Abstract
Bacterial meningitis has a high mortality rate and can be challenging to diagnose and manage. This study aimed to evaluate the effect of diosmetin in a rat model of Streptococcus pneumoniae meningitis and to investigate the mechanism of action.
Forty rats included a treatment group (n=30) that underwent intracisternal injection with S. pneumoniae, and a sham group (n=10) that underwent intracisternal injection with normal saline. In the treatment group, four days before the inoculation of the bacteria, rats were pre-treated with oral diosmetin 100 mg/kg (n=10) and 200 mg/kg (n=10), and the negative control was pre-treated with normal saline (n=10). Bacterial meningitis was confirmed one day after inoculation by cerebrospinal fluid (CSF) bacterial titer and neurological score. In rat brain tissue, levels of inflammatory mediators were determined by enzyme-linked immunosorbent assay (ELISA) and western blot for protein kinase B (Akt), phosphoinositide 3-kinase (PI3K), myeloid differentiation primary response 88 (MyD88), and nuclear factor-κB (NF-κB), and the TUNEL assay for apoptosis was performed.
In the diosmetin-treated group compared with negative control group, the CSF bacterial titer and the level of pro-inflammatory mediators, and the neurological score, were significantly reduced (p<0.01). In the rat hippocampal tissue, levels of Akt, PI3K, MyD88 and NF-κB, and the number of TUNEL-positive apoptotic cells were significantly reduced in the diosmetin-treated group compared with negative control group (p<0.01).
In a rat model of bacterial meningitis due to S. pneumoniae, diosmetin reduced neuroinflammation, and neuronal apoptosis by modulating the PI3K/AKT/NF-κB signaling pathway.
MeSH Keywords: Apoptosis; Inflammation; Meningitis, Bacterial
Background
Bacterial meningitis is a severe infection with a high mortality rate, particularly in developing countries, where the prevalence is up to 0.9% [1]. Streptococcus pneumoniae is the most common causative organism of bacterial meningitis [2]. Patients that survive the initial infection may suffer from long-term neurological impairment, including hearing loss, seizures, learning and memory deficit [3]. The findings from previously published studies indicate that inflammatory pathways play an important role in the development of bacterial meningitis. Nuclear factor kappa B (NF-κB) that translocates in the nucleus of immune cells has been shown to enhance the production of inflammatory mediators [4]. Although inflammation is a protective response of body that helps in the eradication of bacterial, it also causes neuronal degeneration [5]. Optimal management of bacterial meningitis requires the reduction of neuronal apoptosis and inflammation that is associated with the effects of the immune response and bacterial toxins. In bacterial meningitis, it is difficult to manage all these events by a single drug.
Diosmetin is a flavonoid isolated from the leaves of Olea europaea L [6]. Diosmetin is a traditional Chinese medicine that has been used to treat several types of cancer and has been shown to act by inhibiting the activity of the CYP1B1 and CYP1A1 enzymes [7]. Diosmetin has been shown to inhibit the proliferation of human oral squamous carcinoma SCC-9 cells in vitro [8]. Other pharmacological properties of diosmetin are anti-inflammatory, estrogenic, anti-oxidant, antimicrobial, and anticancer properties [9,10]. Also, diosmetin has been shown to reduce the symptoms of diabetes in rats with streptozotocin-induced diabetes due to its strong antioxidant properties [11].
Therefore, this study aimed to evaluate the effect of diosmetin in a rat model of Streptococcus pneumoniae meningitis and to investigate the mechanism of action.
Material and Methods
Rats used in the animal model
Female Wistar rats were obtained from Shanghai Medical College, Shanghai, China. All the animals were housed under standard conditions and underwent 7 days of acclimatization. The animals were given free access to a standard chow diet and tap water. The study protocol was approved by the Institutional Animal Care and Use Committee of the Peoples’ Hospital of Yinan, China (IACUC/PHY/2017/11) and the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International for animal experimentation were followed.
Chemical reagents
Diosmetin was purchased from Sigma-Aldrich (St. Louis, MO, USA). The enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α) were obtained from Abcam (Cambridge, UK). Akt, PI3K, and MyD88 were purchased from Cell Signaling Technology (Danvers, MA, USA) and NF-κB, nucleolin and β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Infection with Streptococcus pneumoniae
A standard strain of Streptococcus pneumoniae serotype 3 was used in this study. Blood agar plates were used to culture the bacterial strain overnight and a further logarithmic phase was achieved by incubating the bacteria for 18 hr at 37°C. Centrifugation of bacteria was done at 5000×g for 15 min and a nephelometer was used to resuspend the bacteria in saline solution at 1×104 CFU/mL.
