Abstract
Objective
Most suicidality literature in Huntington disease (HD) is based on natural history studies or retrospective reviews, but reports on risk factors from clinical trials are limited.
Methods
We analyzed 609 participants from 2CARE, a randomized, double-blind, placebo-controlled clinical trial with up to 5 years of follow-up, for risk factors related to suicidality. The primary outcome variable was the time from randomization until the first occurrence of either suicidal ideation or attempt. We also considered time from randomization until the first suicide attempt as a secondary outcome variable.
Results
Depression, anxiety, bipolar disorder, antidepressant or anxiolytic use, and prior suicide attempt at baseline were associated with time to ideation or attempt. Baseline employment status, marital status, CAG repeat length, tetrabenazine use, and treatment assignment (coenzyme Q10 or placebo) were not associated with suicidality. Time-dependent variables from the Unified Huntington's Disease Rating Scale Behavioral Assessment were associated with time to suicidal ideation or attempt, driven mainly by items related to depressed mood, low self-esteem/guilt, anxiety, suicidal thoughts, irritability, and compulsions. Variables associated with time to suicide attempt alone were generally similar.
Conclusion
These data suggest psychiatric comorbidities in HD are predictive of suicidal behavior while participating in clinical trials, reinforcing the importance of clinical surveillance and treatment towards lessening risk during participation and perhaps beyond. Designing a composite algorithm for early prediction of suicide attempts in HD may be of value, particularly given anticipated trials aimed at disease modification are likely to be long-term.
Clinicaltrials.gov identifier
Behavioral disturbances in Huntington disease (HD) frequently have a substantial effect on patient and family quality of life.1 Suicidal behavior, in particular, is a potentially devastating feature of HD. It is common; estimates of lifetime prevalence range as high as 20% for premanifest/manifest mutation carriers, with rates up to 12-fold greater than those in the general population, prompting investigations of risk factors towards better identification and treatment.2,3 Many of these inquiries are retrospective reviews of clinical cases or are taken from observational natural history cohorts, but analyses of suicidality risk factors in the context of clinical trials are limited. Given the need to maximize participant safety in a condition with substantial psychiatric features and the anticipation of studies with long-term follow-up for interventions aimed at disease modification, understanding suicidality in HD clinical trials is critical.
2CARE was a randomized, double-blind, placebo-controlled clinical trial of coenzyme Q10 in early-stage HD.4 It enrolled 609 participants followed for up to 5 years, thereby providing a valuable longitudinal dataset to follow clinical evolution in HD. In the present analysis, we report variables associated with suicidal ideation or behavior during the 2CARE study. We aimed to determine if suicidal ideation or behavior, or risk factors associated with suicidal ideation or behavior, in a randomized, double-blind, placebo-controlled trial differed from those reported in the literature.
Methods
The 2CARE trial enrolled 609 participants at 48 sites between 2008 and 2012. Participants were randomly assigned to receive coenzyme Q10 2,400 mg/d or matching placebo. They had evaluations at baseline, months 1, 3, and 6, and every 6 months thereafter. The maximum follow-up time was 60 months (5 years), but the trial was halted for futility in July 2014. As a result, at study termination, 91% of the participants had completed 1 year of follow-up, 83% had completed 2 years, 69% had completed 3 years, 52% had completed 4 years, and 34% had completed 5 years. Details regarding study design and results have been published.4
Suicidal ideations or attempts were identified via reporting of adverse events from site personnel to the medical monitor, with whom plans to stabilize the participant were discussed on a case-by-case basis. Active inquiries into participant status were also triggered by answers on the Unified Huntington's Disease Rating Scale Behavioral Assessment (UHDRS Behavioral), specifically questions 28a and 28b (“Within the past month, how often have you thought about hurting yourself or ending it all?” and “Do you have a plan in mind to end it all? Have you taken any steps towards carrying out your plan?”).5 Scores of 2 or higher (where 2 reflects minimum severity of “sometimes thinking about suicide—at least once a month” or “seriously consider suicide but has no plan”) were automatically sent to the medical monitor electronically, prompting the medical monitor to contact the site for discussion about status.
