Abstract
Background:
The literature on drugs used for combined general anesthesia and epidural analgesia (CGE) in lumbar operations is scarce. The purpose of the study was to compare the addition of either dexmedetomidine or fentanyl to bupivacaine for epidural analgesia in combination with general anesthesia with regard to efficacy and adverse events in such operations.
Materials and Methods:
This prospective, randomized, double-blinded study was conducted on 80 patients who were scheduled for an elective lumbar disc operation, age 20–65 years, of either sex and American Society of Anesthesiologists physical status I or II. They were randomly allocated into one of the two groups – group bupivacaine-dexmedetomidine (BD) (n = 40): patients who received CGE with 15 mL of bupivacaine 0.20% plus 50 μg of dexmedetomidine and group bupivacaine-fentanyl (BF) (n = 40): patients who received CGE with 15 mL of bupivacaine 0.20% plus 50 μg fentanyl. The primary outcome was time to first analgesic requirement, whereas the secondary outcomes were the total opioid consumption and pain scores during the first 24 h. The incidence of adverse postoperative (PO) effects related to the study drugs, such as sedation, nausea and vomiting, pruritus, shivering, and respiratory depression, was also documented.
Results:
Patients in the BD group experienced a significantly prolonged pain-free period, lower total opioid consumption, and lower pain scores than patients in the BF group (P < 0.001). Patients in the BD group showed a significantly lower intraoperative heart rate and mean blood pressure (P < 0.001). Regarding adverse events, there were greater PO sedation scores (P < 0.001) and less frequent episodes of PO nausea and vomiting in the BD group. In addition, patients in the BD group showed less pruritis and shivering. There were no reported cases of respiratory depression in either group.
Conclusion:
CGE with bupivacaine plus dexmedetomidine provided better PO pain control than bupivacaine plus fentanyl, with fewer adverse events overall.
Keywords: Bupivacaine, dexmedetomidine, epidural analgesia, fentanyl, lumbar surgery
Introduction
There is considerable acceptance for the use of epidural analgesia, which has minimal side effects, in spine surgery, taking into consideration the proper selection of patients and surgeons;[1] epidural analgesia provides an acceptable hemodynamic profile, especially when combined with general anesthesia.[2] However, most previous relevant studies have not addressed the type of drugs used for epidural analgesia in such operations.
The beneficial effects of adding opioids, such as fentanyl, to local anesthetics on postoperative (PO) pain control have been demonstrated in the literature.[3,4,5] Unfortunately, side effects such as respiratory depression, pruritus, nausea and vomiting, and urinary retention may occur.[6]
The highly selective α2-adrenoreceptor agonist dexmedetomidine is known to decrease sympathetic central nervous system outflow and to exert sedative, anxiolytic, and analgesic effects. Additionally, dexmedetomidine lacks most of the side effects of opioids.[7]
The purpose of this study was to compare the addition of dexmedetomidine or fentanyl to bupivacaine for epidural analgesia in combination with general anesthesia in elective lumbar disc operations with regard to PO pain control, hemodynamic stability, and adverse effects.
Materials and Methods
After approval of the research by the ethical committee of the Faculty of Medicine, Ain-Shams University, and its registration in the ClinicalTrials.gov (registration number: NCT03438240) database, this prospective, randomized double-blinded study was conducted on 80 patients who were scheduled for first-time elective lumbar discectomy or laminectomy, age 20–65 years old, of either sex and physical status American Society of Anesthesiologists (ASA) I or II. Informed consent with full explanation of the procedure was obtained from patients before starting.
Patients who refused to participate, had a body mass index (BMI) >30 kg/m2, needed an emergency lumbar disc operation, were ASA physical status >II, or had major illnesses (e.g., cardiac, respiratory, renal, hepatic), coagulation abnormalities, hypovolemia, a history of increased intracranial pressure, convulsions, spinal stenosis, infection at the needle insertion site, other contraindications to epidural procedure, an allergy or contraindications to the drugs used in the study, a history of addiction or alcohol abuse, a psychiatric illness, or mental retardation were excluded from the study.
