Abstract
An emerging body of literature has examined cortical activity during walking and balance tasks in older adult and Parkinson’s disease (PD) participants, specifically using functional near infrared spectroscopy (fNIRS) or electroencephalography (EEG) devices. This review aimed to provide an overview of this developing area, in order to inform disease-specific mechanisms underlying walking or balance deficits. Medline, PubMed, PsychInfo and Scopus databases were searched. Articles that described cortical activity during walking and balance tasks in older adults and those with PD were screened by the reviewers. Thirty-seven full-text articles were included for review, following an initial yield of 566 studies. This review summarizes study findings, where increased cortical activity appears to be required for older adults and further for PD participants to perform walking and balance tasks, but specific activation patterns vary depending upon task-demands. Studies attributed cortical activation to compensatory mechanisms for underlying age-or PD-related deficits in automatic movement control. However, a lack of standardization within the reviewed studies was evident from the wide range of study protocols, instruments, regions of interest, outcomes and interpretation of outcomes that were reported. Unstandardized data collection, processing and reporting limited the clinical relevance and interpretation of study findings. Future work to standardize approaches to cortical activity measurement during walking and balance tasks in older adult and PD populations with fNIRS and EEG systems is needed, which will allow direct results comparison and ensure robust data collection/reporting. Based on the reviewed articles we provide clinical and future research recommendations.
Keywords: Maturitas, Special Issue, Modern medicine for healthy ageing
1. Introduction
Parkinson’s disease (PD) causes walking and balance deficits [1, 2] that lead to increased falls, reduced mobility and quality of life [3]. In general, 60% of older adults (>80years old) have gait disorders [4] which cause 17% of falls [5], with higher incidences in PD [6]. While some gait impairments in PD, such as slow gait, may relate to primary pathophysiology (i.e. bradykinesia), others, such as increased gait variability may be compensatory in nature [7]. Animal model evidence denotes that voluntary movements are derived from motor commands projecting from the cortex to the brainstem and spinal cord [8], [9–12]. Goal-directed behaviors, such as walking, are always accompanied by automatic processes of postural control involving balance adjustment and muscle tone regulation [8] that rely more on subcortical structures (i.e. basal ganglia and brain stem) [8]. PD impacts subcortical pathways leading to dysfunctional automatic movement control, which is suggested to be accompanied by a compensatory shift to more voluntary cortical control [10, 13, 14]. Therefore, walking may rely heavily on compensation from cortical structures in PD, which may also increase motor performance variability.
In parallel, other studies focus on the role of cognition in balance and gait dysfunction in PD [15]. Extensive associative behavioral studies have linked cognition to walking and balance performance [16, 17], however these previous experiments have not investigated the neural mechanisms involved. Changes in brain structure and connectivity with ageing and PD impact cognitive processes, walking and balance [18, 19], likely due to involvement of common neural centers. Specifically, white matter structural changes, lower hippocampal and anterior cortex volume relate to executive-attentional deficits with links to walking and balance impairment [15, 20–22]. Therefore, disease-specific impairments of brain activity, motor control and cognition potentially mediate task performance. Examining underlying cortical activity involved in walking and balance in older adults and PD will allow further clarification of disease-specific links between these features.
Traditionally, brain structure and function have been studied using imaging techniques, such as functional magnetic resonance imaging [18, 23, 24]. The majority of studies have focused on investigation of motor regions (such as supplementary motor area (SMA), premotor (PMC), primary motor (M1) and sensorimotor cortices (SMC)) [25], with only a few recent studies examining the involvement of cognitive regions (such as prefrontal cortex (PFC)) [26–28]. Unfortunately, imaging results are limited as the head has to remain still and assays of walking and balance are used to mimic task performance (e.g. virtual reality or mental imagery), or studies use simple, single-segment motor tasks (e.g. finger tapping or button pressing) to infer cortical or sub-cortical activity related to general motor control.
Recent technological advances have allowed investigation of cortical activity during real-time walking or balance tasks in older adults and PD using functional near infra-red spectroscopy (fNIRS) or electroencephalography (EEG). However, this emerging body of literature has yet to be compiled for comprehensive methodological evaluation and interpretation. Greater understanding of cortical activity involved in walking and balance with age and PD will allow PD-specific cortical targets for intervention development to be uncovered. Therefore, we focused this review on the following: 1) cortical activity during real-time walking and balance tasks in older adult and PD subjects; 2) study protocols including cortical activity instrumentation and outcomes; and 3) clinical and future research recommendations.
2. Methods
2.1. Search strategy
Key search terms and synonyms are displayed within Figure 1. All key terms were matched and exploded with medical subject headings (MeSH). Databases searched included Medline and PsychInfo, Scopus and PubMed (Figure 2). Studies were deemed relevant if they incorporated terminology that focused on cortical activity during a walking or balance task in older adults or PD in the title, abstract or keywords. Initial title screen for relevant articles was performed by the reviewer (SS). Titles and abstracts were then further screened by three reviewers (SS, RV, RM). A final full-text review was performed if further clarity was required for articles meeting review criteria (Table 1).
Figure 1.

Search string used for study acquisition. This illustrates the four key terms used for this review and the synonyms used for each.
Figure 2.
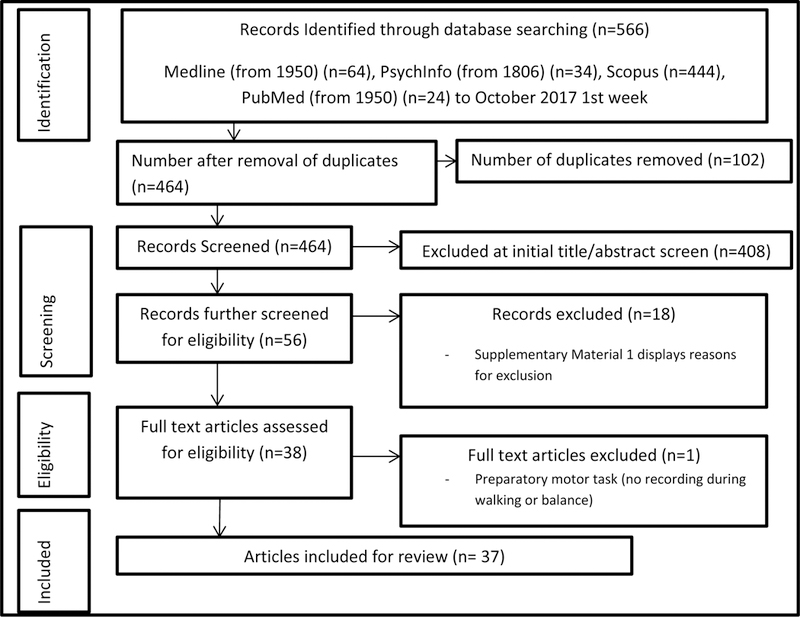
PRISMA flow chart of study design. This illustrates the yield of the search strategy at each stage of the study selection process.
Table 1 –
Article Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Studies were included if they reported cortical activity during a walking or balance task (such as; walking, turning, obstacle crossing, standing, standing with perturbation etc.). | • Articles were excluded if they involved simple motor tasks (i.e. button pressing, finger tapping/movement, wrist/elbow extension or flexion, a single step) as these were not considered to be walking or balance tasks. |
| • Whereby articles included another clinical cohort (e.g. multiple Sclerosis) or a static imaging task, only data pertaining to older adult or PD participants during a walking or balance task was reviewed. | • Only articles written in English were considered and abstracts, case studies, reviews, commentaries, discussion papers, editorials or conference proceedings were excluded. |
| • Experiments that involved assays of walking or balance (e.g. motor imagery or virtual reality) without task performance, or that involved pre and post-task cortical measurement rather than during task recording were also excluded, as these may not represent activity that actually occurs during the task. | |
| • Articles related to animal models (monkey, rat or mouse) were excluded with separate key terms. | |
2.2. Data extraction
Data was extracted and synthesized into tables by the reviewer (SS) and confirmed by the other reviewers (RV, RM, DM, PF) (Table 2, 3). Extracted data included first author and year of publication, population characteristics, cohorts, task, type of measurement device, regions of interest (ROI), signal pre-processing, outcome measures, key findings and interpretation.
Table 2.