All available data for the entire cohort (n = 609) were used for analysis. The primary outcome variable in this secondary analysis of the 2CARE database was the time from randomization until the first occurrence of either suicidal ideation or a suicide attempt. We also considered time from randomization until the first suicide attempt as a secondary outcome variable. Kaplan-Meier curves were used to describe the distribution of time to event. Cox proportional hazards models were used to examine the associations between demographic and clinical characteristics and time to event. Potential risk factors that were measured longitudinally, in particular variables derived from the UHDRS and body mass index, were treated as time-dependent covariates in these models. Times were censored at the date of the last follow-up visit for those who did not experience the endpoint of interest. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Standard protocol approvals, registrations, and patient consents
The study from which the present analysis was derived, 2CARE (ClinicalTrials.gov identifier number NCT00608881), was approved by the institutional review boards at 48 sites in the United States, Canada, and Australia. All participants provided written informed consent. The National Institute of Neurological Disorders and Stroke–appointed independent data and safety monitoring board monitored the progress of the trial.
Data availability statement
De-identified 2CARE participant data of the type used in the present article will be shared indefinitely if requested, as will the analysis design plan and statistical analysis plan used to create this article.
Results
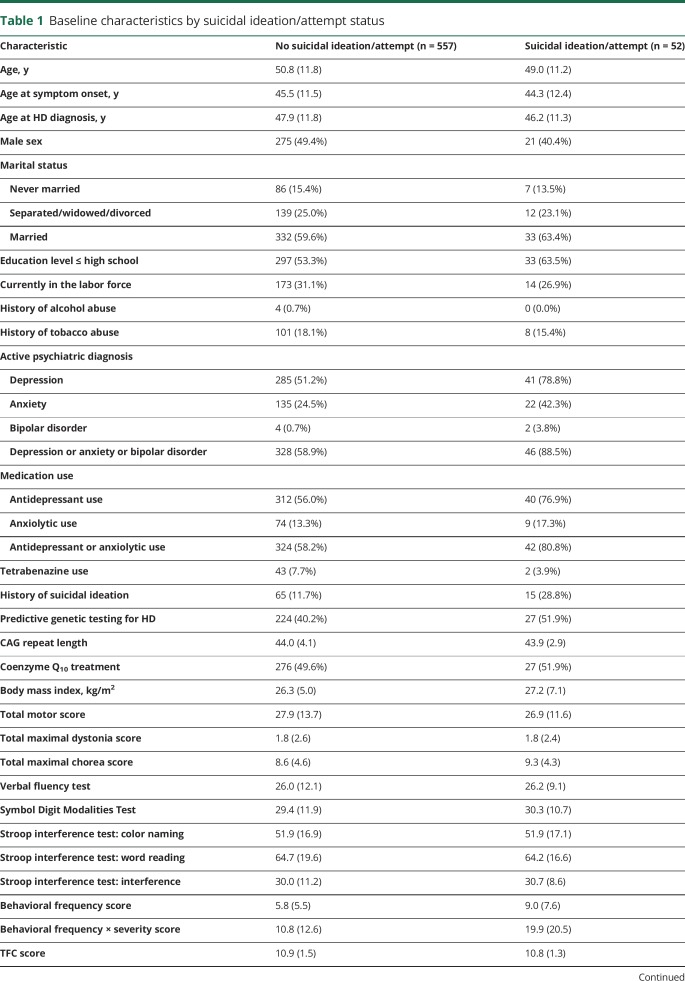
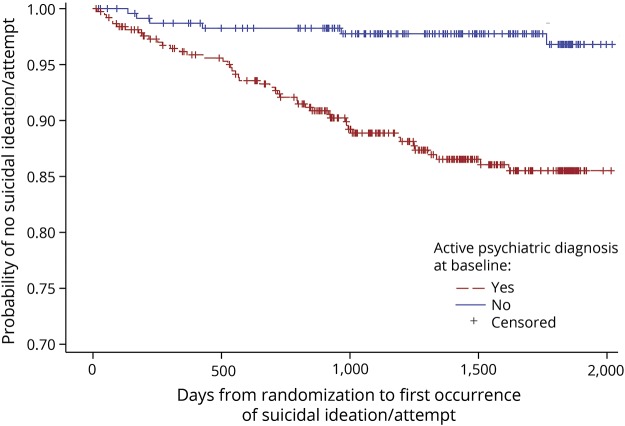
