Abstract
Background
We examined NK cell phenotypes and functions after seven years of ART and undetectable viral loads (<50 copies/ml) with restored CD4 T-cell counts (≥500 cells/μl) and age-matched healthy-HIV-uninfected individuals from the same community.
Methods
Using flow-cytometry, NK cell phenotypes were described using lineage markers (CD56+/-CD16+/−). NK cell activation was determined by expression of activation receptors (NKG2D, NKp44 and NKp46) and activation marker CD69. NK cell function was determined by CD107a, granzyme-b, and IFN-gamma production.
Results
CD56 dim and CD56 bright NK cells were lower among ART-treated-HIV-infected than among age-matched-HIV-negative individuals; p = 0.0016 and p = 0.05 respectively. Production of CD107a (P = 0.004) and Granzyme-B (P = 0.005) was lower among ART-treated-HIV-infected relative to the healthy-HIV-uninfected individuals. NKG2D and NKp46 were lower, while CD69 expression was higher among ART-treated-HIV-infected than healthy-HIV-uninfected individuals.
Conclusion
NK cell activation and dysfunction persisted despite seven years of suppressive ART with “normalization” of peripheral CD4 counts.
Keywords: Natural killer cells, NK cell activation, NK degranulation, NK Cytolytic function, NK cell dysfunction, Antiretroviral therapy, 107a, Granzyme-B, Interferon gamma, NKG2D, NKp46, Sub-Saharan Africa
1. Introduction
Natural killer (NK) cells have exceptional roles in host immune response to infection including bridging the gap between the innate and adaptive immune systems [1], recognizing and killing virally infected cells, as well as recognizing and clearing cancerous and stressed cells [2,3]. Different infections alter the NK cell compartment, for example, unique NK cell signatures have been primed by Cytomegalovirus (CMV) and Varicella zoster viruses [4,5]. Insights into recent work have further highlighted the ability of NK cells to have memory like phenotypes to different viral infections [6,7].
HIV infection is associated with several changes in the NK cell compartment, including phenotypic and functional abnormalities that contribute to difficulty in the control of HIV progression [8]. Upon HIV infection, NK cells rapidly divide and produce huge amounts of IFN-γ cytokine as a way of controlling the infection [9]. Strong NK cell activity and cytotoxic receptor expression are associated with preservation of CD4 T-cells and lower viral set point [10]. ART was shown to restore NK cell numbers with a mature phenotype in HIV-infected individuals although NK cell phenotypes and the ability to produce IFN-ɣ and cytotoxicity remained impaired after one year of ART [[11], [12], [13]]. We previously demonstrated persistence of the pro-inflammatory subset of NK cells (CD56++ CD16+), after four years of successful ART with viral suppression [14]. Moreover, persistent pro-inflammatory subsets were associated with suboptimal CD4 count recovery [14].
NK cells have been shown to kill Mtb-infected cells directly or through antibody-dependent cellular cytotoxicity (ADCC) [15], and confer protection against Mtb infection as was shown in mice with T-cell deficiencies [16]. Longitudinal studies among healthy individuals in South Africa have shown that NK cell mediated cytotoxicity remains key to successful immune control of Mycobacterium tuberculosis infection, therefore peripheral NK cell phenotypes and function may inform disease progression and treatment responses [17]. Our earlier studies on NK cell phenotypes after four years of ART did not examine NK cell function recovery. We postulate that NK cell function may take more than four years of ART to recover to levels comparable to healthy HIV-uninfected individuals, particularly among patients that initiated ART at CD4 counts below 350 cells/μl. There is paucity of data on recovery of NK cell function, among other innate immune dysfunctions associated with HIV disease, particularly in Africa where other co-infections like tuberculosis, and CMV remain endemic. We hypothesized that innate immune dysfunction, for example, NK cell dysfunction may not recover fully during ART, thereby affecting recovery of adaptive T-cell responses. Incomplete recovery of NK cell repertoire could contribute to the persistent T-cell function abnormalities previously described in our cohort of ART-treated adults, even when CD4 counts were restored to levels above 500 cells/μl [18]. With emerging evidence that NK cell function is key to protection against progression from latent Mtb infections to active tuberculosis, understanding recovery of NK cell function during ART is essential to the control of Mtb infection among ART-treated HIV-infected adults in sub-Saharan Africa where Tb remains a leading opportunistic infection and a leading cause of death [19,20].