Intracisternal cannula implantation
Implantation of an intracisternal cannula was performed. All the animals were anesthetized by intraperitoneal injection of 10% chloral hydrate. Implantation of the cannula was performed in the right lateral cerebral ventricle of the rats. The location of the cannula implantation was 3.8 mm rostral to the lambdoid suture of the skull, 2 mm lateral to the right side from the midline of the skull, and 2.5 mm from the skull surface. Following implantation, all the animals underwent recovery for 3 days in the cages.
Induction of bacterial meningitis
All the animals were anesthetized and 20 μL of cerebrospinal fluid (CSF) was removed by intracisternal puncture. An equal volume of S. pneumoniae of 1×104 CFU/mL concentration was inoculated into the rats. Meningitis developed one day after the inoculation of bacteria and confirmed by culturing 5 μL of CSF. Also, assessment of the severity of the disease was done using a neurological scoring system: 1, does not turn upright when supine; 2, turns upright when supine within 30 s; 3, minimal ambulatory activity; 4, turns upright when supine within <5 s; 5, normal.
Forty rats included a treatment group (n=30) that underwent intracisternal injection with S. pneumoniae, and a sham group (n=10) that underwent intracisternal injection with normal saline. In the treatment group, four days before the inoculation of the bacteria, rats were pre-treated with oral diosmetin 100 mg/kg (n=10) and 200 mg/kg (n=10), and the negative control was pre-treated with normal saline (n=10). After the rats were euthanized, the brain was removed from all the animals 24 hr after the inoculation of bacteria and the brain was stored at −80°C.
Determination of pro-inflammatory mediators
Brain tissue was homogenized in phosphate-buffered saline (PBS) and centrifuged or 15 min at 12000×g. ELISA kits were used to determine the levels of IL-1β, IL6, and TNF-α, according to the manufacturer’s instructions.
Western blot
Cells from isolated rat brain were lysed in buffer containing protease inhibitor after washing in PBS solution to extract the total protein. The lysate was centrifuged for 10 min at 12000×g and 4°C. A BCA kit was used to estimate the concentration of total protein, according to the manufacturer’s instructions. sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to fractionate the proteins using a polyvinylidene fluoride (PVDF) membrane. The primary antibodies used included anti-p-Akt (dilution, 1: 1000), anti-Akt (dilution, 1: 1000), anti-p-PI3K (dilution, 1: 1000), anti-PI3K (dilution, 1: 1000), anti-MyD88 (dilution, 1: 1000), anti-β-actin (dilution, 1: 1000), anti-NF-κB (dilution, 1: 500) and anti-nucleolin (dilution, 1: 500), which were incubated with the membrane at 4°C overnight. This was followed by incubation with the anti-mouse horseradish peroxidase-conjugated secondary antibody. The enhanced chemiluminescence (ECL) method was used for the estimation of band intensity.
TUNEL apoptosis assay
Brain tissue from each rat was fixed in 4% paraformaldehyde, followed by embedding in paraffin wax and tissue sections were cut at 5 mm thickness onto glass slides. The Dead End™ Fluorometric TUNEL System kit (Promega Corp., Madison, WI, USA) was used to perform the TUNEL fluorescence assay, according to the manufacturer’s instructions. Slides stored in the dark before the cell nuclei were stained using Hoechst fluorescence stain (Thermofisher Scientific, Waltham, MA, USA). NIS-Elements BR image processing and analysis software (Nikon, Tokyo, Japan) were used to quantify the TUNEL-positive cells in the hippocampal region.
Electrophoretic mobility shift assay (EMSA) for NF-κB DNA-binding
An electrophoretic mobility shift assay (EMSA) kit was used to measure the NF-κB DNA-binding activity. Extracted DNA was incubated with a p65 biotin end-labeled NF-κB oligonucleotide probe (forward: 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; reverse: 5′-G CCT GGG AAA GTC CCC TCA ACT-3′) in binding buffer for 15 min at room temperature. Then, the p65 biotin-labeled oligonucleotide complex was incubated for a further 15 min. Gel electrophoresis was used for the separation of the sample in Tris buffer solution and ECL was used to detect the bands.