Of 609 randomized participants, 557 (91%) did not experience suicidal ideation or attempts, while 52 (9%) reported at least one instance of ideation or attempt (table 1). Twenty-two suicide attempts occurred among 21 participants (3.4%), with 5 completed (0.8%). The median (interquartile range) follow-up time was 50.6 months (32.6–60.9 months). Higher percentages of participants with a suicidal ideation or attempt had active psychiatric diagnoses (88.5% vs 58.9%), use of psychiatric medications (80.8% vs 58.2%), and prior suicidal ideation (28.8% vs 11.7%) at baseline. The figure displays the Kaplan-Meier estimate of the cumulative distribution of time to first suicidal ideation or attempt by presence of an active psychiatric diagnosis at baseline. Total functional capacity (TFC) scores at baseline were essentially identical between those who did and did not have a future suicidal ideation or attempt. Detailed results concerning the UHDRS Behavioral scale at baseline for participants with and without suicidal ideation or attempt are provided in table e-1 (doi.org/10.5061/dryad.2hp4hq3).
Table 1.
Baseline characteristics by suicidal ideation/attempt status
Figure. Kaplan-Meier estimate of the cumulative distribution of time to suicidal ideation/attempt by active psychiatric diagnosis at baseline.
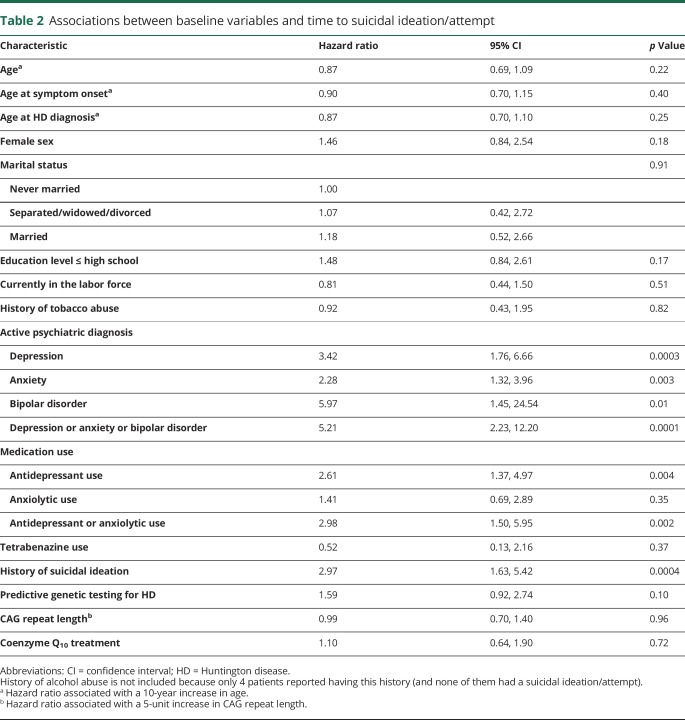
Among baseline variables, active depression, anxiety, bipolar disorder, and prior suicidal ideation were significantly associated with time to suicidal ideation or attempt (table 2). Antidepressant and antidepressant or anxiolytic use were also significantly associated with time to suicidal ideation or attempt, though anxiolytic use alone was not. Notable baseline variables that were not significantly associated with time to suicidal ideation or attempt included undergoing predictive genetic testing, current employment status, marital status, CAG repeat length, tetrabenazine (TBZ) use, and treatment assignment (coenzyme Q10 or placebo).