Extracted data from studies
| Author | Participants | Walking or Balance Tasks | Dual Tasks | Measurement device and ROIs | Outcome measures | Signal pre-processing |
|---|---|---|---|---|---|---|
| fNIRS | ||||||
| Beurskens et al., 2014 | 15 YA - aged 24.5 ± 3.3 years 10 OA - aged 71.0 ± 3.8 years |
Treadmill: NW Task length: 30s blocks |
Motor DT: walk while checking X in boxes with a pen on a piece of paper Cognitive DT: walk with secondary complex visual task |
fNIRS DYNOT Imaging System (1.8 Hz) LED Light Interoptode distance: 2.2–2.5cm 14 channels: PFC |
Change in oxygenated and de-oxygenated haemoglobin | • Visual inspection with moving standard deviation and spline interpolation to remove artefacts • Pre-colouring filter for temporal correlations • De-trending for physiological noise with wavelet-minimum description length Baseline condition: sitting |
| Chaparro et al. 2017 | 12 OA – aged 63.1 ± 4.4 years, 3m / 9f 10 people with Multiple Sclerosis |
Treadmill: NW, walk with partial (30%) body weight supported Task length: 30s blocks |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIR Imager 1000 (2Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated haemoglobin | • Visual inspection for movement artefacts • Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing while counting 10s |
| Chen et al. 2017 | 90 OA – aged 78.1 ± 5.5 years, 44m / 46f | Overground: NW, obstacle (lasers elliptical-shaped randomly triggered by walking) crossing Task length: NR |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIR Imager 1000 (2 Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated haemoglobin | • Visual inspection for movement artefacts • Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing while counting 10s |
| Clark et al., 2014a | 14 OA – aged 77.1 ± 5.6 years | Treadmill and overground: NW with bare feet, textured insoles or normal shoes. Task length: 60–120s |
Cognitive DT: walk with a secondary verbal fluency task | fNIRS Niro 200NX LED Light Interoptode distance: 3cm 2 channels: PFC |
Tissue oxygenation index (TOI); a ratio of oxygenated haemoglobin to total haemoglobin (sum of oxygenated and deoxygenated haemoglobin) | • NR Baseline condition: walking with normal shoes |
| Clark et al., 2014b | 16 OA – aged 77.2 ± 5.6 years, 8m / 8f | Overground: NW, obstacle (6 small shoes evenly placed) crossing, weighted vest (10% body weight) and diminished lighting. Task length: 5 × 18m laps |
Motor DT: walk while carrying a tray with three rolls of tape stacked on it Cognitive DT: walk with a secondary verbal fluency task |
fNIRS Niro 200NX (2 Hz) LED Light Interoptode distance: 3cm 2 channels: PFC |
Tissue oxygenation index (TOI); a ratio of oxygenated haemoglobin to total haemoglobin (sum of oxygenated and deoxygenated haemoglobin) | • NR Baseline condition: standing still |
| Doi et al. 2013 | 16 OA - aged 75.4 ± 7.2 years, 10m / 6f | Treadmill: NW Task length: 20s blocks |
Cognitive DT: walk with a secondary letter fluency task | fNIRS Spectratech OEG-16 LED Light Interoptode distance: 3cm 16-channels: PFC |
Change in oxygenated and de-oxygenated haemoglobin | • Signals low-pass filtered with cut-off frequency of 0.05Hz Baseline condition: standing 10s |
| Eggenberger et al., 2016 | 33 OA – 74.9 ± 6.9 years, 12m / 21f | Treadmill: NW at different speeds (usual walking speed calculated using 4m walk test overground, added 2km/hr for fast speed) and pre vs. post 8-week exercise intervention Task length: 30s blocks |
fNIRS (1Hz) Oxiplex TS Tissue Spectrometer (1Hz) LASER Light Interoptode distance: 2–3.5cm 2 channels: PFC |
Change in oxygenated and deoxygenated haemoglobin | • Visual inspection for movement artefacts • Blocks averaged to minimise physiological noise • De-trended and transformed by subtracting 60s moving average Baseline condition: slow walking (0.2km/hr) 1min |
|
| Fraser et al., 2016 | 19 YA – aged 21.8 ± 1.9 years, 7m / 12f 14 OA – aged 66.9 ± 5.3 years, 2m / 12f |
Treadmill: NW Task length: 2min blocks |
Cognitive DT: walk with secondary N-back task (different task difficulties: 0–9 items back) |
fNIRS CW6 TechEn LASER Light Interoptode distance: 2.8cm 14 channels: PFC |
Change in oxygenated and deoxygenated haemoglobin | • NR Baseline condition: standing 5s |
| Harada et al., 2009 | 15 OA – aged 63.0 ± 4.0, 2m / 13f | Treadmill: NW at different speeds (started 2km/hr, increased by 1.2km/hr every 3min until reached 85% total heart rate) to impact heart rate (30, 50 and 70% total heart rate) Task length: 60s blocks |
fNIRS OMM-2001 (5.3 Hz) LASER Light Interoptode distance: 3cm 42 channels: PFC, SMA, PMC, SMC |
Change in oxygenated haemoglobin | • NR Baseline condition: standing 10s |
|
| Hernandez et al., 2016 | 8 OA – aged 61.0 ± 4.0 years, 2m / 6f 8 People with multiple Sclerosis |
Overground: NW Task length: 3 × 14ft loop |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIR Imager 1000 (2Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated haemoglobin | • Visual inspection for movement artefacts • Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing 10s |
| Holtzer et al., 2011 | 11 YA – aged 24.0 years, 4m / 7f 11 OA – aged 78.5 years, 4m / 7f |
Overground: NW Task length: 6 × 15ft loop |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS Custom built system LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated haemoglobin | • Signals low-pass filtered with cut-off frequency of 0.14Hz • Independent component analysis removed noise and signal drift Baseline condition: standing 5s |
| Holtzer et al., 2015 | 348 OA – aged 76.8 ± 6.8 years, 143m / 205f | Overground: NW Task length: 3 × 14ft loop |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIR Imager 1000 (2 Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated, deoxygenated haemoglobin, oxygenated index (oxy-deoxy) and total haemoglobin (oxy + deoxy) (Only oxygenated haemoglobin analysed) |
• Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing while counting 10s |
| Holtzer et al., 2016 | 167 OA (no NGA) – aged 74.4 ± 6.0 years, 82m / 85f - 29 OA (central NGA) – aged 79.6 ± 7.4 years, 9m / 20f - 40 OA (peripheral NGA) – aged 77.0 ± 6.3 years, 23m / 17f |
Overground: NW Task length: 3 × 14ft loop |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIR Imager 1000 (2 Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated, deoxygenated haemoglobin, oxygenated index (oxy-deoxy) and total haemoglobin (oxy + deoxy) (Only oxygenated haemoglobin analysed) |
• Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing while counting 10s |
| Holtzer et al., 2017a | 318 OA – aged 76.8 ± 6.7 years, 139m / 179f | Overground: NW Task length: 3 × 14ft loop |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIR Imager 1000 (2 Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated, deoxygenated haemoglobin, oxygenated index (oxy-deoxy) and total haemoglobin (oxy + deoxy) (Only oxygenated haemoglobin analysed) |
• Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing while counting 10s |
| Holtzer et al., 2017b | 314 OA – aged 76.8 ± 6.7 years, 138m / 176f | Overground: NW Task length: 3 × 14ft loop |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIR Imager 1000 (2 Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated haemoglobin | • Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing while counting 10s |
| Mahoney et al. 2016 | 126 OA – aged 74.4 ± 6.1 years, 57m / 69f 26 Parkinsonian syndromes (PS) - aged 81.2 ± 5.9 years, 13m / 15f, UPDRS: 11.08 ± 3.60 117 mild Parkinsonian signs (MPS) –aged 77.5 ± 6.7 years, UPDRS: 3.21 ± 2.49 |
Standing: NS with forward counting task Task length: 10s |
fNIRS fNIR Imager 1000 (2 Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated haemoglobin | • Visual inspection for movement artefacts • Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing 2s |
|
| Maidan et al., 2015 | 11 OA – aged 71.2 ± 6.0 years, 4m / 7f 11 PD – aged 66.2 ± 10.0 years, 8m / 3f, UPDRS-III 42.8 ± 9.3; disease duration 9.2 ± 5.5 years, ON medication |
Overground: NW + turning 180º (anticipated and unanticipated) Task length: 6s |
fNIRS OxyMon MKIII (10Hz) LASER Light Interoptode distance: 3.5cm 6 channels: PFC |
Change in oxygenated haemoglobin | • Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: walking 6s before freezing episode |
|
| Maidan et al., 2016 | 38 OA – aged 70.4 ± 0.9 years, 20m / 18f 68 PD – aged 71.7 ± 1.1 years, 46m / 22f, UPDRS-III 32.9 ± 1.7; disease duration 9.1 ± 0.7 years, ON medication |
Overground: NW, obstacle (30cm width x 20cm depth x 10cm height) crossing Task length: 30s |
Cognitive DT: walk while serially subtracting 3s from a 3-digit number | fNIRS PortaLite (10 Hz) LED Light Interoptode distance: 3–4cm 6 channels: PFC |