Statistical analysis
All data were expressed as the mean ± standard error of the mean (SEM). The statistical analysis was performed using one-way analysis of variance (ANOVA). Post hoc comparison of means was performed using Dunnett’s post hoc test and GraphPad Prism version 6.1 (GraphPad Software, La Jolla, CA, USA). A P-value <0.05 was considered to be statistically significant.
Results
Diosmetin reduced the bacterial titer and neurological score in the rat model of bacterial meningitis
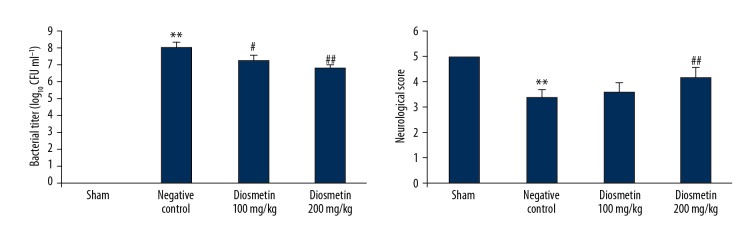
The effect of diosmetin treatment on the bacterial titer and neurological score in Streptococcus pneumonia-induced meningitis rat model is shown in Figure 1. The bacterial titer was significantly enhanced in the CSF (p<0.01), and the neurological score was significantly reduced in the negative control group compared with the sham group (p<0.01). However, treatment with diosmetin significantly reduced the bacterial titer in the cerebrospinal fluid (CSF) and the neurological score was significantly enhanced compared with negative control group of rats (p<0.01).
Figure 1.
Effect of diosmetin on the bacterial titer and neurological score in Streptococcus pneumoniae meningitis in a rat model. Mean ±SEM (n=10). ** p<0.01 vs. the sham group; # p<0.05, ## p<0.01 vs. the negative control group.
Diosmetin reduced the concentration of pro-inflammatory cytokines in the rat model of bacterial meningitis
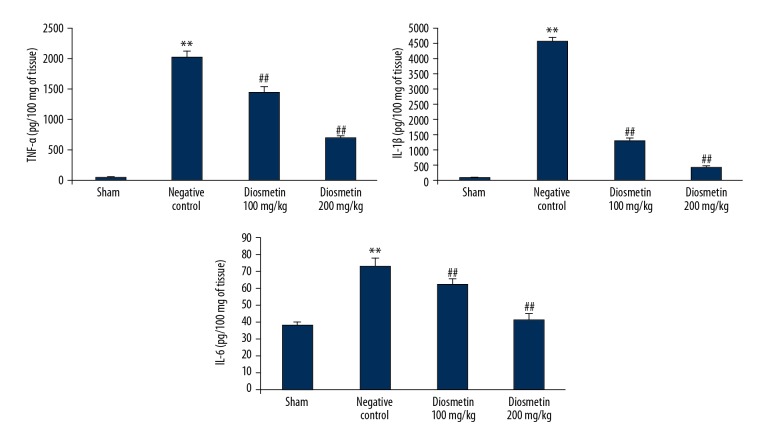
The effect of diosmetin on the concentration of the pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 in the hippocampal tissue of the S. pneumoniae-induced meningitis rat model is shown in Figure 2. The levels of TNF-α, IL-1β, and IL-6 in the hippocampal tissue of the negative control group were increased when compared with the sham group. However, there was a significant decrease in the concentration of TNF-α, IL-1β, and IL-6 in the hippocampus tissue of the diosmetin-treated group compared with the negative control group (p<0.01).
Figure 2.
Effect of diosmetin on the concentration of pro-inflammatory mediators including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 in tissue from the hippocampus in Streptococcus pneumoniae meningitis in a rat model. Mean ±SEM (n=10). ** p<0.01 vs. the sham group; # p<0.05, ## p<0.01 vs. the negative control group.