Table 2.
Associations between baseline variables and time to suicidal ideation/attempt
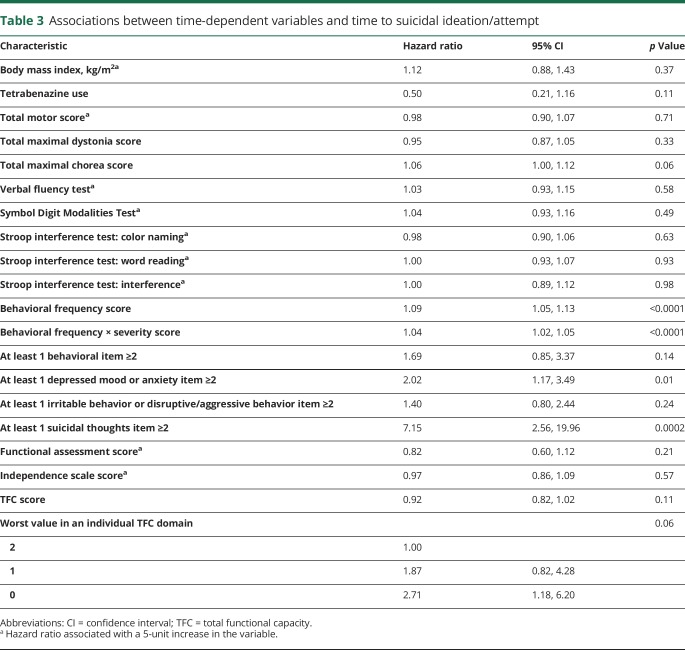
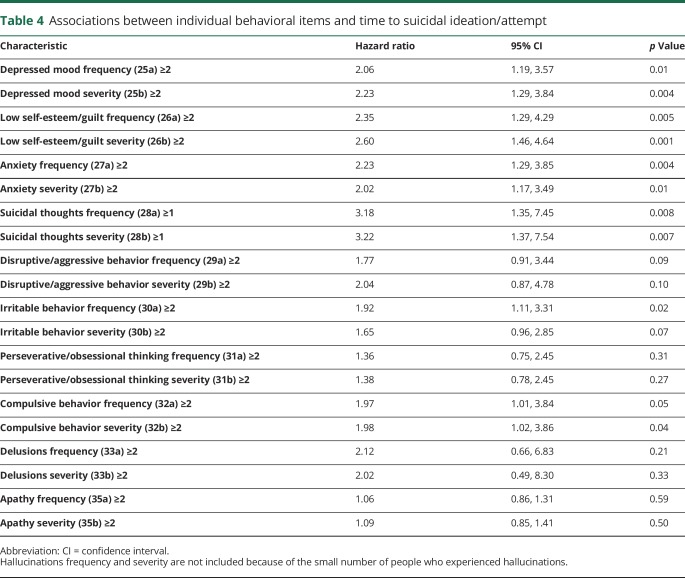
For time-dependent UHDRS variables, none of the motor, cognitive, or functional scores were significantly associated with time to suicidal ideation or attempt (table 3). UHDRS Behavioral Assessment scores (behavioral frequency, behavioral frequency × severity) were associated with time to suicidal ideation or attempt; these associations were driven mainly by items related to depressed mood, low self-esteem/guilt, anxiety, suicidal thoughts, irritability, and compulsive behavior (table 4). Other behaviors such as disruptive/aggressive behavior, perseverative/obsessional thinking, delusions, and apathy were not associated with time to suicidal ideation or attempt.
Table 3.
Associations between time-dependent variables and time to suicidal ideation/attempt
Table 4.
Associations between individual behavioral items and time to suicidal ideation/attempt
The findings concerning risk factors for suicide attempts were generally similar to those for the composite endpoint of suicidal ideation or attempts; detailed results are presented in supplemental tables e-2–e-4 (doi.org/10.5061/dryad.2hp4hq3).