This paper therefore, describes the NK cell phenotypes and function among individuals with successful HIV treatment, viral suppression and CD4 counts above 500 cells/μl after seven years of therapy within an African cohort. In addition to the distribution of NK cell phenotypes, we describe the expression of NK activating receptors NKG2D, NKp46 and NKp44, cytokine production, as well as their cytotoxic functions as expressed by CD107a and Granzyme b production. Our results provide insight on recovery of the innate immune system among HIV-infected adults after long-term ART and the potentially associated risk of active tuberculosis and common viral infections. Our comparisons between ART-treated individuals that have attained otherwise normal CD4 counts with age-matched HIV-uninfected individuals from the same community, provide clinicians with contextual data on persistent host susceptibility to common infections. This will inform potential interventions geared towards optimisation of immune recovery of the HIV-infected adults on life-long ART in sub-Saharan Africa.
2. Materials and methods
2.1. Study design and participants
It was a comparative cross-sectional study which utilised cryopreserved PBMCs of all the 30 optimal responders to ART, defined as HIV-infected ART-treated adults who had attained a CD4 T-cell count ≥500 cells/μl after seven years of suppressive ART within the Infectious Diseases Institute (IDI) HIV treatment research cohort located at Mulago National referral hospital.
2.2. Cohort description
In April of 2004 and April 2005, the IDI HIV treatment research cohort was founded, enrolling and initiating a total of 559 consecutive ART-naïve HIV-infected patients. HIV treatment drugs were offered by the Global Fund (a generic combined formulation of stavudine [d4T], lamivudine [3TC], and nevirapine [NVP] and the US President's Emergency Plan for AIDS Relief (a combined formulation of zidovudine [ZDV] and 3TC plus efavirenz [EFZ] or NVP). Tenofovir [TDF] was given as the drug of choice to patients that had toxicities to ZDV. Cotrimoxazole prophylaxis was given to all people living with HIV in accordance to the national guidelines at the time. Group counselling sessions were carried out at least three times to support adherence to ART. Patients returned to the clinic monthly for physician's review of adherence to medication, toxicities, and acute infections. HIV RNA viral loads, complete blood counts and CD4 lymphocyte counts were measured 6 monthly [21]. Optimal responders, consecutively enrolled into this immune recovery study, were individuals in the highest quartile of CD4 increase (a quartile with mean CD4 increase of 823 cells/μl) who did not have any opportunistic infection in the six months preceding immune recovery study [18]. These were compared with 30 age-matched HIV-uninfected adults within the communities served by Mulago National referral hospital routine HIV testing program. These were healthy asymptomatic individuals that had negative HIV test results within the routine HIV testing program.
2.3. Ethical considerations
Ethical approval of the study was sought from the institutional review board of School of Biomedical Sciences Makerere University College of Health Sciences. All participants provided written informed consent.
2.4. Monoclonal antibodies and flow cytometric analysis
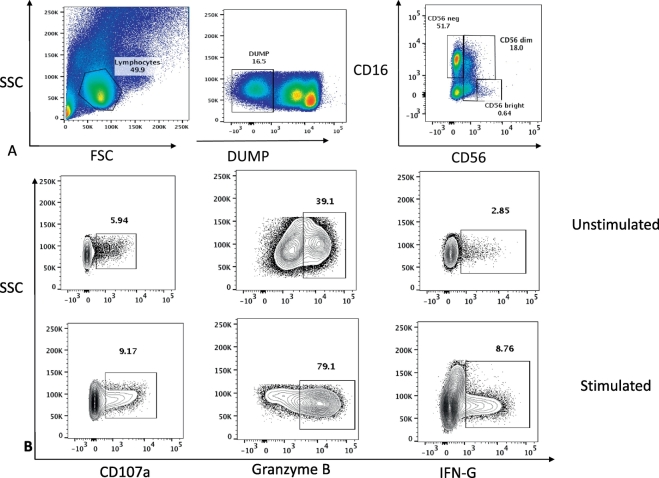