Diosmetin reduced the level of Akt, PI3K, MyD88 and NF-κB in the rat model of bacterial meningitis
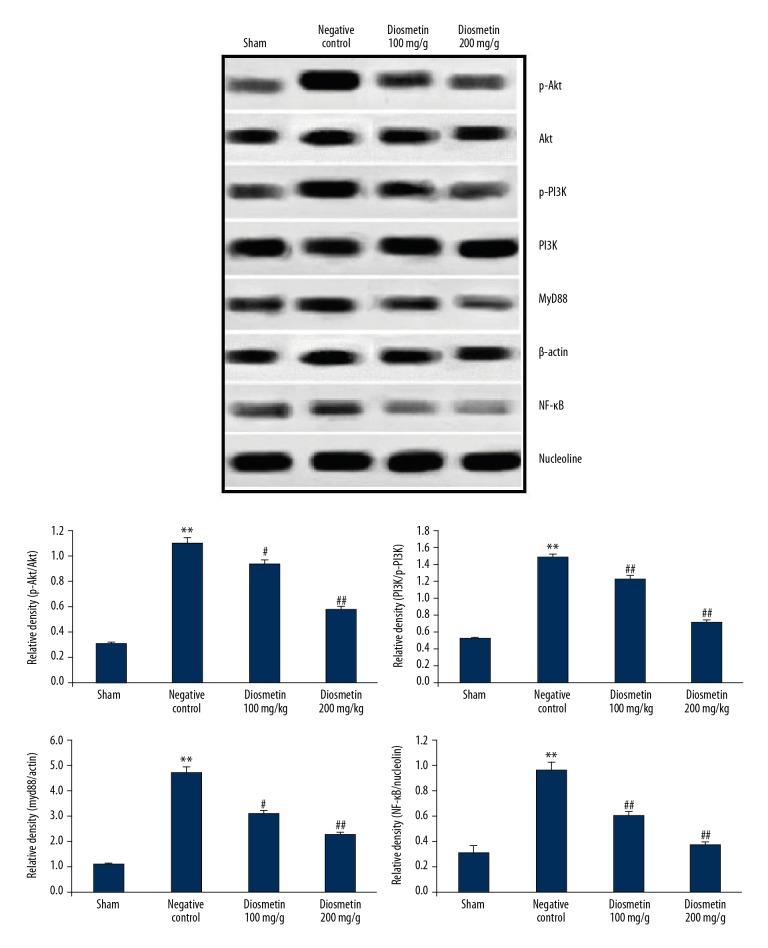
Figure 3 shows the effect of diosmetin treatment on the level of Akt, PI3K, MyD88, and NF-κB in the hippocampal tissue of the S. pneumoniae-induced meningitis rat model. There was a significant increase in the level of Akt, PI3K, MyD88, and NF-κB in the hippocampus tissue of negative control group compared with the sham group (p<0.01). However, treatment with diosmetin reduced the levels of Akt, PI3K, MyD88, and NF-κB proteins in the hippocampal tissue of S. pneumoniae-induced meningitis in the rat model.
Figure 3.
Effect of diosmetin on the expression levels of Akt, PI3K, MyD88 and NF-kB in tissue from the hippocampus in Streptococcus pneumoniae meningitis in a rat model. Mean ±SEM (n=10). ** p<0.01 vs. the sham group; # p<0.05, ## p<0.01 vs. the negative control group.
Diosmetin decreased apoptosis in the rat model of bacterial meningitis
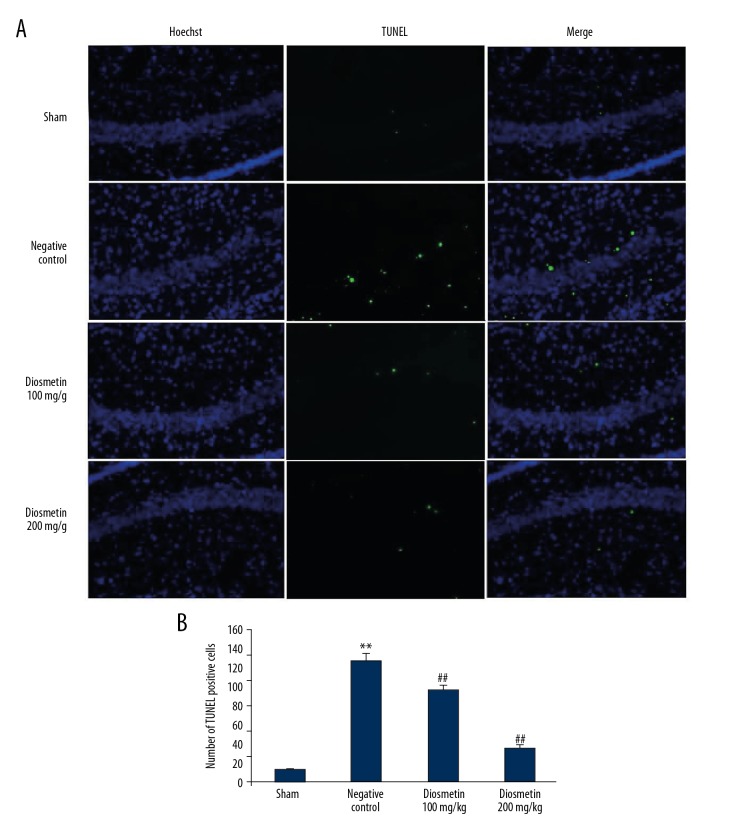
Figures 4A, 4B show the effect of diosmetin on neuronal apoptosis in the hippocampal tissue of the S. pneumoniae-induced meningitis rat model. There was a significant increase in the number of TUNEL-positive cells in the hippocampal tissue of the negative control group compared with the sham group (p<0.01). However, treatment with diosmetin significantly reduced the number of TUNEL-positive cells in the hippocampal tissue when compared with the negative control group (p<0.01).