Patients were randomly allocated by computer-generated lists via the closed-envelope method into one of the following groups – group 1 [bupivacaine-dexmedetomidine (BD) (n = 40): patients received combined general anesthesia and epidural analgesia (CGE) with 15 mL of bupivacaine 0.20% plus 50 μg of dexmedetomidine (Precedex 200 μg/2 mL vial; Hospira, Inc., Lake Forest, IL, USA) brought to a total volume of 16 mL in saline. Group 2 [bupivacaine-fentanyl (BF)] (n = 40): patients received combined CGE with 15 mL of bupivacaine 0.20% plus 50 μg of fentanyl in a total volume of 16 mL.
All patients underwent thorough preoperative evaluation on the day before surgery and were instructed about the usage of patient-controlled analgesia (PCA). On arrival to the operating room, an 18-G intravenous (IV) cannula was secured, and standard electrocardiograph, non-invasive blood pressure, and pulse oximetry (SpO2) monitoring were established. Baseline heart rate (HR), mean blood pressure (MBP), and SpO2 readings were also obtained.
Ringer's lactate 10 mL/kg was administered to all patients; then, the epidural block was performed in the sitting position with an 18-G Tuohy needle through a midline approach at the second intervertebral level above the herniated disc (e.g., at the L3–L4 intervertebral space for an L5–S1 hernia) under complete aseptic conditions. After identification of the epidural space with loss of resistance using the saline technique, a 20-G catheter was threaded 3–4 cm beyond the epidural needle with its tip directed up.
The patients received a 3-mL test dose of 2% lidocaine with 1:200,000 adrenaline to exclude the subarachnoid placement of the catheter after the trial for the aspiration of blood or cerebrospinal fluid (CSF). If blood was aspirated, the catheter was removed, and another trial was performed in a different space. If CSF was aspirated, the patient was excluded from the study. In the following 2 or 3 min, patients were asked for signs of either intravascular injection (an abnormal metallic taste, tinnitus, dizziness, or rapid HR) or subarachnoid injection (investigated by the ability of the patients to move their legs and the absence of hypotension). If there were no signs, the catheter was fixed, and the patients were placed in the supine position.
Then, general anesthesia was induced. After preoxygenation, IV fentanyl 1 μg/kg was administered slowly, followed by propofol 1.5 mg/kg, which was slowly injected and titrated until the loss of verbal contact with the patient after IV atracurium 0.5 mg/kg was given to facilitate intubation. Once the endotracheal tube had been secured in place, end-tidal CO2 monitoring was established using capnography, and ventilation was adjusted to maintain normocapnia.
Anesthesia was maintained with isoflurane, with end-tidal isoflurane at 1.5% the minimum alveolar concentration. Atracurium was supplemented at 0.1 mg/kg according to nerve stimulator monitoring.
After anesthesia was induced and the airway was secured, the patient was carefully and gradually rolled on to the prone position frame and supported so as to not impede the arterial or venous circulation or increase the pressure of the abdomen. The HR, MBP, and SpO2 were monitored closely just before and after positioning to avoid significant adverse events.
Ten minutes after this positioning, epidural block was administered to patients in group BD, who received epidural anesthesia with 15 mL of bupivacaine 0.20% plus 50 μg of dexmedetomidine in a total volume of 16 mL, whereas patients in group BF received epidural anesthesia with 15 mL of bupivacaine 0.20% plus 50 μg of fentanyl in a total volume of 16 mL. The drugs were prepared by an anesthesiologist who was not included in the study.
During the operation, the depth of anesthesia was monitored by the bispectral index, which was maintained within 45 ± 5 by regulating the isoflurane concentration in both the groups.
The hemodynamic parameters (HR, MBP) were monitored continuously, and recordings were collected every 3 min after the injection of epidural drugs. After 10 min, hypotension (defined as MBP falling more than 20% mmHg from baseline) was treated with 250 mL of Ringer's lactate and/or 3–6 mg of IV ephedrine in bolus doses, and a HR <50 beats/min was treated with 0.5 mg of IV atropine. The patients were monitored intraoperatively by an anesthesiologist who was not aware of the drugs used in the study.
After skin closure, a top-up dose was given in the form of 10 mL of the previously injected solution according to the group, and the epidural catheter was removed carefully and slowly. If epidural tearing occurred during the procedure, the patient was excluded from the study.