The following fluorochrome-conjugated antibodies were used in this study: CD3 BV510, CD14 BV510, CD19 BV510, CD56 PE, CD16 PECY7, CD38 BV605, NKG2D PERCPCY5.5, NKp44 PE-CY7, NKp46 BV450, CD107a APC, CD8 APC CY7, CD69 BV785, HLADR BV711 for surface antigens; GRANZYME B Pacific Blue and IFN-γ BV488 for intracellular staining. Cryopreserved PBMC from ART-treated HIV-infected individuals and age-matched healthy HIV-negative adults from the same community were snap thawed and washed in pre-warmed complete Roswell Park Memorial Institute (cRPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS). The cells were rested for 4 h in 5 ml of R10 at 37 °C in a 5% CO2 incubator. After resting, cells were washed with R10 and the numbers of viable cells were counted. Surface staining was done at 4 °C for 30 min with saturating concentrations of different combinations of antibodies in the presence of fixable live/dead stain (Invitrogen). Cells were then fixed and acquired using the LSRII flow cytometer (BD Bioscience FACSDiva8.0) and data analysed using FlowJo software (Tree Star, Version 10.1). Natural Killer cells were distinguished by their surface receptor expression of CD56+/− and the CD16+/− (Fig. 1). The subsets were assessed according to the intensity of expression of the CD56 marker; CD56+ as the dim NK cells and CD56++ as the bright NK cells. CD3 expression was used to exclude T cells, CD19 for the exclusion of B cells and CD14 was used to exclude the monocytes. Fluorescence minus one controls (FMOs) were applied to standardise the gating and compensation controls to correct for spectral overlap. At least 100,000 events of CD56+CD16+ were acquired and analysed (Fig. 1). Comparisons and pictures were made using Graph Prism 6. The Mann Whitney test for non-parametric variables was used to compare proportions of the different cell subsets and their functions among the two groups. A significant difference was obtained with p values < 0.05.
Fig. 1.
Gating strategy for Natural Killer (NK) cell phenotypes and function markers (CD107a, Granzyme B and Interferon gamma (IFN-G).
2.5. Titration of NK cell stimulation
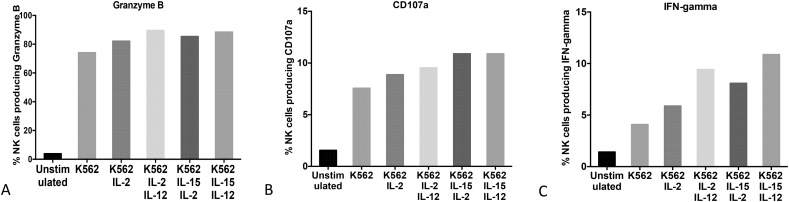
In our optimisation experiment we added 2 × 106 PBMCs to the unstimulated well, [K562 alone (5:1 ratio)], [K562 (5:1 ratio) and IL-2 (100 IU/ml)], [K562 (5:1ratio), IL-2(100 IU/ml) and IL-12 (50 ng/ml)], [K562(5:1ratio), IL-2(100 IU/ml) and IL-15 (100 ng/ml)] and finally [K562(5:1ratio), IL-12 (50 ng/ml) and IL-15 (100 ng/ml)]. From our analysis, the combination of [K562 (5,1ratio), IL-12 (50 ng/ml) and IL-15 (100 ng/ml)] was the best because it consistently gave the highest percentage of granzyme B production, CD107a, IFN-gamma and total NK cells numbers (Fig. 2).
Fig. 2.
Optimisation of Natural Killer (NK) cell stimulation. PBMCs were cultured with medium alone or with combinations of different stimulants (K562, IL-2, IL-12 and IL-15) for 6 h. A shows percentage of Granzyme B production, B shows percentage IFN-gamma production and C shows percentage expression of CD107a.
2.6. NK cell functional assays
PBMC were incubated with MHC-devoid K562 cells (5:1 ratio) in combination with IL-12 (50 ng/ml) and IL-15 (100 ng/ml) recombinant proteins for 6 h at 37 °C in the presence of CD107a- APC antibody (BD Biosciences). To determine interferon gamma production, we added Monensin, 1/1500 concentration, Brefeldin A 1/250 concentration – (BD Biosciences) to each of the above wells and these were incubated for 6 h at 37 °C with 5% CO2. An additional well containing PMA 50 ng/mL, and ionomycin 1 μg/mL (catalogue No. P8139)- SIGMA) was used as the positive control. At the end of the incubation, cells were washed with BD Pharm staining buffer (Cat. No. 554656), fixed and permeabilized to allow specific anti-cytokine fluorescence antibody conjugates to enter into the cell. Samples were acquired on an LSRII using BD FACS Diva 8.0 sofware (BD Bioscience) and data analysed using FlowJo software (Tree Star, Version 10.1). Fluorescence minus one controls (FMOs) were applied to standardise the gating and compensation controls to correct for spectral overlap.