Change in oxygenated haemoglobin | • Signals band-pass filtered with cut-off frequency of 0.01–0.14Hz • De-trended with wavelet-minimum filter and correlation Baseline condition: standing 5s |
| Maiden et al. 2017 | 49 PD – aged 71.7 ± 1.1 years, 33m / 16f, UPDRS-III 31.8 ± 2.1, disease duration 9.7 ± 0.9, ON medication | Overground: NW + turning 180º Task length: 5× 30m loop |
fNIRS PortaLite (10 Hz) LED Light Interoptode distance: 3–4cm 6 channels: PFC |
Change in oxygenated, deoxygenated haemoglobin (Only oxygenated haemoglobin analysed) |
• Signals band-pass filtered with cut-off frequency of 0.01–0.14Hz • De-trended with wavelet-minimum filter and correlation Baseline condition: standing 5s |
|
| Mirelman et al. 2017 | 23 YA - 30.9 ± 3.7 years, 10m / 13f 20 OA - 69.7 ± 5.8 years, 10m / 10f |
Overground: NW, obstacle (30cm width x 20cm depth x 10cm height) crossing Task length: 5 × 30m loop |
Cognitive DT: walk while serially subtracting 3s from a 3-digit number | fNIRS PortaLite (10 Hz) LED Light Interoptode distance: 3–4cm 6 channels: PFC |
Change in oxygenated haemoglobin | • Signals band-pass filtered with cut-off frequency of 0.01–0.14Hz • De-trended with wavelet-minimum filter and correlation Baseline condition: standing 20s |
| Nieuwhof et al., 2016 | 14 PD – aged 71.2 ± 5.4 years, 7m / 7f, disease duration 5.7 ± 3.3 years, ON medication | Overground DT only Task length: 40s |
Cognitive DT: walk with secondary tasks of; 1) Count forward 2) serially subtracting 3s 3) reciting forward digit-spans, set to maximum length in sitting |
fNIRS PortaLite (10 Hz) LED Light Interoptode distance: 3–4cm 6 channels: PFC |
Change in oxygenated and deoxygenated haemoglobin | • Moving standard deviation and spline interpolation to remove artefacts • De-trended and low-pass filtered at 0.1Hz Baseline condition: standing 20s |
| Osofundiya et al., 2016 | 10 OA (non-obese) – aged 80.6 ± 7.5 years 10 OA (obese) – aged 80.5 ± 6.8 years |
Overground: NW, precision stepping on randomly presented floor targets Task length: 30s |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS NIRO 200 NX (5 Hz) LED Light Interoptode distance: 3cm 2 channels: PFC |
Change in oxygenated haemoglobin and total haemoglobin | • NR Baseline condition: relative to zero |
| Rosso et al. 2017 | 6 YA – 26.0, 4m / 2f 10 OA – 73.5, 3m / 7f |
Standing: NS Task length: 121s |
Cognitive DT: standing with secondary choice-reaction time attention task | fNIRS CW6 Real-time LASER Light Interoptode distance: 2.8cm 15 channels: PFC, temporal and motor cortices. |
Change in oxygenated and deoxygenated haemoglobin | • NR Baseline condition: standing 30s |
| Takeuchi et al. 2016 | 16 YA – aged 25.9 ± 4.4 years, 11m / 5f 15 OA- aged 71.7 ± 3.3 years, 10m / 5f |
Overground: NW Task length: 10–30s |
Cognitive/motor DT: walk while completing a secondary smartphone game task | fNIRS WOT NIRS (5Hz) LASER Light Interoptode distance: 3cm 16 channels: PFC |
Change in oxygenated and de-oxygenated haemoglobin (Only oxygenated haemoglobin analysed) |
• Moving average filter to remove artefacts; time window 5s • Signals band-pass filtered with cut-off frequency of 0.01–0.5Hz • Blocks averaged to minimise physiological noise Baseline condition: standing |
| Verghese et al., 2017 | 166 OA – aged 75.0 ± 6.1 years, 81m / 85f | Overground: NW Task length: 3 × 14ft loop |
Cognitive DT: walk while repeating alternating letters of the alphabet | fNIRS fNIRS Imager 1000 (2Hz) LED Light Interoptode distance: 2.5cm 16 channels: PFC |
Change in oxygenated haemoglobin | • Signals low-pass filtered with cut-off frequency of 0.14Hz Baseline condition: standing while counting 10s |
| Wang et al. 2016 | 22 YA – aged 24.4 ± 1.6 years, 13m / 9f 39 OA – aged 70.5 ± 7.7 years, 25m / 14f |
Standing: NS Task length: 10min |
fNIRS TH200 & OXYMON MK III (10Hz) LED Light Interoptode distance: 3.5cm 10 Channels: PFC, SMC |
Functional connectivity of cortical regions by wavelet phase coherence of change in oxygenated haemoglobin | • Signals band-pass filtered with cut-off frequency of 0.005–2Hz • Moving average filter to remove artefacts • Wavelet transformed Baseline condition: sitting 20min |
|
| EEG | ||||||
| Chang et al. 2016 | 31 OA 15 low risk fallers – aged 68.4 ± 2.6 years, 5m / 10f16 high risk fallers – aged 70.2 ± 2.2 years, 7m / 9f | Standing; continuous perturbations (floor translation) with and without virtual reality Task length: 0.5s blocks |
EEG Quik-Cap (1000Hz) 32 channels: only Fz, Cz, Pz, Oz analysed. Reference channels: A1, A2, forehead |
Power spectral density: Theta (4–7Hz) Alpha (8–12Hz) Beta (12–30Hz) Gamma (30–40Hz) |
• Signals band-pass filtered with cut-off frequency of 0.1–0.5Hz; Welch method in 128 windows with 50 overlaps | |
| Fujiwara et al. 2012 | 13 YA – aged 22.2 ± 4.8 years, 7m / 6f 12 OA – aged 65.5 ± 3.6 years, 6m / 6f |
Standing: perturbation (floor translation) Task length: 3s blocks |
EEG Ag-AgCL cup electrodes (1000Hz) 3 channels: Cz, Fz, Pz. Reference channel: Fpz and forehead. |
Contingent negative variation (CNV) obtained by averaging EEGs at perturbation warning and response time points and tracking signal between points (Cz only) | • Signals high-pass filtered with cut-off frequency of 40Hz • Trials with eye blinks or movement artefacts removed; voltage exceeding ± 100 µV |
|
| Handojoseno et al. 2015 | 16 PD with FoG – aged 64.0 ± 7.3 years, H&Y 2.3 ± 0.7, UPDRS III; 40.1 ± 12.2 | Overground: Timed-up-and-go task (over 5m) Task length: 1–2s |
EEG Gold cup electrodes (500Hz) 4 channels: SMA (Fz), precentral gyrus (Cz), parieto-occipital junction (P4), occipital cortex (O1). Reference channels: T3, T4, TCz |
Power spectral density: Delta (1–4Hz) Theta (4–8Hz) Alpha (8–13Hz) Beta (13–30Hz) Gamma (30–60Hz) |
• Signals amplified with common rejection ration >95 dB • Signals band-pass filtered with cut-off frequency of 0.15–100Hz • Normalised power spectrum as percentage of total power in frequency window of 0.5–60Hz |
|
| Huang et al. 2017 | 12 YA - aged 25.3 ± 1.3 years, 7m / 5f 12 OA - aged 65.8 ± 1.0 years, 7m / 5f |
Standing: NS, stabilometer force-matching task Task length: 80s |
EEG Ag-AgCl electrodes (1000Hz) 30 channels: frontal (Fp1, Fp2, Fz, F3, F4, F7, F8), sensorimotor (Cz, C3, C4, CPz, CP3, CP4) and parietal-occipital (PC3, PC4, Pz, P3, P4, Oz, O1, O2). Others: FCz, FC3, FC4, T3, T4, T5, T6, TP7, TP8. Reference channel: Fz and forehead |
Event-related potential (ERP) - Component amplitudes - Functional connectivity |
• Regression analysis removed eye movement and blink artefacts • Signals low-pass filtered with cut-off frequency of 40Hz / 48 dB roll-off • Segmented into 700ms epochs |
|
| Maekawa et al. 2013 | 15 OA – aged 71.2 ± 6.0 years, 7m / 8f | Standing: perturbation (floor transition) Task length: 10s |
EEG Ag-AgCL cup electrodes (1000Hz) 3 channels: Cz, Fz, Pz. Reference channel: Fpz and forehead. |
Contingent negative variation (CNV) obtained by averaging EEGs at perturbation warning and response time points and tracking signal between points (Cz only) | • Signals band-pass filtered with cut-off frequency of 0.05–100Hz | |
| Malcolm et al. 2015 | 17 YA – age = 27.2 ± 4.6 years, 9m / 8f 16 OA - age = 63.9 ± 4.0 years, 7m / 9f |
Treadmill: NW Task length: 4min |
Cognitive DT: walk while completing a secondary visual Go/No-Go task | EEG BioSemi ActiveTwo (512Hz) 72 channels: Fp1, AF7, AF3, F1, F3, F5, F7, FT7, FC3, FC5, FC1, C1, C3, C5, T7, TP7, CP5, CP3, CP1, P1, P3, P5, P7, P9, PO7, PO3, O1, Iz, Oz, POz, CPz, FPz, FP2, AF8, AF4, AFz, Fz, F2, F4, F6, F8, FT8, FC6, FC4, FC2, FCz, Cz, C2, C4, C6, T8, TP8, CP6, CP4, CP2, P2, P4, P6, P8, P10, PO8, PO4, O2 |
Event-related potential (ERP) (FCz, Cz, Pz only) | • Online signals band-pass filtered with cut-off frequency of 0.15–100Hz (24 dB/octave) • Offline signals band-pass filtered with cut-off frequency of 1–30Hz • Eye movement and muscle noise removed with ± 75 µV threshold • Nearest neighbour spline correction for interpolation of trials with <6 bad channels • >6 bad channel trials excluded • Re-referenced to average reference • Epochs of 800ms post-stimulus and 50ms pre-stimulus |
| Marcar et al. 2014 | 2 YA – both aged 26 years 2 OA – aged 71 and 78 years |
Overground: NW Task length: 50 steps forward |
Motor DT: walk with secondary tasks of; 1) Carrying an empty water glass 2) Carrying a full water glass 3) Repeatedly tapping finger against thumb Cognitive DT: walk while serially subtracting 3s |
EEG ActiCap32 (250Hz) 32 channels: frontal cortex (Fp1, Fp2, F7, F3, Fz, F4 & F8), central cortex (C3, Cz & C4), parietal cortex (P7, P3, Pz, P4 & P8), occipital cortex (PO9, O1, Oz, O2 & PO10) Reference channels: Fz and FCz. |
Power spectral density: Alpha Beta Gamma |