Oral suction was performed, and reversal agents (atropine 0.02 mg/kg and neostigmine 0.04 mg/kg) were administered after adequate recovery of the neuromuscular blockade. Patients were extubated when they were able to open their eyes on verbal command, and the T4/T1 ratio was 90%. After extubation, an IV PCA system was connected to the patient (Accufuser Plus® 100 mL; Woo Young Medical Co, Korea). PCA was prepared with 60 mL of normal saline containing 60 mg of morphine, and the system was programmed to give a 0.5 mL bolus dose with a lockout interval of 8 min. There was no basal rate. PCA was discontinued at 24 h after surgery, and at that time, oral analgesics began.
The patients were then transferred to the postanesthesia care unit (PACU), where an anesthetist and a nurse unaware of the study protocol observed the patients. The hemodynamic parameters were recorded in the PACU at 1-, 5-, 10-, 20-, and 30-min intervals. The patients were transferred to the ward on meeting standard discharge criteria.
The time to the first analgesic requirement and the total opioid consumption over the first 24 h postoperatively were recorded. Pain scores were evaluated by a blinded observer anesthesiologist at the time of arrival in the PACU and 10, 20, and 30 min and 1, 2, 4, 6, 8, 10, 12, 16, and 24 h thereafter using a visual analog scale (VAS) (0–10 cm: 0 = no pain, 10 = the worst pain possible). The patients were instructed about the usage of the PCA system and the VAS preoperatively.
Examination of the motor function was done in the immediate PO period using Bromage score indicating the below:
Bromage I: The patient is unable to move legs or feet.
Bromage II: The patient is unable to flex knees, but with free movement of feet.
Bromage III: The patient is just able to flex knees with free movement of feet.
Bromage IV: The patient is able to move legs and feet freely.
Sedation was assessed using the Ramsay sedation scale (RSS) just before surgery, immediately after extubation (considered time zero) and at 2, 12, and 24 h postoperatively [Table 1].[8]
Table 1.
Ramsay sedation scale
| Score | Definition |
|---|---|
| 1 | Anxious and agitated or restless or both |
| 2 | Cooperative, oriented, and tranquil |
| 3 | Responds to commands only |
| 4 | Brisk response to a light glabellar tap or loud auditory stimulus |
| 5 | Sluggish response to a light glabellar tap or loud auditory stimulus |
| 6 | No response to a light glabellar tap or loud auditory stimulus |
Performed using a series of steps: observation of behavior (score 1 or 2), followed (if necessary) by assessment of response to voice (score 3), followed (if necessary) by assessment of response to loud auditory stimulus or light glabellar tap (scores 4–6)
The severity of postoperative nausea and vomiting (PONV) during the first 24 h was recorded and classified as no PONV, mild PONV, moderate PONV, and severe PONV[9] if it occurred; PONV was treated with 0.1 mg/kg of IV ondansetron. Other adverse events, such as pruritus, shivering, and respiratory depression (respiratory rate <10/min), were recorded.
The primary outcome was the time to the first analgesic requirement, whereas the secondary outcomes were the total opioid consumption in the first 24 h postoperatively, the VAS score, and the incidence of PO adverse effects related to the study drugs.
Sample size calculation
The PASS program was used to calculate the sample size, with an alpha error of 5% and a power of 80%. The results from a previous study (Soliman and Eltaweel, 2016) showed that in the dexmedetomidine group, the need for opioids was detected among 9.5% of patients compared with 31.7% of patients in the fentanyl group. As such, the required sample size was 40 patients per group, for a total of 80 patients.
Statistical methods
The collected data were coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences software version 22.0, 2013; IBM Corp., Chicago, IL, USA). Descriptive statistics were determined for quantitative data as mean ± standard deviation for quantitative normally distributed data, median and interquartile range for quantitative non-normally distributed data, and number and percentage for qualitative data.
Inferential analyses were performed for quantitative variables using Shapiro–Wilk test for normality testing, independent t-test in cases of two independent groups with normally distributed data, and Mann–Whitney U-test in cases of two independent groups with non-normally distributed data. For qualitative data, inferential analyses for independent variables were performed using Chi-square test for differences between proportions and Fisher's exact test for variables with small expected numbers. P value <0.050 was considered significant.
Results
Among 98 patients who were screened for eligibility, 11 patients were excluded because they did not meet the protocol inclusion criteria, 5 patients refused to participate in the study, and 2 others were excluded due to other causes (postponement of the surgery). After they provided their consent, a total of 80 patients were randomly allocated to the study groups (40 patients in each group) and completed the study [Figure 1]. There were no significant differences between the studied groups regarding the demographic or basal characteristics [Table 2].