3. Results
3.1. Disparities in the NK cell numbers and phenotype distribution in ART-treated HIV-infected individuals
We assessed the NK cell phenotypes and functions from PBMC of HIV-infected individuals that had been on ART for at least seven years and these were compared with age-matched healthy HIV-uninfected individuals from the same community. The HIV-infected individuals had a nadir median CD4 count of 97 (11, 158) cells/μl at ART initiation and a median CD4 count of 607 (759, 996) cells/μl with viral load < 50 copies/ml at enrolment in this study; denoted as Optimal Immune responders. Of 30 ART-treated optimal responders, 26(87%) were female, median age was 40 IQR (38–46) years, BMI median was 22.57 IQR (20, 25), 93% were free from hypertension and 97% had no diabetes. Of the 30 healthy HIV-uninfected individuals, 63% (19/30) were female, their median age was 36 IQR (33–42) years, median Body Mass Index (BMI) 25.95(22, 30), 8% were hypertensive and 4% were diabetic. All participants had no fevers at the time of enrolment in the study [18].
3.1.1. NK cell phenotypes
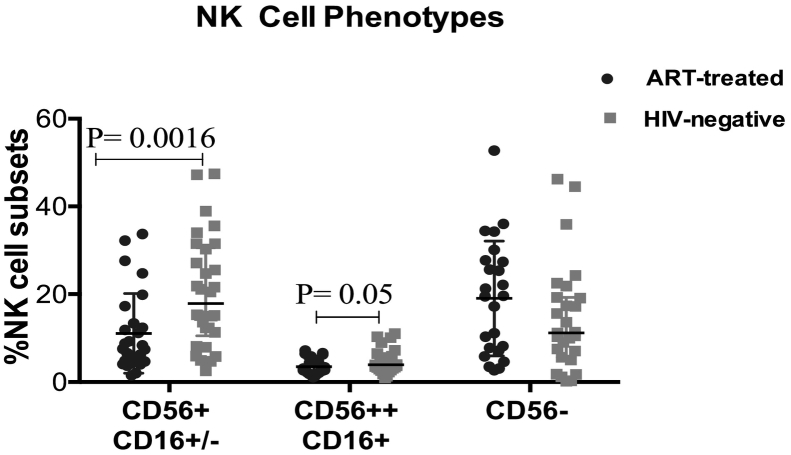
Percentages of the CD56+ (CD56dim) NK cells were significantly lower (P = 0.0016) in ART-treated HIV-infected individuals than in healthy HIV-uninfected individuals despite having a CD4 count of >500 cells/μl after seven years of ART. Similarly, proportions of CD56++ (CD56 bright) NK cells were significantly lower (P = 0.05) in ART-treated individuals than in healthy HIV-uninfected individuals (Fig. 3). Total NK cell numbers (CD56+ CD16+/−) and the CD56- phenotype were comparable among the two groups; P = 0.397 and P = 0.117.
Fig. 3.
Phenotypic evaluation of Natural Killer (NK) cells among HIV-infected individuals with CD4 T-cells ≥ 500 cells/μl after seven years of ART relative to age-matched healthy HIV-negative individuals from the same community. CD56 + CD16+/− represents the CD56 dim NK cells, CD56++CD16- represents CD56 bright NK cells and CD56-CD16+ represents CD56 negative NK cells. The Mann–Whitney U test for non-parametric tests was used for comparisons of the two groups with statistical significance at p values ≤ 0.05.
3.1.2. NK cell receptor expression
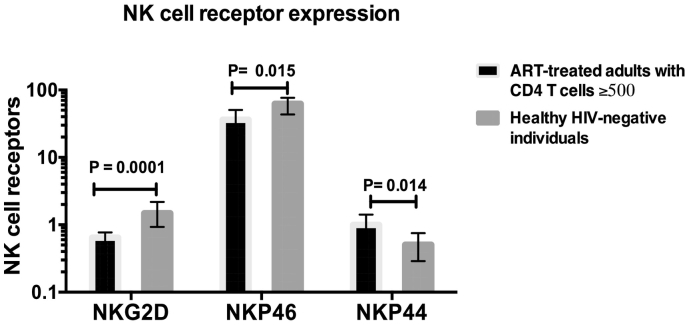
Of the total NK cell phenotypes, the percentage expressing the natural cytotoxic receptor NKP46 was significantly lower in ART-treated HIV-infected individuals than among the healthy HIV-uninfected adults; P = 0.015. Also, the c-type lectin receptor NKG2D was found to be significantly lower in the ART-treated individuals than in their HIV-uninfected counterparts; P = 0.0001 (Fig. 4). Expression of the NKP44 natural cytotoxic receptor was significantly higher among the ART-treated HIV-infected individuals than in the HIV-uninfected individuals; P = 0.014 (Fig. 4).
