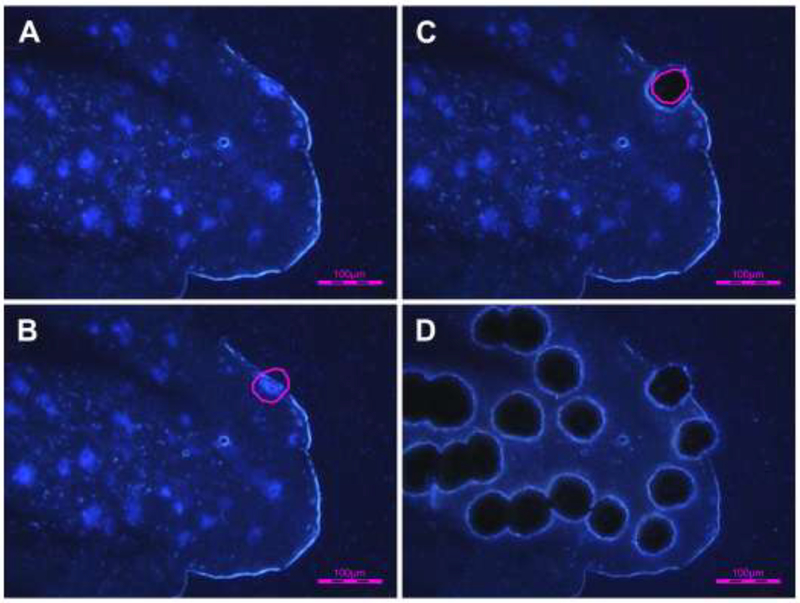
L6 DRGs were embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek USA, Torrance, CA) and stored at −80 °C until use. Samples were sectioned at a thickness of 10 μm and mounted on PEN membrane slides (Leica Microsystems, Wetzlar, Germany). Tissue sections were dipped sequentially in 75%, 95%, and 100% ethanol for 30 s each, followed by xylene for 2 min. The sections were then air-dried. LCM was performed using an LMD6000 (Leica Microsystems) to dissect the FB-labeled bladder afferent neurons (Fig. 2). Excised cells were individually captured in the caps of 0.5 mL Eppendorf tubes and lysed. RNA isolation was performed using an RNeasy® Plus Micro Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Real-time PCR was performed using the MX3000P system (Stratagene, La Jolla, CA). The primers sequences used were as follows: Actb (NM_007393.5; forward primer: GCCCTGAGGCTCTTTTCCAG; reverse primer: TGCCAC AGGATTCCATACCC); TRPV1 (NM_001001445.2; forward primer: TACTTTTCTTTGTACAGTCACT; reverse primer: TCAATCATGACAGCATA GAT); TRPC1 (NM_011643; forward primer: GCGAACAGCAAAGCAATGAC; reverse primer: GATGTACCAGAACAGAGCAAAGCA); TRPC3 (NM_019510; forward primer: GGAGAGCGATCTGAGCGAAGT; reverse primer: GGGAGCCATTTGTCTCTAGCA); and TRPC6 (NM_013838; forward primer: ACTACATTGGCGCAAAACAGAA; reverse primer: AGAAAGACCAAAGATAGCCCAGAA). The cycle conditions comprised a 10-min polymerase activation and 45 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s followed by dissociation from 55 °C to 95 °C. The reactions were performed in triplicate and the relative quantities of mRNA were normalized to the neuronal cell control gene s-actin (Actb). Real-time PCR data were analyzed by the DCp (difference in crossing points) method as R=2^ (Cp sample - Cp control) to generate the relative expression ratio (R) of each target gene relative to that of Actb. We also determined the specificity of the cDNA using real-time PCR to verify that our primer/probe sets did not amplify genomic DNA.
Figure 2. Laser-capture microdissection (LCM) of Fast Blue-labeled bladder afferent neurons.
A-D show the same DRG section before and after LCM. A: before LCM; B: bladder afferent neurons outlined for LCM; C: after LCM, showing a microdissected neuron; D: after LCM showing multiple microdissected neurons.
Statistical analysis
One-way analysis of variance with post hoc Mann–Whitney U-test was used to calculate the statistical significance among groups. A P value < 0.05 was considered to be statistically significant.