Figure 1.
CONSORT patient flow chart
Table 2.
Demographic and basal characteristics of the studied groups
| Variables | BD (n=40) | BF (n=40) | P |
|---|---|---|---|
| Age (years) | 43.2±7.4 | 41.5±7.4 | 0.298* |
| Sex | |||
| Male | 27 (67.5%) | 23 (57.5%) | 0.356** |
| Female | 13 (32.5%) | 17 (42.5%) | |
| BMI | 28.1±1.5 | 28.5±2.0 | 0.259* |
| ASA | |||
| I | 35 (87.5%) | 31 (77.5%) | 0.239** |
| II | 5 (12.5%) | 9 (22.5%) | |
| Duration of operation (min) | 102.6±5.9 | 103.9±5.7 | 0.341* |
BD: Bupivacaine-dexmedetomidine; BF: Bupivacaine-fentanyl; BMI: Body mass index; ASA: American Society of Anesthesiologists Physical Status Data are presented as mean±standard deviation or number, n (%), as appropriate P<0.05 was considered statistically significant *Independent t-test **Chi-square test
The time to the first analgesic requirement was significantly prolonged in the BD group compared with the BF group. In addition, the total opioid consumption was significantly lower in the BD group than in the BF group over the first 24 h postoperatively [Table 3].
Table 3.
Time to first analgesic requirement and total opioid consumption in the studied groups
| Variables | BD (n=40) | BF (n=40) | P* |
|---|---|---|---|
| Time to first analgesic requirement (min) | 392.7±34.8 | 296.9±24.5 | <0.001** |
| Total opioid consumption (mg) | 18.9±3.4 | 23.3±3.2 | <0.001** |
BD: Bupivacaine-dexmedetomidine; BF: Bupivacaine-fentanyl Data are presented as mean±standard deviation *Independent t-test **P<0.05 was considered statistically significant
Regarding the VAS scores, there were no significant differences between the studied groups from hours 0 to 2, after which the VAS score became significantly lower in the BD group than in the BF group [Figure 2].
Figure 2.
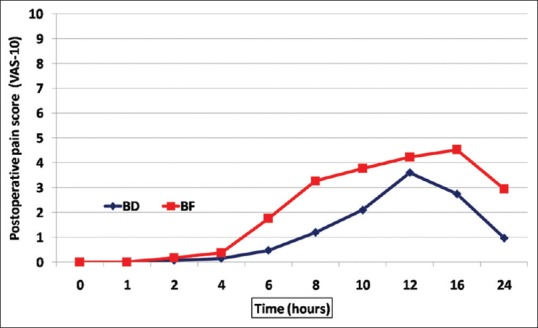
Postoperative visual analog scale (VAS) scores in both groups
Regarding the hemodynamic parameters, there were no significant differences between the studied groups regarding the basal or immediately postintubation HR or MBP, whereas the intraoperative and PO HR and MBP were significantly lower in the BD group than in the BF group [Figures 3 and 4].
Figure 3.
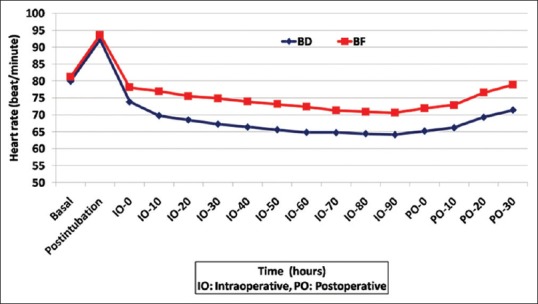
Intraoperative and postoperative HR in both groups
Figure 4.
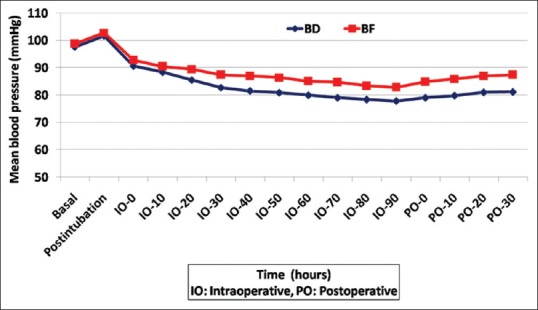
Intraoperative and postoperative MBP in both groups
Regarding motor examination, there was no affection of motor function in either group. PO RSS scores were significantly lower in the BD group than in the BF group [Table 4].