Fig. 4.
Evaluation of Natural Killer (NK) cell receptor expression for NKG2D, NKp46 and NKp44 among HIV-infected individuals with CD4 T cells ≥5 00 after seven years of ART and their age-matched healthy HIV-negative counterparts.
3.2. NK cell functions of cytolysis, degranulation and interferon gamma production
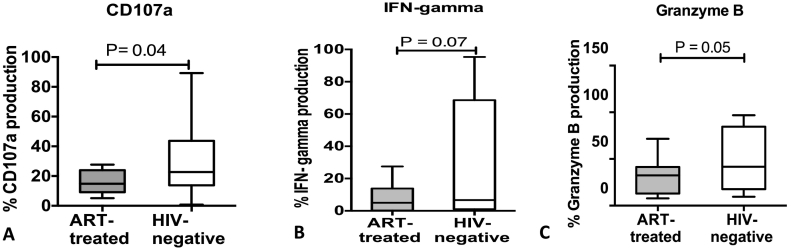
The NK cytolytic and degranulation function was assessed by measuring the levels of granzyme b production and the expressions of the CD107a. The cytolytic CD56dim NK cells produced significantly lower levels of Granzyme B in ART-treated HIV-infected adults than their age-matched healthy HIV-uninfected counterparts; P = 0.004. Furthermore, the levels of CD107a expression were significantly lower in the ART-treated adults with CD4 > 500 cells/μl after seven years of ART, relative to their age-matched HIV-uninfected counterparts; P = 0.005 (Fig. 5).
Fig. 5.
Natural Killer (NK) cell function among ART-treated HIV-infected individuals with CD4 T-cells ≥ 500 cells/μl after seven years of ART relative to age-matched healthy HIV-negative individuals. A shows proportion of NK cells expressing CD107a, B shows proportion of NK cells producing interferon gamma, and C shows proportion of NK cells producing Granzyme B. The Mann–Whitney U test for non-parametric tests was used to compare the two groups with statistical significance at p values ≤0.05.
4. Expression of activation markers in NK cell phenotypes
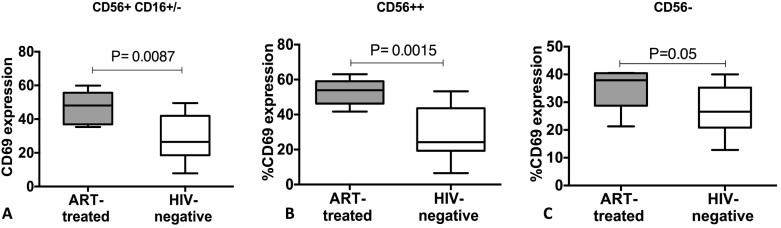
NK cells recent activation status, as denoted by CD69 expression, was evaluated on the Total NK cells (CD56+ CD16+/−), CD56dim NK cells (CD56+ CD16+) and CD56bright NK cells CD56++CD16−). Recent activation status of the entire pool of circulating NK cells was significantly higher in ART-treated HIV-infected individuals than in healthy HIV-uninfected individuals despite having a CD4 count of > 500 cells/μl and seven years of ART; Total NK cells (P = 0.008), CD56dim (P = 0.01) and CD56 bright (P = 0.0015), as shown in Fig. 6. Although the dysfunctional CD56- population were comparable both in the HIV-infected and HIV-uninfected population, the expression of CD69 was higher in the HIV-infected than in the HIV-uninfected population (P = 0.0015), Fig. 6.
Fig. 6.
CD69 expression on the NK cell subsets. A shows the proportion of CD56dim NK cells expressing CD69, B shows the proportion of CD56 bright NK cells expressing CD69, and C shows the proportion of CD56 negative NK cells expressing CD69. The boxes and whiskers represent medians, 5th and 95th percentiles. The Mann–Whitney U test for non-parametric tests was used to compare ART-treated HIV-infected and healthy HIV-uninfected individuals, with statistical significance at p values ≤0.05.