• Signals band-pass filtered with cut-off frequency of 0.5–80Hz (24–48 dB/octave) • Eye blink and movement artefact removed with independent component analysis and Analyzer 2.04 software |
| Ozdemir et al. 2016 | 10 YA - aged 26.20 ± 2.77 years 6m / 4f 9 OA - aged 81.42 ± 6.30 years, 3m / 6f |
Standing: NS, sway platform Task length: 30s |
Cognitive DT: walk with secondary N-back task (set task difficulty to 2 stimuli back) |
EEG ActiCap system (1000Hz) 64 Channels: frontal (F3, F1, Fz, F2, F4), central-frontal (FC5, FC3, FC1, FC2, FC4, FC6), central (C3, C1, Cz, C2, C4), central-parietal (CP3, CP1, CPz, CP2, CP4) and parietal (P3, P1, Pz, P2, P4) cortices. |
Power spectral density: Delta (1–4Hz) Theta (4–7Hz) Alpha (8–12Hz) Beta (14–24Hz) Gamma (30–50Hz) |
• Signals band-pass filtered with cut-off frequency of 0.1–50Hz • Subtracted mean and divided by the standard deviation of the signal |
| Shine et al. 2014 | 24 PD with FoG (“OFF” medication) – aged 69.0 ± 8.4, H&Y 2.7 ± 0.5, UPDRS III 40.2 ± 11.1, OFF medication | Overground: Timed-up-and-go task (over 5m) Task length: 1–2s |
EEG Gold cup electrodes (500Hz) 4 channels: SMA (Fz), precentral gyrus (Cz), parieto-occipital junction (P4), occipital cortex (O1). Reference channels: T3, T4, TCz |
Power spectral density: Alpha (8–13Hz) Beta (13–30Hz) Theta (4–8Hz) Delta (0.5–4Hz) Cross talk; Cross spectrum analysis Cross frequency power ratio: Delta:Beta, Theta:Beta |
• Signals amplified with common rejection ration >95 dB • Signals band-pass filtered with cut-off frequency of 0.15–100Hz |
|
| Shoushtarian et al 2011 | 11 PD no gait initiation difficulties – aged 64.1 (7.8) years, 7m / 4f, UPDRS III 28.8 (9.7), H&Y 2.2 (0.9), disease duration 7.4 (3.6) years, OFF medication 9 PD with gait initiation difficulties – aged 67.2 (6.3), 8m / 1f, UPDRS III 28.2 (14.3), H&Y 1.9 (0.6), disease duration 7.7 (4.4) years, OFF medication 12 YA – aged 25.5 ± 7.4 years 8 OA – aged 62.6 (9.2) years |
Over-ground: gait initiation task Task length: 3 steps forward |
EEG Quick-cap NuAmps 40 electrode (1000Hz) 7 channels: Fz, FCz, C3, Cz, C4, CPz, Pz Reference channels: mastoids and EOG below left eye |
Movement related potentials (MRP) – Slope (early or late) – Peak amplitude |
• Signals band-pass filtered with cut-off frequency of 0.05–100Hz • Epochs 2s before and after task • Blinks removed using spatial filter in edit 4.4 software |
|
| Tilley et al. 2017 | 8 OA – aged 75.8 ± 6.8 years | Overground: NW (outside) Task length: 15min |
EEG Emotive EPOC+ (1024Hz) 16 Channels: AF3, AF4, F3, F4, F7, F8, FC5, FC6, T7, T8, P7, P8, O1, and O2. Reference channels P3 and P4 |
Affective Suite outputs: excitement, engagement, frustration | • NR |
[All data entered as fully as possible from the reviewed studies. NR: Not Reported, PD: Parkinson’s disease, OA: older adult, H&Y: Hoehn and Yahr, NGA, neurological gait abnormalities, NW: normal walking, NS: normal (quiet) standing, DT: dual-task. Cortical areas – M1, primary motor cortex; PFC, prefrontal cortex; PMC, premotor cortex; SAC, sensory association cortex; SMA, supplementary motor area; SMC, sensorimotor cortex; SPL, superior parietal lobule; S1, primary somatosensory cortex]
Table 3.
Key findings and results interpretation extracted from studies
| Author | Key findings and interpretation | Associative findings: cognitive or behavioural |
|---|---|---|
| fNIRS | ||
| Beurskens et al., 2014 | • Decreased PFC activation in OA with DT walking compared to NW. Interpreted as a potential shift of resources to other brain regions with a DT. |
• NR |
| Chaparro et al. 2017 | • Increased PFC activity in OA with DT or partial body weight support walking compared to NW. Interpreted as greater attentional demands being required while dual-tasking in OA. |
• NR |
| Chen et al. 2017 | • Increased PFC activity in OA with DT walking compared to NW. Interpreted as compensatory reallocation of resources during complex tasks (DT and obstacle negotiation) required greater cortical activation than NW, particularly in those with mobility issues. |
• Slow gait related to increased PFC activity when negotiating obstacles under NW and DT conditions. |
| Clark et al., 2014a | • Decreased PFC activity with textured insoles or no shoes compared to with shoes. Interpreted as enhanced somatosensory input reduced PFC activity during gait in OA; more automatic processing. |
• NR |
| Clark et al., 2014b | • Increased PFC during standing phase. Little PFC activity change with DT compared to NW. Interpreted as availability and use of neural resources important for optimised gait during DTs in OA. |
• Larger increase in PFC activity during walks with DTs was linked to smaller declines in gait speed and to decreases, rather than increases, in step length variability. |
| Doi et al. 2013 | • Increased PFC activity with DT walking in OA with mild cognitive impairment Interpreted as DT walking requires PFC activation and confirm the clinical relevance of DT walking. |
• PFC activity positively correlated with executive function. |
| Eggenberger et al., 2016 | • Reduced PFC activation during NW in OA following exercise intervention compared to baseline. Interpreted as reduction in PFC activation freeing attentional resources to be applied to gait or other processes during NW, which could improve mobility and reduce falls. |
• NR |
| Fraser et al., 2016 | • Increased PFC activation in YA and OA with DT walking compared to NW, no group difference. Interpreted as similar PFC response to DTs in YA and OA. |
• NR |
| Harada et al., 2009 | • Increased PFC and SMA activity during walking at fast speeds in OA compared to XX. Interpreted as PFC, SMA and SMC control gait speed in OA, with PFC contribution depending on age-related decline in gait. |
• Greater PFC activation in those with worst gait in OA. • Lower SMA activation in those with worse gait in OA. • SMC and SMA activation correlated with gait speed. |
| Hernandez et al., 2016 | • Increased PFC activation with DT walking compared to NW in OA. Interpreted as compensating for difficulties with divided-attention walking by increasing PFC activity. |
• NR |
| Holtzer et al., 2011 | • Increased PFC activity with DT walking compared to NW, with greater activation in YA compared to OA. Interpreted as underutilisation of the PFC in OA compared to YA during walking with attention-demanding conditions. |
• NR |
| Holtzer et al., 2015 | • Increased PFC activation during NW compared to standing and counting • Increased PFC activation during DT walking compared to NW in OA. • Increased PFC activation was maintained for several trials during DT, whereas during NW activation attenuated after two trials. Interpreted as identifying online brain mechanisms underpinning walking in OA that may help to create assessment or intervention for mobility deficits. |
• Increased activation in PFC related to gait and DT performance. |
| Holtzer et al., 2016 | • Increased PFC activity during DT walking compared to NW in OA (all groups). • Central NGA related to attenuated changes in PFC activity during DT compared to NW. • Greater PFC activity in peripheral NGA during DT compared to NW. Interpreted as compensatory function of the PFC to support gait within OA with NGA. |
• Increased PFC activity related to slower gait velocity in OA. • Increased PFC activity during DT in Central NGA compared to a seated cognitive task. • Greater PFC activity in peripheral NGA during DT related to faster gait speed. |
| Holtzer et al., 2017a | • Increased PFC activity with DT walking compared to NW in OA. Interpreted as stress in older men influenced PFC activity levels during walking, which may impact falls risk. |
• Increased PFC activity related to gender and stress levels in OA. |
| Holtzer et al., 2017b | • Increased PFC activity with DT compared to NW in OA. Interpreted as attention-demanding walking requiring PFC activity that is susceptible to fatigue in OA. |
• Increased PFC activity related to fatigue that worsened with repeated trials in OA. |
| Mahoney et al. 2016 | • Increased PFC activity in PS and MPS compared to OA during NS with counting. • Activation patterns were similar within all groups. Interpreted as the PFC having an important role in balance control in PD participants, which could help to refine interventions. |
• NR |
| Maidan et al., 2015 | • Increased PFC activity only in those PD participants who experienced a FoG episode during anticipated turns, but not unanticipated turns. • Other PD participants decreased in PFC activity when turning. • No change in PFC activity with turning in OA. Interpreted as support for association between FoG episodes and PFC activity in PD, with links between motor planning, information processing and FoG. Alterations in executive control with PD may lead to FoG. |