Table 4.
Postoperative RSS in the studied groups
| Time (h) | BD (n=40) | BF (n=40) | P* |
|---|---|---|---|
| PO 0 | 3.0 (3.0-3.0) | 2.0 (1.0-2.0) | <0.001** |
| PO 2 | 3.0 (2.0-3.0) | 2.0 (1.0-2.0) | <0.001** |
| PO 12 | 2.0 (2.0-2.0) | 2.0 (1.0-2.0) | <0.001** |
| PO 24 | 2.0 (2.0-2.0) | 2.0 (1.0-2.0) | <0.001** |
PO: Postoperative; BD: Bupivacaine-dexmedetomidine; BF: bupivacaine-fentanyl Data are presented as median (IQR) *Mann–Whitney test **P<0.05 was considered statistically significant
Although nonsignificant, PONV was less frequent in the BD group than in the BF group; two patients (5%) experienced mild PONV in the BD group, while seven patients (17.5%) experienced mild PONV in the BF group (P = 0.154). No cases of moderate or severe PONV were detected in either group.
Regarding other adverse events, the incidence of intraoperative bradycardia and hypotension was significantly higher in the BD group than in the BF group (P = 0.003, 0.012, respectively). On the other hand, the incidence of PO pruritus and shivering was significantly lower in the BD group than in the BF group (P = 0.026 and 0.002, respectively) [Figure 5]. No cases of respiratory depression were reported in either group.
Figure 5.
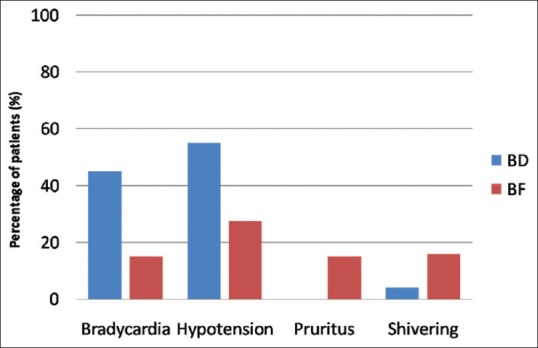
Adverse events in the studied groups
Discussion
This study shows that CGE with bupivacaine plus dexmedetomidine provided better PO pain control than bupivacaine plus fentanyl, with fewer side effects overall. Multiple randomized trials have compared general anesthesia with regional anesthesia for lumbar laminectomy and discectomy operations. These studies demonstrated no identifiable differences in outcomes regarding morbidity and mortality, but they showed some advantages for using regional anesthesia.[10,11,12]
However, general anesthesia is still considered the most common method for providing anesthesia in such operations, as it provides better airway management in the prone position with the possibility of extending the duration of anesthesia according to the length of the operation.[10]
On the other hand, general anesthesia has some drawbacks; it may subject patients to frequent intraoperative episodes of hypertension, thus increasing blood loss, which may in turn lead to a prolonged surgical time, an increased need for blood transfusions, and delayed wound healing.[13,14]
Khajavi et al.[2] showed in their study that CGE analgesia could be a better alternative to general anesthesia alone in lumbar disc operations because the combination carries the advantages of both methods.
The administration of epidural opioids under general anesthesia was examined by Bourke et al. for laminectomy, and they found that it provided better PO pain control with fewer doses required for analgesia.[3]
However, opioids are usually associated with an increased incidence of PONV, shivering, and pruritis. Recently, it was found that opioids could result in PO hyperalgesia with a paradoxical increase in the intensity of pain and subsequent opioid consumption.[15]
Using other adjuvants as α2-adrenoreceptor agonists could decrease the occurrence of such complications.[16] In 1989, Bonnet et al. used epidural clonidine for the first time.[17] Dexmedetomidine is a much more selective α2-adrenoreceptor agonist than clonidine,[18] and it has been demonstrated to achieve favorable results when used epidurally.[19]
To the best of our knowledge, no previous studies have compared the epidural administration of either dexmedetomidine or fentanyl with local anesthetic under general anesthesia for lumbar disc operations.
This study shows that patients in the dexmedetomidine group experienced a significantly longer pain-free period and lower opioid consumption than patients in the fentanyl group. Additionally, VAS scores were significantly lower in the dexmedetomidine group than in the fentanyl group (P < 0.001).