5. Discussion
We examined populations of circulating NK cell phenotypes, receptor expression and functions of cytotoxicity and cytokine production among HIV-infected individuals after seven years of suppressive ART. This cross-sectional study demonstrated the persistent perturbations in NK cell phenotypes and functions among patients that restored peripheral CD4 T-cells to >500 cells/μl in a Ugandan cohort. These findings could mirror the outcomes of long term ART treatment in different Sub-Saharan cohorts where individuals started ART with nadir CD4 counts below 350 cells/μl.
We observed significantly lower proportions of the cytolytic CD56dim (CD56+) and cytokine producing CD56bright (CD56++) NK cells in the HIV-infected individuals relative to age-matched HIV-uninfected individuals. NK cells are known to work efficiently in the clearance of viruses and tumor forming cells [22]. Therefore, few cytolytic and Interferon gamma producing NK cells would imply persistent risk of succumbing to viral infections and cancers of viral origin. Similarly the affected ART-treated adults may not have sufficient NK cell response to control replication of residual HIV-1 virus. The lack of control of the residual HIV virus likely contributes to the ongoing NK cell activation status in ART-treated HIV-infected adults we observed. In Spain, Frias et al. observed similar finding of lower CD56dim NK cells in the HIV-infected individuals than in HIV-uninfected healthy donors even after approximately eight years of ART with undetectable viral load and CD4 T-cell count >500 cells/μl [23]. Similarly, Alter et al. observed an early depletion of CD56 bright NK cell subset during acute HIV infection followed by a continual reduction in the CD56 dim NK cells subset after one year of infection, which did not normalise to levels in HIV-uninfected controls after six months of ART [24]. After 15 years of ART, in South Carolina –USA, Luo et al. reported similar NK cell phenotype distribution among ART-treated and HIV-uninfected individuals [25]. It is therefore likely that with longer durations of effective ART, the NK cell compartment may recover to levels comparable with HIV-uninfected individuals. A better understanding of the magnitude of perturbations of the NK cells in the reticuloendothelial system may provide more insight on what is required to design more effective therapeutic strategies to optimize immune recovery.
We also found that expression of activating natural cytotoxic receptors of NKP46 and NKG2D was higher among HIV-infected adults. NKp46, however, was lower among ART-treated HIV-infected adults relative to their age-matched HIV-negative counterparts. NKp44 receptor, highly expressed on recently activated NK cells [26], was higher among the ART-treated HIV-infected adults; implying persistent activation of the NK cell pool despite seven years of effective ART. Similarly, CD69, a marker for recent NK cell activation was high in the HIV-infected relative to HIV-uninfected individuals. Persistently high levels of NK cell activation, as measured by CD69, were significantly higher in the HIV positive individuals compared to the HIV-negative individuals after an average of three years of ART [27]. NK cells expressing NKp46 carry out an important role of NK cell cytotoxicity against tumor cells and virally infected cells. Effective NK cell cytolytic function requires delicate balance between the activating and inhibitory receptors [8]. Downregulation of the receptors during chronic HIV infection has been associated with risk of development non-AIDS-defining cancers [28,29]. Low NKp46 receptor expression was previously reported after three years of HIV treatment in a European cohort, where it improved with increasing duration of ART [23]. Persistent immune activation has been associated with development of non-AIDs illnesses which are emerging as major causes of death in adults aging with HIV [30,31]. The ongoing immune activation despite effective ART and a CD4 > 500 cells/μl is not fully explained. We postulate that it may be driven by residual viral replication lymph nodes and other tissues.
We further assessed NK cell function following stimulation of NK cells with MHC- devoid K562 cells, IL-12 and IL-15 recombinant proteins. We measured the expression of CD107a, a molecule found within membrane-bound lytic lysosomal vesicles encompassing proteins such as granzyme and perforin. CD107a is a known functional marker for NK cell cytolytic activity upon cell stimulation [32]. We observed significantly lower cytolytic activity of NK cells as measured by granzyme B production and CD107a in the ART-treated HIV-infected individuals than in their HIV-uninfected counterparts despite the seven years of effective ART. Alter et al. also observed high levels of CD107a in HIV-infected viremic individuals, which recovered after six months of ART [33]. Our Ugandan cohort started ART when they were critically ill with a nadir CD4 count of <200 cell/μl a state in which the whole repertoire of the immune system had been severely compromised. We postulate that the status of the immune system at the point of ART initiation has an effect on the recovery of the NK cell CD107a function and granzyme B production. More so, the effects of persistent immune activation a phenomenon that has been observed in this group of patients, can further compromise NK cell functional recovery despite the seven years of ART. Production of IFN-gamma, after stimulation with an extra NK-tropic cytokine IL-15, IL-12 and K562, was lower in the HIV-infected than HIV-uninfected individuals. Inability to restore NK cell interferon gamma production despite the presence of CD161+/CD56+ mature NK cells in pro- portions was previously reported [11]. Further studies looking at the NK cell gene signatures among the HIV-infected individuals may provide a better understanding of the biological pathways that remain deranged during ART in sub-Saharan Africa.