• NR |
| Maidan et al., 2016 | • Increased PFC activation during NW in PD compared to OA. • Increased PFC activity during DT compared to NW in OA, but not PD. • Increased PFC activity during obstacle crossing in PD and less so in OA. Interpreted as higher activation during NW suggests that the PFC already plays an important role in gait. The activation beyond NW depends on the nature of the task. |
• NR |
| Maiden et al. 2017 | • Increased PFC activation with NW, but decreased activity with turning in PD. Interpreted as compensatory attempt to improve performance in those with worst ambulation by increasing PFC activity. |
• PD participants with worst ambulation (<1m/sec speed) had greater activation of PFC during turning. |
| Mirelman et al. 2017 | • Increased PFC activation with NW compared to standing in OA. • Both YA and OA increased PFC activity during DT and obstacle crossing. • Lower PFC activation across all walking conditions in YA compared to OA. Interpreted as PFC activation with walking being dependant on age and task, with more cognitive resources required by OA even with NW. |
• NR |
| Nieuwhof et al., 2016 | • Increased PFC activation in PD with DTs compared to rest, but little difference between DTs. • No change in HHb concentrations. Interpreted as the different DTs may not have differed in terms of difficulty which meant that PFC activation was not different between the tasks in PD. |
• NR |
| Osofundiya et al., 2016 | • Increased PFC activity with DT and precision stepping compared to NW and sitting in all of the OAs. • Greater PFC activation associated with obesity. Interpreted as DT and precision stepping have a higher neural costs in OA. |
• NR |
| Rosso et al. 2017 | • Increased PFC and temporal area activation during NS and DT in OA compared to YA. • When controlling for NS activation both groups had a reduction in activation with DT, with greater activation for the postural task compared to the cognitive task alone. Interpreted as neural resources required for balance are reduced under DT conditions. |
• NR |
| Takeuchi et al. 2016 | • No difference in PFC activation during DT between YA and OA. Interpreted as PFC activation lateralisation of motor and cognitive aspects aids in efficient task performance in YA and OA. |
• PFC activation related to selective aspects of gait and the DT in YA and OA, which differed between groups. |
| Verghese et al., 2017 | • Increased PFC activation during DT compared to NW in OA. Interpreted as neurobiological processes being implicated in early pathogenesis of falls in OA. |
• Increased PFC activation during DT predicted falls in OA, but NW velocity and letter rate did not. |
| Wang et al. 2016 | • Higher global, SMC and PFC connectivity in NS in OA compared to YA. • Functional connectivity of PFC, and SMC decreased in NS compared to sitting in OA, but not YA. Interpreted as differences in brain activity modes is required as a defence mechanisms during postural control that may reduce postural instability in OA. |
• NR |
| EEG | ||
| Chang et al. 2016 | • Cortical activity increased for balance control in OA with reduced visual and somatosensory input. • Gamma and Beta bands in parietal-occipital region facilitate cortical modulation and sensorimotor integration. • Theta band in frontal-central region involved in error detection during perception • Alpha band in occipital lobe processes visual challenges Interpreted as inefficient central modulation in OA during balance tasks may contribute to falls. |
• NR |
| Fujiwara et al. 2012 | • Late CNV amplitude negatively increased and peaked just before response in YA. • Late CNV amplitude had no negative increase in OA and peaked in mid-point between warning and response. • No adaptive changes found in OA in late CNV. Interpreted as reduced activation of the frontal lobe may contribute to decreased balance adaptability in OA. Various brain regions may be used by OA to adapt dynamic balance response compared to YA. |
• NR |
| Handojoseno et al. 2015 | • Increased theta oscillations in cortex during transition to FoG which remained high over central region (SMA and SMC) during FoG. • Increased beta synchronisation prior to FoG. • Coherence in gamma band at pairwise O1-Cz and Cz-Fz during FoG. Interpreted as a combination of different aspects of EEG signals can classify FoG episodes in PD. |
• NR |
| Huang et al. 2017 | • Strength of functional connectivity increased in whole brain and fronto-sensorimotor network in the balance condition in YA and OA. • Greater synchronised likelihood of ERPs in the fronto-sensorimotor network in OA than YA in both stance conditions. • OA did not deactivate functional connectivity of temporal-parietal-occipital network with increasing balance load, unlike YA. • Functional connectivity of the PFC was maintained in OA with balance load. Interpreted as OA still capable of increasing allocation of neural resources for balance control especially at PFC and under DT. |
• NR |
| Maekawa et al. 2013 | • Late CNV was recognised by the 3rd or 4th perturbation, occurring ~1000ms after the warning and peaked ~300ms before response. Interpreted as repeated perturbations can improve frontal lobe function that improves balance in OA. |
• Balance performance and muscle activity related to CNV peak timing. |
| Malcolm et al. 2015 | • ERP modulations within processing motor stream were at later stages (increased P3 amplitude) of inhibitory network while walking. • Frontally distributed P3 topography in OA, increased from sitting to NW, and then DT. • Minimal variation in P3 topography throughout trials in OA but more dynamic in YA. Interpreted as delay and attenuation of ERP modulations in OA indicating age-related loss of flexible resource allocation during DT walking |
• NR |
| Marcar et al. 2014 | • Decreased gamma band activity (power) over frontal cortex in OA with motor and cognitive DTs compared to NW, whereas the opposite occurred in YA. • One motor DT increased gamma band activity over the frontal cortex in OA compared to NW, but decreased activity in YA. Interpreted as increased activity of the frontal cortex in OA is required for one motor DT due to them finding that task more difficult and the task requiring more processing. |
• NR |
| Ozdemir et al. 2016 | • Less theta oscillations in OA during DT compared to YA. • Gamma oscillations over the central and central-parietal cortices increased during the dual task, sway platform balance task in OA. Interpreted as increased allocation of attentional resources being required for postural control in OA. |
• NR |
| Shine et al. 2014 | • Increased theta band within central and frontal channels associated with FoG episodes, compared to NW. • Increased theta frequency coupling between central and frontal channels was associated with FoG episodes compared to NW. • Increased theta activity coupled with significant alpha activity in the parietal and frontal channels with FOG episode compared to NW. • Increased cross-frequency coupling in central channel associated with FoG episodes compared to NW. Interpreted as abnormal oscillations in the brain are associated with FoG episodes in PD. |
• NR |
| Shoushtarian et al 2011 | • No difference in MRP between the two PD groups • Reduced activation at Cz in PD and OA compared to YA Interpreted as gait generation is related to cortical disturbances in PD. |
• Reduced stride length related to greater MRP within the early phase in PD with gait initiation difficulties |
| Tilley et al. 2017 | • Increased cortical activity during NW in OA. Interpreted as open outdoor space related to positive brain signals, whereas stress built up in urban built up areas in OA. |
• Increased cortical activity related to the qualitative themes of their walk and environmental factors. |
[NR: Not reported, PD: Parkinson’s disease, OA: older adult, NGA, neurological gait abnormalities, YA: Young Adult, NW: normal (comfortable speed) walking, NS: normal (quiet) standing, DT: dual-task. Cortical areas – M1, primary motor cortex; PFC, prefrontal cortex; PMC, premotor cortex; SAC, sensory association cortex; SMA, supplementary motor area; SMC, sensorimotor cortex; SPL, superior parietal lobule; S1, primary somatosensory cortex]
3. Results
3.1. The evidence base
The initial search yielded 556 articles following duplicate exclusion (Figure 2) [29]. Initial title and abstract screening resulted in 56 articles of interest of which 37 were identified for inclusion by three reviewers (SS, RV, RM), with consensus of article inclusions following consultation with the other adjudicating reviewers (DM, PF, MM). Reasons for exclusion of studies are included as Supplementary Material 1.