These findings agree with the results of a study performed by Soliman and Eltaweel, who found that the addition of dexmedetomidine to epidural bupivacaine improved PO analgesia compared with the addition of fentanyl to bupivacaine in patients undergoing total knee replacement.[20] Additionally, Paul et al. demonstrated similar results in their study.[21]
The analgesic properties of dexmedetomidine at the spinal cord level can be explained by stimulation of the α2-AR receptors in the substantia gelatinosa of the dorsal horn, which results in inhibition of the release of substance P from the nociceptive neurons.[22]
Although we used relatively small doses of dexmedetomidine and fentanyl, patients in both the groups experienced a low HR and MBP after the initiation of epidural analgesia; however, there were significant differences between patients who received dexmedetomidine and those who received fentanyl.
This may be due to the effect of combining general anesthesia and epidural analgesia, and this effect may be exacerbated by placing patients in the prone position. However, the effect was managed effectively with atropine, fluids, or ephedrine, as indicated.
The results of previous studies are conflicting. The studies by Soliman and Eltaweel[20] and Paul et al.[21] showed similar findings, whereas another study conducted by Bajwa et al.[23] reported no significant difference in the HR or MBP between patients who received dexmedetomidine as an adjuvant to ropivacaine and patients in the control group. In a study performed by Eskkender et al.,[24] there was a significant reduction in the HR but an insignificant reduction in the MBP in patients in the dexmedetomidine group.
There was no affection of the motor function in the immediate PO period; this may be attributed to the bupivacaine concentration used in this study (0.2%). A previously published study had reported minimal affection of motor function using this bupivacaine concentration.[25]
Patients in the dexmedetomidine group had higher sedation scores than patients in the fentanyl group. This effect is due to inhibition of the release of norepinephrine in the locus coeruleus as a result of the stimulation of the presynaptic α2-adrenoreceptors.[26] Similar findings were reported by Soliman and Eltaweel[20] and Paul et al.[21]
Nausea and vomiting is a major PO concern. Although the difference was insignificant in this study, PONV was less frequent in the dexmedetomidine group than in the fentanyl group. Additionally, the incidence of pruritus and shivering was significantly lower in the dexmedetomidine group. There were no reported cases of PO respiratory depression in either group.
Soliman and Eltaweel's study[20] showed a lower incidence of PONV, pruritus, urinary retention, and respiratory depression in the dexmedetomidine group than in the fentanyl group. In a study performed by Bajwa et al.,[23] while epidural fentanyl was associated with a higher incidence of PONV than was epidural dexmedetomidine, there was no difference in the incidence of pruritus or respiratory depression between the two groups.
One limitation of our study is the duration of the study, which was limited to the first 24 h postoperatively. Additionally, we did not measure the effect of the modality on the hospital stay duration.
Conclusion
CGE with bupivacaine plus dexmedetomidine provided better PO pain control than bupivacaine plus fentanyl, with fewer side effects overall.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Demirel CB, Kalayci M, Ozkocak I, Altunkaya H, Ozer Y, Acikgoz B. A prospective randomized study comparing perioperative outcome variables after epidural or general anesthesia for lumbar disc surgery. J Neurosurg Anesthesiol. 2003;15:185–92. doi: 10.1097/00008506-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Khajavi MR, Asadian MA, Imani F, Etezadi F, Moharari RS, Amirjamshidi A. General anesthesia versus combined epidural/general anesthesia for elective lumbar spine disc surgery: A randomized clinical trial comparing the impact of the two methods upon the outcome variables. Surg Neurol Int. 2013;4:105. doi: 10.4103/2152-7806.116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourke DL, Spatz E, Motara R, Ordia JI, Reed J, Hlavacek JM. Epidural opioids during laminectomy surgery for postoperative pain. J Clin Anesth. 1992;4:277–81. doi: 10.1016/0952-8180(92)90128-n. [DOI] [PubMed] [Google Scholar]