Participants were asked whether they felt they were on active drug or placebo at the end of their participation in the study. These data were missing for 174 participants. Of the 435 participants who answered this question, 208 of 419 with no suicide attempt (49.6%) thought that they were on placebo, compared to 6 out of the 16 with a suicide attempt (37.5%). Also, 199 out of the 400 with no suicidal ideation or attempt thought that they were on placebo (49.8%), compared to 15 out of the 35 (42.9%) with a suicidal ideation or attempt.
Discussion
The extensive longitudinal data from the 2CARE trial affords the opportunity to analyze potential risk factors for suicidal ideation and behavior for those participating in a randomized, double-blind, placebo-controlled trial. Our results are consistent with some observations in the literature, with differences outlined in table e-5 (doi.org/10.5061/dryad.2hp4hq3).6–8,10,23 Comparing suicidal incidence in 2CARE to those in retrospective studies and large cohorts in the literature is not straightforward, as there is variation in disease stage within cohorts, setting (North America, Australia, Europe), reliance on historical self-report vs prospective observation, and variability in access to behavioral health services.9
Our data are generally consistent with prior reports from case histories and observational studies regarding psychiatric comorbidities (depression, anxiety) and antidepressant use being associated with suicidality, highlighting the importance of making these observations in considering a participant's risk for suicidal ideation or behaviors (table e-5: doi.org/10.5061/dryad.2hp4hq3). Higher scores for depression on the UHDRS Behavioral items demonstrated associations with suicidality in multiple studies, and similarly were associated with time to suicidal ideation or attempt in 2CARE. It is notable that low self-esteem/guilt, anxiety, and compulsive behavior were all strongly associated with time to suicidal event. Apathy and obsessive thinking were not associated with time to suicidal event. This may reflect relatively few 2CARE participants having more severe disease, which may in turn carry lesser ability to contemplate or act on suicidal considerations, or indicate something intrinsic about those behaviors with respect to suicidality. For example, apathy may prevent motivated enactment of a plan, or obsessiveness may diminish cognitive bandwidth to consider things other than the objects of obsessions.
The association between total maximal chorea score and suicidal events, while not statistically significant (p = 0.06), raises issues regarding treatment approaches to suicide risk. It is not clear whether this finding, if confirmed, would suggest that aggressive treatment of chorea might mitigate suicide risk by improving functionality or whether worsening chorea, treated or not, is merely a surrogate for overall progression, including deterioration of behavioral and cognitive stability. It does not appear that this finding represents a confounding effect of the use of TBZ, an antichorea medication carrying theoretical risk of worsening depression; TBZ use was not associated with suicidality in this study. Treatment of chorea was allowed in 2CARE but not required to be consistent in terms of medication choice, dosing, or duration. Use of TBZ could vary by participant preference, clinician discretion, and variable evolution of symptoms, making a uniform connection between TBZ use and suicidality in 2CARE difficult. Of note, Dorsey et al.11 recently found no association between supervised TBZ use and suicidality in a large prospective study. Total motor score was not associated with time to a suicidal event; it is unclear why chorea would intrinsically carry more suicidality risk than dystonia, bradykinesia, gait, or TFC score. It may be the case that chorea, usually present early while function is relatively preserved, carries more weight as an overt signal of progressing disease and in that respect may be destabilizing. Bradykinesia, dystonia, rigidity, and gait dysfunction better correlate with more advanced disease, when suicidal behavior may be more difficult to enact or report. The lack of association between these nonchoreic motor elements and suicidal events may reflect the lesser severity of early-stage 2CARE participants.
While the total TFC score was not associated with time to events, there was a suggestion that the presence of significant functional impairment (i.e., score of 0 in any individual TFC domain) was associated with time to ideation/attempt (table 3). While speculative, it may be that arrival at minimal scoring in any domain is associated with heightened risk, and deserves closer attention when documented than perhaps is currently given. This notion is clinically sensible, as TFC domains relate to broad items of identity and independence (capacity for work, living at home). Of note, no other functional outcome was associated with time to suicidal event.