3.2. Participants
The reviewed articles (n=37) investigated older adults and people with PD with an average age of 66.5 years and 78.2 years, respectively. Both male and female participants were recruited to the majority of studies, although several did not report gender characteristics (Table 2). Generally, PD participants were tested “ON” their parkinsonian medications, although some studies did not report medication status [30, 31]. Fewer studies examined PD compared to older adult subjects.
3.3. Study protocols
3.3.1. Tasks
Table 2 demonstrates that there was little consensus regarding the walking and balance tasks used within the reviewed studies. Studies primarily involved assessment of cortical activity during walking (normal walking; n=8 treadmill, n=22 over-ground) with fewer studies examining balance tasks (n=8 quiet standing, n=4 with floor translation perturbations). The most common manipulation was a dual-task (cognitive or motor) (n=23), with other complex walking tasks also used such as different walking speeds (n=2), obstacle crossing/precision stepping (n=5) or turning tasks (n=2). Cognitive dual-tasks consisted of a variety of speaking tasks, with reciting alternative letters of the alphabet most used (n=10). Three studies used motor dual-tasks that involved carrying items or pressing buttons.
3.3.2. Cortical activity monitoring instruments
Table 2 shows that within the reviewed articles various fNIRS (n=26 studies) [12, 31–56] and EEG (n=11 studies) [30, 57–66] instruments were used, which differed in terms of type/model, sampling frequency and number of channels. Different models of fNIRS devices emitted either LED or laser light, with inter-optode distances that ranged from 2.2cm to 4cm. Similarly, different fNIRS and EEG devices used a range of materials to hold channels in place, such as semi-rigid plastic forms or neoprene head-bands/caps. The sampling frequency of the fNIRS devices varied from 1–10Hz, whereas EEG systems had frequencies that varied from 250–1024Hz. The number of channels also varied considerably between studies, with fNIRS systems ranging from 2–16 channels and EEG systems having a larger range of 3–72 channels.
3.4. Regions of interest
All fNIRS studies examined the PFC, with only two studies also examining other ROIs (e.g. SMA, PMC, SMC). The EEG studies reported data from a range of different ROIs. For example, frontal, parietal, occipital, central and sensorimotor cortices, although not all of the reviewed studies described their results in terms of ROIs (i.e. just reported channels) and several studies combined ROIs (Table 2). Studies also varied in terms of the number of channels devoted to each ROI, for example one study [59] used multiple channels to denote ROIs whereas others used a single channel [30]. Several studies also used the same EEG channels but attributed recordings to different ROIs, e.g. Cz channel was used to report SMC, frontal lobe, central cortex and precentral gyrus.
3.5. Cortical activity pre-processing and outcome measures
Cortical activity pre-processing and outcome reporting was inconsistent (Table 2). Pre-processing for fNIRS involved visual signal inspection, filtering (low or band-pass) with de-trending or moving averages, and averaging across trial blocks. In contrast to previous research, none of the reviewed fNIRS studies used reference channels (inter-optode distance <1.5cm) to account for peripheral tissue signals.
Twenty-three fNIRS studies reported oxygenated hemoglobin relative to static baseline conditions (2s to 20min duration), with three studies using dynamic baselines and few reporting deoxygenated hemoglobin or absolute values (i.e. total hemoglobin or total oxygenation index) (Table 2). One fNIRS study reported functional connectivity of ROIs [56]. Outcomes were primarily calculated from averages over task blocks (1s to 2min duration), which led to primarily intermittent walking or standing bouts being studied.
Table 3 shows that the pre-processing for EEG involved are range of techniques. For example, filtering (cut-offs ranged from 0.05 to 100Hz), regression, independent component analysis (ICA), voltage threshold cut-offs (e.g. ± 75 µV) or spatial filtering to remove blinks or movement artefacts and creating timing epochs for trial periods (e.g. 2sec windows). EEG studies used reference channels at the forehead, around the eye and peripheral regions to remove muscle, eye movement or other physiological signals.
Five of EEG studies reported power spectrum densities (PSD), with few studies reporting cross talk variables, functional connectivity, component amplitudes, event or movement-related potentials (MRP) or contingent negative variation (Table 2). One EEG study used the manufacturer software to obtain generic variables of “excitement”, “engagement” and “frustration” [66]. PSD bandwidths for Alpha, Beta and Gamma were most commonly used (n=5 studies), with theta and delta reported in only three studies. However, between studies bandwidth thresholds varied (i.e. Alpha=8–13Hz [64] vs Alpha=8–12Hz [63]).
3.6. Interpretation of cortical activity outcome measures
Table 3 and Figure 3 detail influence of age and PD on cortical activity outcomes during walking and balance tasks. Control groups, when included, were young adults or otherwise healthy elderly.
Figure 3.
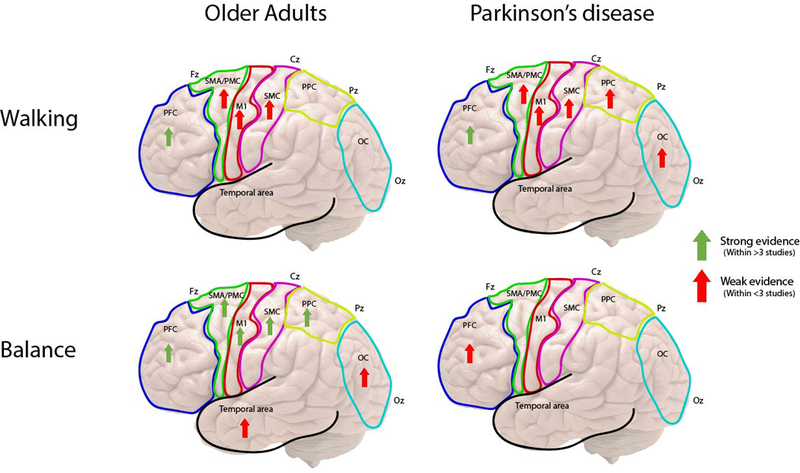
Overview of cortical activation recorded from fNIRS and EEG during walking and balance tasks in older adults and Parkinson’s disease. [Arrows represent findings of increased cortical activity during the separate tasks within specific brain regions]
3.6.1. Walking tasks
Walking at comfortable speed generally increased cortical activity in both older adults and PD compared to control groups (young or older adults, respectively) or baseline measures (standing or sitting), with greatest activation in PD. Specifically, walking led to greater activation of frontal ROIs or networks [61, 66], as well as the PFC [40, 42–44, 47, 48, 50], SMA, PMC and SMC [40]. Although, after initial task exposure, activation levels decreased and tended to return to the baseline in older adults [43]. However, two reviewed studies reported that PFC activation was not increased during walking at comfortable speed in older adults [32, 42] compared to baseline (sitting/standing), and another study demonstrated reduced cortical activity in older adults and PD within the first three steps of walking [65] compared to baseline (standing). In addition, only one study also showed that, following an exercise intervention, PFC activity during walking reduced in older adults compared to before exercise [38].
Dual-task walking generally increased PFC activation compared to baseline (standing or sitting) and single-task walking in both older adults and PD, with greater activation in PD (Table 3). However, three studies reported no increase in cortical activity in older adults when walking under dual-task conditions compared to single-task [32, 49, 54]. Increased PFC activity with dual-task also predicted falls in older adults [55].
During complex walking conditions, such as obstacle crossing, turning, precision stepping and timed-up-and-go (TUG), cortical activation appeared task-specific. Specifically, cortical activity increased in primarily frontal and central ROIs prior to freezing episodes compared to walking during TUG in people with PD [30, 64]. Turning had no effect of PFC activity in older adults, but reduced PFC activity in PD and increased activity in those who experienced freezing during turns [47, 48]. Obstacle crossing and precision stepping increased PFC activity in older adults [34, 50, 52]. Cortical activity also increased during treadmill walking compared to over-ground walking [35].
3.6.2. Balance tasks
Quiet standing generally increased cortical activity in older adults and PD, shown by greater PFC activation compared to sitting [31] and greater functional connectivity of various ROIs compared to controls [56, 59]. However, one study also reported reduced functional connectivity in standing compared to sitting in older adults [56]. Dual-task standing further increased PFC and temporal cortex activity in older adults [53]. Balance under perturbation (floor translation) increased cortical activity compared to normal standing in older adults [60, 63], but activation reduced over time with further perturbation exposure [60].