Results
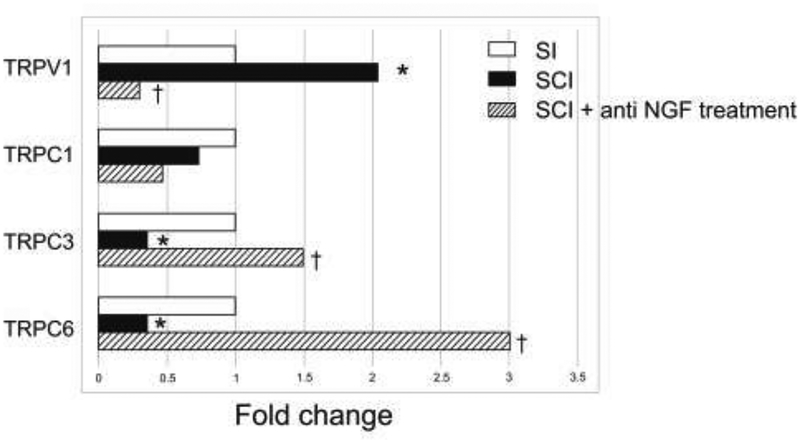
FB-labeled bladder afferent neurons were randomly selected from L6 DRG sections, and 90–100 bladder afferent neurons per sample were laser-captured for the measurement of TRPV1, TRPC1, TRPC3, and TRPC6 mRNA levels. Fifteen samples were obtained from 12 DRGs in the SI group, 20 samples were obtained from 9 DRGs in the SCI group, and 29 samples were obtained from 20 DRGs in the SCI-NGF-Ab group. In each group, one or two samples were obtained from L6 DRG sections of each mouse, and the relative expression to the housekeeping Actb gene was calculated for each sample. The mRNA expression of TRPV1 was higher in vehicle-treated SCI mice than in SI mice. The expression levels of TRPC3 and TRPC6 in vehicle-treated SCI mice were lower than in SI mice. However, in SCI mice treated with anti-NGF antibody, the mRNA expression of TRPV1 was lower and the mRNA levels of TRPC3 and TRPC6 were higher than in vehicle-treated SCI mice (Fig. 3). In addition, there were no significant differences in TRPV1, TRPC1, TRPC3 or TRPC6 expression between SI and SCI-NGF-Ab groups.
Figure 3. The expression of mRNA levels of TRPV1, TRPC1, TRPC3, and TRPC6 receptors in bladder afferent neurons.
The data were expressed as fold changes of relative values of mRNA levels in each of SCI groups vs. the SI group whereas the statistical analysis was performed using the mRNA expression ratio of TRPV1, TRPC1, TRPC3 or TRPC6 against the house keeping gene (Actb) calculated in each sample. In SCI mice, TRPV1 mRNA levels in the L6 DRG (n=20 samples from 7 mice) were higher than in spinal intact (SI) mice (n=15 samples from 8 mice), and TRPC3 and TRPC6 mRNA levels in the L6 DRG were lower than in spinal intact mice. After anti-NGF treatment, TRPV1 levels in SCI mice were lower than in SCI mice without treatment (n=20 samples from 10 mice), and TRPC3 and TRPC6 were higher than in SCI mice without treatment. In addition, there were no significant differences in TRPV1, TRPC1, TRPC3 or TRPC6 expression between SI mice and SCI mice with anti NGF treatment.
*P < 0.05 versus spinal intact (SI) mice, †P < 0.05 versus SCI mice without treatment.
Discussion
The present study showed that, in SCI mice, the expression of TRPV1 in the L6 DRG was increased and the expression levels of TRPC3 and TRPC6 were reduced. Additionally, the results showed that anti-NGF treatment reduced the expression changes in TRPV1 and reversed the expression changes in TRPC3 and TRPC6 in bladder afferent neurons obtained from L6 DRG. These results indicate that NGF plays an important role in the plasticity of TRP channel expression in bladder afferent neurons in SCI mice. We recently reported that anti-NGF Ab treatment, when administered using the same regimen as in this study, improved C-fiber-dependent DO, as indicated by a decrease in non-voiding contractions during bladder filling and the reduced hyperexcitability of capsaicin-sensitive C-fiber bladder afferent neurons in SCI mice [5, 11, 14]. Therefore, NGF-dependent changes in TRP channels, such as the changes in TRPV1, TRPC3, and TRPC6 found in this study, may contribute to the functional alterations of bladder function and bladder afferent activity that underlie SCI-induced DO.