Figure 4.
Effect of diosmetin on neuronal apoptosis in tissue from the hippocampus in Streptococcus pneumoniae meningitis in a rat model. (A) TUNEL-positive cells in tissue from the rat hippocampus. (B) TUNEL-positive cells in tissue from the rat hippocampus. Mean ±SEM (n=10). ** p<0.01 vs. the sham group; # p<0.05, ## p<0.01 vs. the negative control group.
Diosmetin reduced the activity of NF-κB in the rat model of bacterial meningitis
Figure 5 shows the effect of diosmetin on the NF-κB DNA-binding activity in the cerebral cortex tissue of the S. pneumoniae-induced meningitis rat model. There was a significant increase in the NF-κB DNA-binding activity in the cerebral cortex tissue of the negative control group compared with the sham group of rats (p<0.01). However, NF-κB DNA-binding activity in the tissue from the cerebral cortex was significantly decreased in the diosmetin-treated group when compared with the negative control group (p<0.01).
Figure 5.
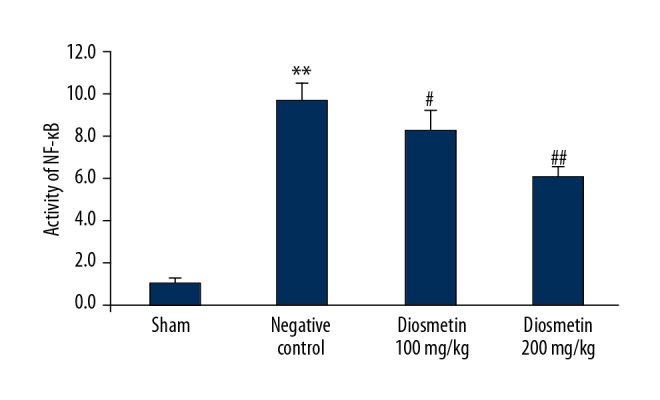
Effect of diosmetin on the NF-κB DNA-binding activity in the cortical tissue in Streptococcus pneumoniae meningitis in a rat model. Mean ±SEM (n=10). ** p<0.01 vs. the sham group; # p<0.05, ## p<0.01 vs. the negative control group.
Discussion
The aim of this study was to evaluate the effect of diosmetin in a rat model of Streptococcus pneumoniae meningitis and to investigate the mechanism of action. The findings showed that diosmetin reduced neuroinflammation and neuronal apoptosis by modulating the PI3K/AKT/NF-kB signaling pathway. Meningitis in rat model was induced by intracisternal inoculation of S. pneumoniae and confirmed by evaluation of the cerebrospinal fluid (CSF) and measurement of the neurological score. The effect of diosmetin on meningitis was determined by measuring the levels of pro-inflammatory cytokines using the enzyme-linked immunosorbent assay (ELISA) and Western blot, and apoptosis of neuronal cells was evaluated using the TUNEL assay on rat brain tissue.
Previously published studies have shown that the release of inflammatory cytokines has an important role in the pathogenesis of bacterial meningitis [12]. There are several inflammatory mediators that are involved in brain injury, including interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α) [13]. The findings from the present study showed that diosmetin treatment significantly reduced the levels of proinflammatory cytokines in the hippocampal tissue in the rat model of bacterial meningitis when compared with the negative control group (p<0.01).