4. Discussion
This structured review summarized current literature on cortical activity during walking and balance tasks in older adults and PD, with a focus on real-time recording of fNIRS and EEG signals. The reviewed studies varied in terms of protocol, ROIs, outcomes and interpretation, with a lack of standardization throughout. Despite this, studies reported clinically relevant information regarding cortical mechanisms underlying mobility in older adults and PD.
4.1. Study protocols
Cortical activity results obtained from small cohorts that use various walking or balance tasks may not generalize to larger cohorts and limit the statistical power of the findings. This was evident within the reviewed studies, as only two PD-related studies had large sample sizes (n>30) and a range of different walking and balance tasks were used (i.e. treadmill or over-ground walking, timed-up–and-go, turning, gait initiation, perturbations etc.), which led to some inconsistent findings. For example, in opposition to larger studies that used simple over-ground continuous walking tasks, those that studied more complex intermittent walking tasks (i.e. treadmill walking or turning) with small sample sizes reported no change or decreased cortical activity [32, 49]. Larger cohort findings may be more robust, particularly for fNIRS results due to physiological variations between individuals [67], indicating that technology may be a limiting factor.
Most reviewed studies involved fNIRS with few using EEG, particularly with PD participants. A mobility-device trade-off appeared, whereby EEG was primarily used for static or controlled tasks (i.e. Timed-up-and-go or gait initiation) which is likely an attempt to avoid movement artefacts [68]. In contrast, fNIRS was used for freely moveable tasks (i.e. prolonged walking over-ground) as it is less prone to noise than EEG [69]. However, fNIRS has a mobility-resolution trade-off. Specifically, sampling frequencies are low and hemodynamic response can take 4–7 seconds [70], which makes real-time examination difficult. Alternatively, EEG provides high-resolution sampling frequency that can examine the activation at multiple brain regions in real-time. However, devices capable of recording with minimal movement artefact have yet to be implemented in older adult and PD research [71–73]. Overall, there is no reported ‘gold standard’ device for monitoring cortical activity during walking or balance tasks.
4.2. Regions of interest
Various ROIs were investigated within older adult and PD participants during walking and balance tasks, which appeared to be based more upon technological limitations rather than task-specific regions. For example, the majority of the fNIRS studies examined the PFC, which is likely due to the headband nature of the devices not allowing examination of other ROIs and problems with wireless streaming of multiple channels from a single device. This limitation was further evident through one study using two fNIRS systems to collect from multiple ROIs [56]. Alternatively, EEG systems allowed multiple ROIs to be included. However, despite collecting data from a large array of EEG channels, studies tended to report data from few selected channels (n=3–4), thus limiting the interpretation of EEG results; some studies suggest a minimum of 35 channels is required for accurate mobile data reporting [74]. In addition, several studies examined data from the same channel (i.e. Cz) but attributed recordings to different ROIs, which further highlights the need for a standardized approach to ensure the correct interpretation of age-or PD-related deficits.
4.3. Pre-processing and Outcome measures
Currently there are no ‘gold standard’ cortical activity outcomes or pre-processing of the signals, which was evident from the wide range of reported metrics and signal processing techniques, particularly within EEG studies. Unstandardized reporting impacted EEG outcome thresholds that likely explain inconsistent findings during the same task in PD [30, 64]. Depending upon the outcome definition/threshold, valuable information may be discarded from, or irrelevant information included within, the analysis. Outcomes also did not appear to be reported in a task-specific manner, although this may be influenced by the software and analysis methods available. Although the majority of fNIRS studies reported the same outcome (HbO2), they also recorded other metrics (HHb, TOI etc.) but did not present these results, limiting the interpretation. Creating optimal strategies for reporting fNIRS and EEG outcomes is difficult with different instruments and methods [75]. Therefore, studies should emphatically report outcomes and explain reasons for thresholds or focusing on particular outcomes. For example, EEG gamma band analysis may be a focus due to links with attentional processing [76, 77].
4.4. Interpretation of outcomes
The reviewed studies primarily examined older adults, with few PD specific studies finding that both ageing and PD consistently influenced cortical activity during walking and balance tasks. Cortical activity tended to increase with walking and balance tasks in both groups compared to baseline conditions (sitting/standing) or controls, particularly within frontal regions and their projected networks (Figure 3). Greater cortical activity increases were required in PD, with attenuation of activation in older adults with repeated task exposure. Despite a lack of consistent secondary cognitive/motor task between studies, dual-tasks exacerbated the increase in cortical activation, particularly in PD, which was suggested to be due to reduced neural resource availability in PD. Several studies attributed increased activation as executive-attentional compensation for either age-or PD-related motor control deficits [41, 45, 48], which supports previous behavioral literature [15–17]. However, to establish this theory examination of relationships between cognitive functions and cortical activity are needed, as well as use of standardized non-spoken dual-task paradigms to avoid data collection issues [70]. Other studies have suggested that older adults may have reduced ability to selectively activate brain regions or mechanisms and integrate these with movement, which leads to over-recruitment of regions [78]. Overall, studies suggest that motor control becomes cortically mediated with age and further with PD pathology, which may be an attempt to bypass or compensate for dysfunctional sub-cortical automatic locomotor circuits.
Recruitment of cortical regions was also related to task demands, with specific walking tasks either increasing or decreasing activation of different ROIs. For example; turning had no effect and reduced PFC activity in older adults and PD, respectively. Whereas obstacle crossing or precision stepping increased PFC activity in older adults, and TUG or treadmill walking increased frontal and central cortical activity in PD. Correspondingly, complex motor tasks have been suggested to require cortical activation [79, 80] and animal models have demonstrated that activation depends on task specifics [81]. Within PD, activation also appeared to depend upon specific motor deficits. A fascinating intermittent phenomenon in PD is freezing of gait (FoG), which is a brief absence or reduction in walking that relates to mobility deficits and falls risk. Increased cortical activity was associated with FoG episodes during walking tasks, which supports theories regarding FoG being caused by increased cognitive task demand [82, 83], as well as bottleneck [84] and interference [85] theories of cognitive input into motor performance.
Unfortunately, few studies reported association between cortical outcomes and behavioral data (Table 3), which limits clinical interpretation. The few reported findings demonstrated that the ability to increase cortical activity related to reduced falls in older adults [55] and may improve with an exercise program [38], with direct clinical implication. Other studies highlighted that increased PFC activity related to worse gait (slower speed), gender, stress and fatigue in older adults [43, 44, 46]. Similarly, increased PFC activity during turning and greater MRP related to worse gait in PD [48, 65]. Balance performance also related to cortical timing metrics in older adults [60]. However, cautious application of these findings to clinical practice is required due to the methodological limitations discussed.
4.5. Clinical and Future Research Recommendations
Cortical activity appears to increase from baseline measures with walking and balance task performance in both older adults and PD, but more so in PD. This may represent cortical compensation for sub-cortical dysfunction with ageing and further with disease-pathology. Therefore, targeting cortical activation, particularly executive-attentional activity at the PFC, with interventions such as transcranial magnetic or direct current stimulation, pharma-logical or cueing strategies may help to alleviate the cortical burden of walking and balance tasks in PD. However, a lack of standardized approach to studying cortical activity during walking and balance tasks limits understanding and application to clinical practice. Therefore, we make the following recommendations for future research;
Use adequately powered sample sizes
Use task-appropriate instrumentation with an adequately justified sampling rate and number of channels for relevant outcomes
Provide detailed information on ROIs and justify the number and location of channels assigned to each ROI
Emphatically report all recorded cortical activity outcomes and provide outcome definitions along with a detailed report of data pre-processing
Use standard walking (ideally overground) or balance tasks that can be compared to previous literature
Use standard secondary tasks that do not interfere with cortical activity instrumentation measurement (i.e. no talking)
Routinely assess relationships between cortical activity and behavioral/cognitive outcomes
5. Conclusions
In conclusion, cortical activity during walking and balance tasks was shown to be sensitive to age and PD, with overall increased activation required for task performance in both groups, but more so in PD. However, current results are limited due to a lack of standardized approach, which future studies must address to ensure accurate and appropriate data interpretation.
Supplementary Material
Acknowledgements
The authors would like to thank Oregon Health and Science University for providing the facilities that allowed the search strategy for this review to be conducted.