It has been demonstrated that the expression of TRP channels such as TRPV1 is known to be involved in the sensitization of C-fiber afferent pathways [4, 17], and that NGF is an important regulator of TRPV1 expression, spatial distribution, and activation thresholds [4, 17]. Previous studies using SCI rats have demonstrated that over-distention of the bladder increases NGF production in the bladder, which could result in the enhancement of C-fiber afferent nerve excitability, leading to neurogenic DO after SCI [8, 9, 15]. Our recent study using SCI mice also showed that NGF was upregulated in the bladder mucosa and the spinal cord, and that the 2 weeks of treatment with anti-NGF Ab reduced the levels of NGF at both sites along with TRPV1 in whole L6-S1 DRG [14]. However, it has not been clarified whether the NGF-dependent change in TRPV1 expression after SCI is induced in bladder afferent neurons because only a small number (6–7%) of L6-S1 DRG neurons innervates the bladder [16]. Thus, this study using LCM techniques further confirmed that the NGF-dependent increase in TRPV1 channels after SCI actually occurs in a bladder-specific population of L6 DRG neurons. In addition, our recent study that used a novel herpes simplex virus (HSV) vector-mediated neuronal labeling technique indicated that SCI induces an expansion of the TRPV1-expressing C-fiber cell population and that the average cell size of TRPV1-expressing cells decreases after SCI, suggesting that SCI induces de novo expression of TRPV1 in small-sized C-fiber bladder afferent neurons, thereby increasing excitability [10].
In contrast to previous studies of TRPV1 channels, little is known about the expression and regulation of TRPC channels in micturition-related afferent pathways. Thus, the present study demonstrated for the first time that SCI induced NGF-dependent alterations of TRPC channels in mouse bladder afferent neurons. In this study, we chose to examine the expression of TRPC1, TRPC3, and TRPC6 because these channels are most abundantly expressed in the cluster of DRG neurons that bring sensory information from the periphery to the spinal cord in adult mice [2]. TRPC1 was expressed in the neurofilament 200-positive large-sized subclass of DRG neurons, while TRPC3 mRNA expression, which stained up to 35% of DRG neurons, was almost exclusively present in non-peptidergic, isolectin B4 (IB4)-positive small-sized neurons that were largely TRPV1-negative [10]. As discussed above, because the expansion of TRPV1-positive bladder afferent neurons after SCI occurred in small-sized neurons in our recent HSV vector-tracing study [16], it is possible to speculate that the reduction of TRPC3/C6 and the increase in TRPV1 are induced in small-sized, non-peptidergic C-fiber bladder afferent neurons after SCI.
Although the functional role of TRPC channels in the control of micturition is not yet clear, the present study showed that SCI reduces the mRNA levels of TRPC3 and TRPC6 in bladder afferent neurons, which are known to be expressed primarily in small-sized, non-peptidergic DRG neurons, [2]. Therefore, TRPC3/6 might play an inhibitory role in the control of cell excitability of non-peptidergic C-fiber bladder afferent neurons; thus, their reduction could contribute to C-fiber hyperexcitability after SCI, though future studies are needed to further support this point. In addition, because this study showed that anti-NGF Ab treatment normalized the expression of TRPV1, TRPC3, and TRPC6, but not TRPC1 in SCI mice, NGF might be less important for the regulation of TRPC1 expression in SCI. Furthermore, our recent study also demonstrated that NGF plays an important role in the hyperexcitability of capsaicin-sensitive bladder afferent neurons due to the reduction of slow-decaying A-type K+ (KA) channel activity in SCI mice [11]. Taken together, these current and previous results provide evidence that NGF-targeting therapies could be effective for the treatment of SCI-induced bladder afferent hyperexcitability and DO via the normalization of ion channels activity, such as that of KA channels [11], and the expression of TRP channels such as TRPV1, TRPC3, and TRPC6.
Conclusion
Anti-NGF antibody treatment reversed the changes in the expression levels of TRPV1 and TRPC3/TRPC6, which were found to increase and decrease, respectively, in a mouse model of SCI. These results suggest that the NGF-dependent changes in genes such as TRPV1, TRPC3, and TRPC6 could be involved in SCI-induced DO and that TRPC3/TRPC6 might have an inhibitory role in the control of bladder afferent activity while TRPV1 overexpression is involved in C-fiber sensitization, leading to the induction of DO after SCI. These findings add to the understanding of the TRP-mediated mechanism underlying neurogenic lower urinary tract dysfunction.