Risk factors for the composite outcome of suicidal ideations/attempts were generally similar to those for suicidal attempts alone. Some commonly observed behaviors in clinic deserve mention in this regard. The hazard ratio (HR) for anxiety was very similar for time to suicidal ideation or attempt (2.28, table 2) and for time to suicide attempt alone (2.13, table e-2: doi.org/10.5061/dryad.2hp4hq3). Ideation can be considered active or passive, where the latter does not involve a specific or imminent plan, but the role of active vs passive ideation in predicting suicide attempts in even non-HD populations appears to be complex and incompletely understood.12 Whether anxiety in HD may in some way differentiate active from passive ideation, and whether that would have prognostic implications for future attempts, is unknown. Our data do not appear to support the hypothesis that heightened anxiety is associated with a passive ideation, which in turn does not translate into actual attempts. Similarly, the HR for bipolar disorder was similar for time to suicidal ideation/attempt (5.97, table 2) and for time to suicide attempt alone (6.27, table e-2: doi.org/10.5061/dryad.2hp4hq3). Variability in mood, including manic behaviors of grandiosity or inflated self-esteem, might interfere with formation of a more specific or motivated plan but not preclude ideation. Conversely, recent data suggest that diminished fear of pain, injury, and death—also conceivable with mania—may help potentiate attempts.13 Whether bipolar behaviors can predict suicide attempts in HD is not clear, though the large HR for suicide attempt and the significant association with suicidal ideation/attempt suggest that clinical surveillance for mood stability is relevant to risk reduction. Although not statistically significant, the HRs for disruptive/aggressive behavior ranged from 1.77 to 2.57 depending on the measure (frequency or severity) and endpoint (ideation/attempt or attempt alone). It is possible that these behaviors involved nonsuicidal self-injury, which has been implicated elsewhere in risk for attempts.14 These considerations will require clarification in future studies, but suggest that a sensitive instrument to predict suicide attempts as early as possible will need to be multifactorial. Future work aimed at establishing a composite algorithm for this may be of value.
Predictive testing for HD was not significantly associated with time to suicidal ideation/attempt (HR 1.59, 95% confidence interval [CI] 0.92–2.74), but there was a stronger suggestion of an association with suicide attempt (HR 2.18, 95% CI 1.01–4.71). This is notable, as predictive testing is widely available and can be of substantial, if not life-changing, consequence. Participants choosing to undergo predictive testing when asymptomatic may be more intrinsically prone to suicidality for reasons that are currently unknown, may have more intense negative feelings about the asymmetry of being relatively healthy but gene-positive, or end up accumulating risk with longer-standing behavioral comorbidities as they wait for phenoconversion. Indeed, Epping and colleagues15 demonstrated longitudinal worsening for 19 of 24 psychiatric measures in 1,305 premanifest participants in the PREDICT-HD study, though impact on suicidality was not described. Almqvist et al.16 reported on “catastrophic events” (suicide attempts, completions, psychiatric hospitalizations) in a large worldwide cohort who underwent predictive testing and noted that existing psychiatric comorbidities within 5 years of testing promoted increased risk. Decruyenaere and colleagues17 followed participants after predictive testing for 5 years and observed a subgroup with long-lasting psychological distress, irrespective of test results, that differed based on motivation for undergoing testing; participants wishing to eliminate uncertainty fared worse than those wanting testing for specific reasons (family planning, career, or relationship decisions). Whether this differentiation influences suicide attempts is not known. A deeper understanding of how predictive testing is discussed, offered, and followed will be essential, particularly with newer treatments in development whose long-term efficacy, while alluring, will not be known for some time.