Recent data from South African HIV-negative cohorts showed that the cohorts' NK cells decreased during progression from latent infection to active disease, and enhanced NK cell cytotoxic responses were associated successful immune control of Mtb infection as evidenced by maintenance latent tuberculosis status [17]. In mouse models with T-cell deficiencies, NK cells conferred protection against Mtb infection [16]. Therefore persistent NK cell activation and dysfunction observed among ART-treated adults in sub-Saharan Africa, where Mycobacterium tuberculosis infection is endemic, presents a potential mechanism for the persistently high levels of active tuberculosis in our HIV treatment cohort [20,34].
Noteworthy, majority of the ART-treated adults with CD4 counts ≥ 500 cells/ul in this study were female, which reflects the sub-Saharan HIV epidemic where women are disproportionately more affected than men. In our previous analysis of immune recovery among ART-treated adults in Uganda, Kenya, and Tanzania over 65% of HIV-treated individuals were female although there was no statistical difference in gender among ART-treated adults with CD4 counts above and below 500 cells/μl after five years of treatment [34,35], yet CD4 counts in the general healthy the Ugandan population tend to be higher in women than men [36]. However differences in NK cell phenotypes among healthy HIV-negative men and women are yet to be studied.
6. Conclusions
Persistent NK cell activation, low cytolytic activity and low cytokine production were observed after seven years of suppressive ART. It is likely that ART-treated HIV-infected individuals remain susceptible to opportunistic infections and malignancies of viral origin. A better understanding of the biological pathways driving the NK cell dysfunction would inform development of novel adjunctive therapies to improve recovery and long term health outcomes of HIV treatment.
Ethics approval and consent to participate
Ethical approval was sought from the School of Biomedical Sciences Makerere University College of Health Sciences Research and Ethics Committee. All participants provided written informed consent for storage and future use of their samples in studies to understand host immune recovery during ART.
Consent for publication
Not applicable.
Availability of data and materials
All data will be made available upon request,
Competing interests
The authors declare that they do not have any competing interests.
Author's contributions
RN, SC, MJ, SRJ, DN contributed to the conceptualization and execution of the study. RN and DN drafted the manuscript. All authors reviewed and approved the manuscript for publication.
Funding and acknowledgements
This work was supported through the DELTAS Africa Initiative [grant# 107743/Z/15/Z], that funded Damalie Nakanjako, Rose Nabatanzi and Lois Bayigga through a group leader award. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [grant #107743/Z/15/Z] and the UK government. The views expressed in this publication are those of the author (s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. The authors also acknowledge funding from the Alliance for Global Health and Science at the University of California, Berkeley, USA and the Wheeler Center for Emerging and Neglected Diseases, University of California, Berkeley, USA that funded our research to further understand recovery of innate immune cells during ART.