Highlights.
Spinal cord injury (SCI) induces alterations of TRP channels in mouse bladder afferent neurons
TRPV1 channel expression in laser-captured bladder afferent neurons is increased after SCI
SCI-induced increase in TRPV1 channels is reversed by anti-nerve growth factor (NGF) treatment
TRPC3 and TRPC6 expressions in laser-captured bladder afferent neurons are decreased after SCI
SCI-induced decreases in TRPC3 and TRPC6 channels are reversed by anti-NGF treatment
Acknowledgments
This work was supported by grants from NIH (NIH P01 DK093424), DOD (W81XWH-17-1-0403), and KAKENHI for Early-Career Scientists (18K16751).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
There are no conflicts of interest to declare.
References
- [1].de Groat WC, Yoshimura N, Plasticity in reflex pathways to the lower urinary tract following spinal cord injury, Experimental neurology 235 (2012) 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Elg S, Marmigere F, Mattsson JP, Ernfors P, Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion, The Journal of comparative neurology 503 (2007) 35–46. [DOI] [PubMed] [Google Scholar]
- [3].Ikeda Y, Zabbarova IV, Birder LA, de Groat WC, McCarthy CJ, Hanna-Mitchell AT, Kanai AJ, Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder, European urology 62 (2012) 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ, p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia, Neuron 36 (2002) 57–68. [DOI] [PubMed] [Google Scholar]
- [5].Kadekawa K, Majima T, Shimizu T, Wada N, de Groat WC, Kanai AJ, Goto M, Yoshiyama M, Sugaya K, Yoshimura N, The role of capsaicin-sensitive C-fiber afferent pathways in the control of micturition in spinal-intact and spinal cord-injured mice, American journal of physiology. Renal physiology 313 (2017) F796–f804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kadekawa K, Yoshimura N, Majima T, Wada N, Shimizu T, Birder LA, Kanai AJ, de Groat WC, Sugaya K, Yoshiyama M, Characterization of bladder and external urethral activity in mice with or without spinal cord injury--a comparison study with rats, American journal of physiology. Regulatory, integrative and comparative physiology 310 (2016) R752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ, Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity, The Journal of urology 181 (2009) 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N, Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats, The Journal of urology 168 (2002) 2269–2274. [DOI] [PubMed] [Google Scholar]
- [9].Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, Yoshimura N, Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats, The Journal of urology 171 (2004) 478–482. [DOI] [PubMed] [Google Scholar]
- [10].Shimizu N, Doyal MF, Goins WF, Kadekawa K, Wada N, Kanai AJ, de Groat WC, Hirayama A, Uemura H, Glorioso JC, Yoshimura N, Morphological changes in different populations of bladder afferent neurons detected by herpes simplex virus (HSV) vectors with cell-type-specific promoters in mice with spinal cord injury, Neuroscience 364 (2017) 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shimizu T, Majima T, Suzuki T, Shimizu N, Wada N, Kadekawa K, Takai S, Takaoka E, Kwon J, Kanai AJ, de Groat WC, Tyagi P, Saito M, Yoshimura N, Nerve growth factor-dependent hyperexcitability of capsaicin-sensitive bladder afferent neurones in mice with spinal cord injury, Exp Physiol 103 (2018) 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC, Yoshimura N, Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 alpha-subunit in rats with spinal cord injury, The Journal of urology 190 (2013) 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vizzard MA, Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury, Prog Brain Res 152 (2006) 97–115. [DOI] [PubMed] [Google Scholar]
- [14].Wada N, Shimizu T, Shimizu N, de Groat WC, Kanai AJ, Tyagi P, Kakizaki H, Yoshimura N, The effect of neutralization of nerve growth factor (NGF) on bladder and urethral dysfunction in mice with spinal cord injury, Neurourol Urodyn (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S, Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats, The Journal of neuroscience: the official journal of the Society for Neuroscience 26 (2006) 10847–10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yoshimura N, Erdman SL, Snider MW, de Groat WC, Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder, Neuroscience 83 (1998) 633–643. [DOI] [PubMed] [Google Scholar]
- [17].Zhang X, Huang J, McNaughton PA, NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels, The EMBO journal 24 (2005) 4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]