Impulsivity, which is thought to influence suicide attempts in general, may be a factor in HD suicidality that was not measurable in our analysis.18 Despite the generally accepted notion that impulsivity is common in HD, it is not well-studied with respect to suicide attempts; it does not have well-validated methodology for study in HD, and may not always be relevant, as indicated in a recent case series where suicidality in patients with HD was more deliberate and not impulsive.19,20 It is important to recognize that alcohol and substance abuse were exclusion criteria in 2CARE; for that reason, our ability to draw conclusions about the relationship between these factors and suicidality in HD is constrained by study design.
The effect of participation in HD clinical trials on suicidal risk is not clear. In 2CARE, the incidence of suicidal ideation was consistent with those in other longer studies,8 though the percentages of participants with attempts (3.4%) and completions (0.8%) were relatively low. There may be a selection bias present in cohorts choosing to participate in research, particularly studies requiring commitment over a long period of time. Participants who enroll and participate in long-term drug trials may be more likely to be relatively functional, have stable lifestyles and good personal support to help ensure fulfillment of study responsibilities, benefit from more routine interaction with and monitoring by clinical personnel for changes in behavioral status, or derive a mood-reinforcing effect from participation in research. Caregiver involvement was required for entry into 2CARE, which may have helped promote stability. Conversely, such a long commitment with the prospect of not being on an active agent could conceivably be daunting or destabilizing; investigators from the CREST-E study, a drug trial of similar size and duration, indicated that this element created problems with recruitment.21 In 2CARE, a smaller percentage of participants with suicidal ideation or attempts thought that they were on placebo compared to those without these events, though the small number of participants with these events prevents substantive conclusions. Whether or not double-blinding and using a control arm influences suicidal risk is also unclear. In a recent open-label HD drug study with 3-year follow-up (Open-HART), no suicidal events were reported in mid-stage participants for the duration of the study period, albeit in a much smaller cohort (n = 64) that may reflect selection bias.22 The psychological factors at play for participation may have ramifications for recruitment, retention, and placebo effects in randomized, double-blind, placebo-controlled trials. Understanding how clinical trial participation affects the behavioral health of participants, including suicidality, deserves further study due to its implications for the well-being of those whose participation is integral to the discovery of safe and effective treatments.
Glossary
- CI
confidence interval
- HD
Huntington disease
- HR
hazard ratio
- TBZ
tetrabenazine
- TFC
total functional capacity
- UHDRS
Unified Huntington's Disease Rating Scale
Appendix. Authors
Study funding
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke Awards (U01 NS052592 & U01 NS052619). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Original 2CARE study funded by NINDS (grants NS052592 and NS052619).
Disclosure
A. McGarry: grant funding from, consultant for Teva Pharmaceuticals, Acadia, and UniQure. M. McDermott, K. Kieburtz, W. Fung, E. McCusker, J. Peng, and E. de Blieck report no disclosures relevant to the manuscript. M. Cudkowicz: consultant for Astra-Zeneca, Cytokinetics, Genentech, Biohaven, and Denali. Go to Neurology.org/N for full disclosures.