References
- 1.Scully E., Alter G. NK cells in HIV disease. Curr. HIV/AIDS Rep. 2016;13(2):85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam V.C., Lanier L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017;44:43–51. doi: 10.1016/j.coi.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pancino G. Natural resistance to HIV infection: lessons learned from HIV-exposed uninfected individuals. J. Infect. Dis. 2010;202(Supplement_3):S345–S350. doi: 10.1086/655973. [DOI] [PubMed] [Google Scholar]
- 4.Schlums H. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell T.M. Varicella zoster virus productively infects human natural killer cells and manipulates phenotype. PLoS Pathog. 2018;14(4):e1006999. doi: 10.1371/journal.ppat.1006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'sullivan T.E., Sun J.C., Lanier L.L. Natural killer cell memory. Immunity. 2015;43(4):634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerwenka A., Lanier L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016;16(2):112. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 8.Mavilio D. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. 2003;100(25):15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terunuma H. Potential role of NK cells in the induction of immune responses: implications for NK cell–based immunotherapy for cancers and viral infections. Int. Rev. Immunol. 2008;27(3):93–110. doi: 10.1080/08830180801911743. [DOI] [PubMed] [Google Scholar]
- 10.De Maria A. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur. J. Immunol. 2003;33(9):2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 11.Azzoni L. Sustained impairment of IFN-γ secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 2002;168(11):5764–5770. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 12.Alter G., Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J. Intern. Med. 2009;265(1):29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chehimi J. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J. Immunol. 2007;179(4):2642–2650. doi: 10.4049/jimmunol.179.4.2642. [DOI] [PubMed] [Google Scholar]
- 14.Bayigga L. High CD56++ CD16-natural killer (NK) cells among suboptimal immune responders after four years of suppressive antiretroviral therapy in an African adult HIV treatment cohort. BMC Immunol. 2014;15(1):2. doi: 10.1186/1471-2172-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esin S., Batoni G. Natural killer cells: a coherent model for their functional role in mycobacterium tuberculosis infection. J. Innate. Immun. 2015;7(1):11–24. doi: 10.1159/000363321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng C.G. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with mycobacterium tuberculosis. J. Immunol. 2006;177(10):7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 17.Roy Chowdhury R. A multi-cohort study of the immune factors associated with M. Tuberculosis infection outcomes. Nature. 2018;560(7720):644–648. doi: 10.1038/s41586-018-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabatanzi R. Low antigen-specific CD4 T-cell immune responses despite normal absolute CD4 counts after long-term antiretroviral therapy an African cohort. Immunol. Lett. 2014;162(2):264–272. doi: 10.1016/j.imlet.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiragga A.N. Quality of data collection in a large HIV observational clinic database in sub-Saharan Africa: implications for clinical research and audit of care. J. Int. AIDS Soc. 2011;14:3. doi: 10.1186/1758-2652-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissberg D. Ten years of antiretroviral therapy: incidences, patterns and risk factors of opportunistic infections in an urban Ugandan cohort. PLoS ONE. 2018;13(11):e0206796. doi: 10.1371/journal.pone.0206796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamya M.R. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2007;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 22.Peppa D. Natural killer cells in human immunodeficiency virus-1 infection: spotlight on the impact of human cytomegalovirus. Front. Immunol. 2017;8:1322. doi: 10.3389/fimmu.2017.01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frias M. Persistence of pathological distribution of NK cells in HIV-infected patients with prolonged use of HAART and a sustained immune response. PLoS ONE. 2015;10(3):e0121019. doi: 10.1371/journal.pone.0121019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter G. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106(10):3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 25.Luo Z. Increased natural killer cell activation in HIV-infected immunologic non-responders correlates with CD4+ T cell recovery after antiretroviral therapy and viral suppression. PLoS ONE. 2017;12(1):e0167640. doi: 10.1371/journal.pone.0167640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitale M. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non–major histocompatibility complex–restricted tumor cell lysis. J. Exp. Med. 1998;187(12):2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisio F. Successfully treated HIV-infected patients have differential expression of NK cell receptors (NKp46 and NKp30) according to AIDS status at presentation. Immunol. Lett. 2013;152(1):16–24. doi: 10.1016/j.imlet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Carroll V., Garzino-Demo A. HIV-associated lymphoma in the era of combination antiretroviral therapy: shifting the immunological landscape. Pathog. Dis. 2015;73(7) doi: 10.1093/femspd/ftv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leal F.E. Role of natural killer cells in HIV-associated malignancies. Front. Immunol. 2017;8:315. doi: 10.3389/fimmu.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guaraldi G., Raggi P. Vol. 263. 2017. Antiretroviral therapies and cardiovascular risk: True or false? Atherosclerosis; pp. 313–314. [DOI] [PubMed] [Google Scholar]
- 31.Ssinabulya I. Subclinical atherosclerosis among HIV-infected adults attending HIV. AIDS care at two large ambulatory HIV clinics in Uganda. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0089537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alter G., Malenfant J.M., Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 2004;294(1–2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Alter G., Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J. Intern. Med. 2009;265(1):29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiragga A.N. A decade of antiretroviral therapy in Uganda: what are the emerging causes of death? BMC Infect. Dis. 2019;19(1):77. doi: 10.1186/s12879-019-3724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanjako D. Frequency and impact of suboptimal immune recovery on first-line antiretroviral therapy within the international epidemiologic databases to evaluate AIDS in East Africa. AIDS. 2016;30(12):1913–1922. doi: 10.1097/QAD.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugada E.S. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin. Diagn. Lab. Immunol. 2004;11(1):29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be made available upon request,