The MyD88/NF-κB signaling pathway has been previously reported to cause neurological injury in bacterial meningitis, and some drugs have been reported to have a beneficial effect on bacterial meningitis by reducing the activity of several signaling pathways, including the MyD88/NF-κB pathway [14]. The results of this study were in keeping with these previous reports as the results showed that treatment with diosmetin reduced the activity of the MyD88/NF-κB signaling pathway in the hippocampal tissue of the rat model of S. pneumonia-induced meningitis.
Histological changes in bacterial meningitis have been reported to show neuronal apoptosis in hippocampal tissue [15]. There are several pathways, including the PI3K/AKT pathway, that have been reported to control cellular apoptosis [16]. In this study, the result of the TUNEL assay showed that the number of TUNEL-positive cells were significantly reduced in the diosmetin-treated rats in the model of bacterial meningitis when compared with the negative control group (p<0.01), and it also reduced the levels of PI3K and AKT protein in the hippocampal tissue in the rat model.
Conclusions
The findings of this study showed that in the rat model of bacterial meningitis due to Streptococcus pneumoniae, diosmetin reduced neuroinflammation and neuronal apoptosis by modulating the PI3K/AKT/NF-κB signaling pathway.
Acknowledgments
The authors thank the Peoples’ Hospital of Yinan, China for providing the facilities for the study.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Yang R, Zhang H, Xiong Y, et al. Molecular diagnosis of central nervous system opportunistic infections and mortality in HIV-infected adults in Central China. AIDS Res Ther. 2017;14:24. doi: 10.1186/s12981-017-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho HK, Lee H, Kang JH, et al. The causative organisms of bacterial meningitis in Korean children in 1996–2005. J Korean Med Sci. 2010;25(6):895–99. doi: 10.3346/jkms.2010.25.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta A, Ibsen LM. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med. 2013;2(4):40–47. doi: 10.5492/wjccm.v2.i4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–69. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meirinhos J, Silva BM, Valentão P, et al. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat Prod Res. 2005;19(2):189–95. doi: 10.1080/14786410410001704886. [DOI] [PubMed] [Google Scholar]
- 7.Androutsopoulos VP, Spyrou I, Ploumidis A, et al. Expression profile of CYP1A1 and CYP1B1 enzymes in colon and bladder tumors. PLoS One. 2013;8(12):e82487. doi: 10.1371/journal.pone.0082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning AM, Walle UK, Walle T. Flavonoid glycosides inhibit oral cancer cell proliferation – role of cellular uptake and hydrolysis to the aglycones. J Pharm Pharmacol. 2005;57(8):1037–42. doi: 10.1211/0022357056514. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Gong XB, Huang LG, et al. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget. 2017;8(19):30723–33. doi: 10.18632/oncotarget.15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge A, Liu Y, Zeng X, et al. Effect of diosmetin on airway remodeling in a murine model of chronic asthma. Acta Biochim Biophys Sin (Shanghai) 2015;47(8):604–11. doi: 10.1093/abbs/gmv052. [DOI] [PubMed] [Google Scholar]
- 11.Juárez-Reyes K, Brindis F, Medina-Campos ON, et al. Hypoglycemic, antihyperglycemic, and antioxidant effects of the edible plant Anoda cristata. J Ethnopharmacol. 2015;161:36–45. doi: 10.1016/j.jep.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 12.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of Pneumococcal meningitis. Clin Microbiol Rev. 2011;24(3):557–91. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W-Y, Tan M-S, Yu J-T, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3(10):136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu D, Lian D, Zhang Z, et al. Brain-derived neurotrophic factor is regulated via MyD88/NF-κB signaling in experimental Streptococcus pneumoniae meningitis. Sci Rep. 2017;7:3545. doi: 10.1038/s41598-017-03861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Liu J, Zhou Z, et al. Diosmetin attenuates Akt signaling pathway by modulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/inducible nitric oxide synthase (iNOS) in streptozotocin (STZ)-induced diabetic nephropathy mice. Med Sci Monit. 2018;24:7007–14. doi: 10.12659/MSM.910764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T, Pang Q, Wang Y, Yan X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int J Mol Med. 2017;40(6):1669–78. doi: 10.3892/ijmm.2017.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]