References
- 1.van Duijn E, Kingma EM, van der Mast RC. Psychopathology in verified Huntington's disease gene carriers. J Neuropsychiatry Clin Neurosci 2007;19:441–448. [DOI] [PubMed] [Google Scholar]
- 2.Orth M, Handley OJ, Schwenke C, et al. Observing Huntington's disease: the European Huntington's Disease Network's REGISTRY. PLoS Currents 2011;2:RRN1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen JS, Hoth KF, Nehl C, Stierman L. Critical periods of suicide risk in Huntington's disease. Am J Psychiatry 2005;162:725–731. [DOI] [PubMed] [Google Scholar]
- 4.Stierman A, McDermott M, Kieburtz K, et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology 2017;88:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huntington Study Group. The Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord 1996;11:136–142. [DOI] [PubMed] [Google Scholar]
- 6.Lipe H, Schultz A, Bird TD. Risk factors for suicide in Huntington's disease: a retrospective case study. Am J Med Genet 1993;48:231–233. [DOI] [PubMed] [Google Scholar]
- 7.Hubers AA, Reedeker N, Giltay EJ, Roos E, van der Mast RC. Suicidality in Huntington's disease. J Affect Disord 2012;136:550–557. [DOI] [PubMed] [Google Scholar]
- 8.Hubers AAM, van Duijn EJ, Roos RAC, et al. Suicidal ideation in a European Huntington's population. J Affect Disord 2013;151:248–258. [DOI] [PubMed] [Google Scholar]
- 9.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington's disease. Neurology 2000;54:452–458. [DOI] [PubMed] [Google Scholar]
- 10.Wetzel HH, Gehl CR, Dellefave-Castillo L, Schiffman KM, Paulsen JS. Suicidal ideation in Huntington disease: the role of comorbidity. Psychiatry Res 2011; 188: 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsey ER, Brocht AF, Nichols PE, et al. Depressed mood and suicidality in individuals exposed to tetrabenazine in a large Huntington Disease observation study. J Huntingtons Dis 2013;2:509–515. [DOI] [PubMed] [Google Scholar]
- 12.Pérez S, Marco JH, García-Alandete J. Psychopathological differences between suicide ideators and suicide attempters in patients with mental disorders. Clin Psychol Psychother 2017;24:1002–1013. [DOI] [PubMed] [Google Scholar]
- 13.Klonsky ED, Saffer BY, Saffer BY. Recent advances in differentiating suicide attempters from suicide ideators. Curr Opin Psychiatry 2017;30:15–20. [DOI] [PubMed] [Google Scholar]
- 14.Ren Y, You J, Zhang X, et al. Differentiating suicide attempters from suicide ideators: the role of capability for suicide. Arch Suicide Res 2018:1–18. [DOI] [PubMed] [Google Scholar]
- 15.Epping E, Kim J, Craufurd D, et al. Longitudinal psychiatric symptoms progress in prodromal Huntington disease: a decade of data. Am J Psychiatry 2016;173:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almqvist EW, Bloch M, Brinkman R, Craufurd MR. A worldwide assessment of the frequency of suicide, suicide attempts, or psychiatric hospitalization after predictive testing for Huntington disease. Am J Hum Genet 1999;64:1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decruyenaere M, Evers-Kiebooms G, Cloostermans T, et al. Psychological distress in the 5-year period after predictive testing for Huntington's disease. Eur J Hum Genet 2003;11:30–38. [DOI] [PubMed] [Google Scholar]
- 18.Boogaerts E, Diaz-Sastre C, Garcia Resa E, et al. Suicide attempts and impulsivity. Eur Arch Psychiatry Clin Neurosci 2005;255:152–156. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PL, Potts GF, Sanchez-Ramos J. Self-reported impulsivity in Huntington's disease patients and relationship to executive dysfunction and reward responsiveness. J Clin Exp Neuropsychol 2017;39:694–706. [DOI] [PubMed] [Google Scholar]
- 20.Cimino OC, Stovall J, Claassen DO. Perseveration and suicide in Huntington's disease. J Huntingtons Dis 2018;7:185–187. [DOI] [PubMed] [Google Scholar]
- 21.Hersch SM, Schifitto G, Oakes D, Bredlau CM, Nahin R, Rosas HD. The CREST-E study of creatine for Huntington disease: a randomized controlled trial. Neurology 2017;89:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGarry A, Kieburtz K, Abler V, et al. Safety and exploratory efficacy at 36 months in open-HART, an open-label extension study of pridopidine in Huntington's disease. J Huntingtons Dis 2017;6:189–199. [DOI] [PubMed] [Google Scholar]
- 23.Honrath P, Dogan I, Wudarczyk O, et al. Risk factors of suicidal ideation in Huntington's disease: literature review and data from Enroll-HD. J Neurol 2018;265:2548–2561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified 2CARE participant data of the type used in the present article will be shared indefinitely if requested, as will the analysis design plan and statistical analysis plan used to create this article.