- 4.Cherng CH, Yang CP, Wong CS. Epidural Fentanyl speeds the onset of sensory and motor blocks during epidural Ropivacaine anesthesia. Anesth Analg. 2005;101:1834–7. doi: 10.1213/01.ANE.0000184131.06529.35. [DOI] [PubMed] [Google Scholar]
- 5.Benzon HT, Wong HY, Belavic AM, Goodman I, Mitchell D, Lefheit T, et al. A randomized double-blind comparison of epidural fentanyl infusion versus patient controlled analgesia with morphine for postthoracotomy pain. Anesth Analg. 1993;76:316–22. [PubMed] [Google Scholar]
- 6.Chaney MA. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891–903. doi: 10.1007/BF03011037. [DOI] [PubMed] [Google Scholar]
- 7.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128–33. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sessler CN, Jo Grap M, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Critical Care. 2008;12(Suppl. 3):S2. doi: 10.1186/cc6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberhart LH, Seeling W, Ulrich B, Morin AM, Georgieff M. Dimenhydrinate and metoclopramide alone or in combination for prophylaxis of PONV. Can J Anaesth. 2000;47:780–5. doi: 10.1007/BF03019481. [DOI] [PubMed] [Google Scholar]
- 10.Sadrolsadat SH, Mahdavi AR, Moharari RS, Khajavi MR, Khashayar P, Najafi A, et al. A prospective randomized trial comparing the technique of spinal and general anesthesia for lumbar disk surgery: A study of 100 cases. Surg Neurol. 2009;71:60–5. doi: 10.1016/j.surneu.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.McLain RF, Kalfas I, Bell GR, Tetzlaff JE, Yoon HJ, Rana M. Comparison of spinal and general anesthesia in lumbar laminectomy surgery: A case-controlled analysis of 400 patients. J Neurosurg Spine. 2005;2:17. doi: 10.3171/spi.2005.2.1.0017. [DOI] [PubMed] [Google Scholar]
- 12.Attari MA, Mirhosseini SA, Honarmand A, Safavi MR. Spinal anesthesia versus general anesthesia for elective lumbar spine surgery: A randomized clinical trial. J Res Med Sci. 2011;16:524. [PMC free article] [PubMed] [Google Scholar]
- 13.Gulur P, Nishimori M, Ballantyne JC. Regional anaesthesia versus general anaesthesia, morbidity and mortality. Best Pract Res Clin Anaesthesiol. 2006;20:249–63. doi: 10.1016/j.bpa.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: A survey of 850 spinal procedures. J Spinal Disord. 1998;11:124–8. [PubMed] [Google Scholar]
- 15.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004. doi: 10.1093/bja/aeu137. [DOI] [PubMed] [Google Scholar]
- 16.Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2013;64:301–7. doi: 10.4097/kjae.2013.64.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet F, Boico O, Rostaing S. Postoperative analgesia with extradural clonidine. Br J Anaesth. 1989;63:465–9. doi: 10.1093/bja/63.4.465. [DOI] [PubMed] [Google Scholar]
- 18.Kamibayashi T, Maze M. Clinical uses of alpha-2 adrenergic agonists. Anaesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Salgado PF, Sabbage AT, Silva P. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anaesthesia. Rev Assoc Med Bras. 2008;54:110–5. doi: 10.1590/s0104-42302008000200011. [DOI] [PubMed] [Google Scholar]
- 20.Soliman R, Eltaweel M. Comparative study of dexmedetomidine and fentanyl as an adjuvant to epidural bupivacaine for postoperative pain relief in adult patients undergoing total knee replacement: A randomized study. J Anesthesiol Clin Sci. 2016;5:1. [Google Scholar]
- 21.Paul A, Nathroy A, Paul T. A comparative study of dexmedetomidine and fentanyl as an adjuvant to epidural bupivacaine in lower limb surgeries. J Med Sci. 2017;37:221–6. [Google Scholar]
- 22.Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–82. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- 23.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskandar AM, Ebeid AM. Effects of epidural dexmedetomidine and low-volume bupivacaine on postoperative analgesia after total knee replacement. Ain-Shams J Anaesthesiol. 2014;7:193–7. [Google Scholar]
- 25.Mehta S, Gajbhare MN, Kamble NP. Comparison of epidural analgesia using 0.2% bupivacaine and 0.2% ropivacaine for the management of postoperative pain in major orthopedic surgery. Anesth Essays Res. 2018;12:586–91. doi: 10.4103/aer.AER_62_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maze M, Regan JW. Role of signal transduction in anaesthetic action: Alpha 2 adrenergic agonists. Ann NY Acad Sci. 1991;625:409–22. doi: 10.1111/j.1749-6632.1991.tb33868.x. [DOI] [PubMed] [Google Scholar]