Funding
This work has been supported by grants from the National Institute of Health via Career Development Award 5R00HD078492–04 (PI Mancini) and a PCO Pilot Grant Award (PI Mancini). This research was also supported in part by Sao Paulo Research Foundation (FAPESP, grant #2014/22308–0) as a postdoctoral fellowship to Rodrigo Vitorio.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Galna B, Lord S, Burn DJ, Rochester L, Progression of gait dysfunction in incident Parkinson’s disease: impact of medication and phenotype, Movement disorders : official journal of the Movement Disorder Society 30(3) (2015) 359–67. [DOI] [PubMed] [Google Scholar]
- [2].Schoneburg B, Mancini M, Horak F, Nutt JG, Framework for understanding balance dysfunction in Parkinson’s disease, Movement Disorders 28(11) (2013) 1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM, Gait and cognition: a complementary approach to understanding brain function and the risk of falling, Journal of the American Geriatrics Society 60(11) (2012) 2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB, Epidemiology of gait disorders in community-residing older adults, Journal of the American Geriatrics Society 54(2) (2006) 255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rubenstein LZ, Falls in older people: epidemiology, risk factors and strategies for prevention, Age and ageing 35 Suppl 2 (2006) ii37–ii41. [DOI] [PubMed] [Google Scholar]
- [6].Gazibara T, Pekmezovic T, Kisic-Tepavcevic D, Svetel M, Tomic A, Stankovic I, Kostic VS, Incidence and prediction of falls in Parkinson’s disease: a prospective cohort study, European journal of epidemiology 30(4) (2015) 349–352. [DOI] [PubMed] [Google Scholar]
- [7].Peterson DS, Horak FB, Neural Control of Walking in People with Parkinsonism, Physiology (Bethesda, Md.) 31(2) (2016) 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Takakusaki K, Neurophysiology of gait: from the spinal cord to the frontal lobe, Movement disorders : official journal of the Movement Disorder Society 28(11) (2013) 1483–91. [DOI] [PubMed] [Google Scholar]
- [9].Takakusaki K, Oohinata-Sugimoto J, Saitoh K, Habaguchi T, Role of basal ganglia–brainstem systems in the control of postural muscle tone and locomotion, Progress in brain research 143 (2004) 231–237. [DOI] [PubMed] [Google Scholar]
- [10].Wu T, Hallett M, Chan P, Motor automaticity in Parkinson’s disease, Neurobiology of disease 82 (2015) 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hanakawa T, Neuroimaging of standing and walking: special emphasis on Parkinsonian gait, Parkinsonism & Related Disorders 12 (2006) S70–S75. [Google Scholar]
- [12].Clark DJ, Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies, Frontiers in Human Neuroscience 9 (2015) 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu T, Hallett M, A functional MRI study of automatic movements in patients with Parkinson’s disease, Brain : a journal of neurology 128(Pt 10) (2005) 2250–9. [DOI] [PubMed] [Google Scholar]
- [14].Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJ, Albin RL, Müller ML, Gait speed in Parkinson disease correlates with cholinergic degeneration, Neurology 81(18) (2013) 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yogev-Seligmann G, Hausdorff JM, Giladi N, The role of executive function and attention in gait, Movement disorders : official journal of the Movement Disorder Society 23(3) (2008) 329–42; quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morris R, Lord S, Bunce J, Burn D, Rochester L, Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease, Neuroscience and biobehavioral reviews 64 (2016) 326–45. [DOI] [PubMed] [Google Scholar]
- [17].Kelly VE, Eusterbrock AJ, Shumway-Cook A, A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications, Parkinson’s disease 2012 (2012) 918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB , Motor Control and Aging: Links to Age-Related Brain Structural, Functional, and Biochemical Effects, Neuroscience and biobehavioral reviews 34(5) (2010) 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tian Q, Chastan N, Bair W-N, Resnick SM, Ferrucci L, Studenski SA, The brain map of gait variability in aging, cognitive impairment and dementia—A systematic review, Neuroscience & Biobehavioral Reviews 74(Part A) (2017) 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Agosta F, Canu E, Stefanova E, Sarro L, Tomić A, Špica V, Comi G, Kostić VS, Filippi M, Mild cognitive impairment in Parkinson’s disease is associated with a distributed pattern of brain white matter damage, Human brain mapping 35(5) (2014) 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sehm B, Taubert M, Conde V, Weise D, Classen J, Dukart J, Draganski B, Villringer A, Ragert P, Structural brain plasticity in Parkinson’s disease induced by balance training, Neurobiology of aging 35(1) (2014) 232–239. [DOI] [PubMed] [Google Scholar]
- [22].Rosenberg-Katz K, Herman T, Jacob Y, Kliper E, Giladi N, Hausdorff JM, Subcortical Volumes Differ in Parkinson’s Disease Motor Subtypes: New Insights into the Pathophysiology of Disparate Symptoms, Frontiers in human neuroscience 10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, De Jager P, Csiszar A, Laurienti PJ, Hausdorff JM, Chen WG, Ferrucci L, Rosano C, Studenski SA, Black SE, Lipsitz LA, Aging, the Central Nervous System, and Mobility in Older Adults: Neural Mechanisms of Mobility Impairment, The Journals of Gerontology: Series A 70(12) (2015) 1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM, Neuroimaging of mobility in aging: a targeted review, The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 69(11) (2014) 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K, Aging of human supraspinal locomotor and postural control in fMRI, Neurobiology of aging 33(6) (2012) 1073–84. [DOI] [PubMed] [Google Scholar]
- [26].Bakker M, De Lange FP, Helmich RC, Scheeringa R, Bloem BR, Toni I, Cerebral correlates of motor imagery of normal and precision gait, Neuroimage 41(3) (2008) 998–1010. [DOI] [PubMed] [Google Scholar]
- [27].Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L, Brain activity during walking: A systematic review, Neuroscience and biobehavioral reviews 57 (2015) 310–27. [DOI] [PubMed] [Google Scholar]
- [28].la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K, Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison, Neuroimage 50(4) (2010) 1589–98. [DOI] [PubMed] [Google Scholar]
- [29].Moher D, Liberati A, Tetzlaff J, Altman DG, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS medicine 6(7) (2009) e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Handojoseno AM, Shine JM, Nguyen TN, Tran Y, Lewis SJG, Nguyen HT, Analysis and prediction of the freezing of gait using EEG brain dynamics, IEEE Transactions on Neural Systems and Rehabilitation Engineering 23(5) (2015) 887–896. [DOI] [PubMed] [Google Scholar]
- [31].Mahoney JR, Holtzer R, Izzetoglu M, Zemon V, Verghese J, Allali G, The role of prefrontal cortex during postural control in Parkinsonian syndromes a functional near-infrared spectroscopy study, Brain Research 1633 (2016) 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Beurskens R, Helmich I, Rein R, Bock O, Age-related changes in prefrontal activity during walking in dual-task situations: A fNIRS study, International Journal of Psychophysiology 92(3) (2014) 122–128. [DOI] [PubMed] [Google Scholar]
- [33].Chaparro G, Balto JM, Sandroff BM, Holtzer R, Izzetoglu M, Motl RW, Hernandez ME, Frontal brain activation changes due to dual-tasking under partial body weight support conditions in older adults with multiple sclerosis, Journal of NeuroEngineering and Rehabilitation 14(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen M, Pillemer S, England S, Izzetoglu M, Mahoney JR, Holtzer R, Neural correlates of obstacle negotiation in older adults: An fNIRS study, Gait & Posture 58 (2017) 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Clark DJ, Christou EA, Ring SA, Williamson JB, Doty L, Enhanced somatosensory feedback reduces prefrontal cortical activity during walking in older adults, Journals of Gerontology -Series A Biological Sciences and Medical Sciences 69(11) (2014) 1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Clark DJ, Rose DK, Ring SA, Porges EC, Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults, Frontiers in Aging Neuroscience 6(AUG) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Doi T, Makizako H, Shimada H, Park H, Tsutsumimoto K, Uemura K, Suzuki T, Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: A fNIRS study, Aging Clinical and Experimental Research 25(5) (2013) 539–544. [DOI] [PubMed] [Google Scholar]
- [38].Eggenberger P, Wolf M, Schumann M, de Bruin ED, Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults, Frontiers in Aging Neuroscience 8(APR) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fraser SA, Dupuy O, Pouliot P, Lesage F, Bherer L, Comparable cerebral oxygenation patterns in younger and older adults during dual-task walking with increasing load, Frontiers in Aging Neuroscience 8(OCT) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Harada T, Miyai I, Suzuki M, Kubota K, Gait capacity affects cortical activation patterns related to speed control in the elderly, Experimental Brain Research 193(3) (2009) 445–454. [DOI] [PubMed] [Google Scholar]
- [41].Hernandez ME, Holtzer R, Chaparro G, Jean K, Balto JM, Sandroff BM, Izzetoglu M, Motl RW, Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis, Journal of the Neurological Sciences 370 (2016) 277–283. [DOI] [PubMed] [Google Scholar]
- [42].Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J, fNIRS study of walking and walking while talking in young and old individuals, Journals of Gerontology -Series A Biological Sciences and Medical Sciences 66 A(8) (2011) 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J, Online fronto-cortical control of simple and attention-demanding locomotion in humans, NeuroImage 112 (2015) 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Holtzer R, Schoen C, Demetriou E, Mahoney JR, Izzetoglu M, Wang C, Verghese J, Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions, European Journal of Neuroscience 45(5) (2017) 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR, Neurological Gait Abnormalities Moderate the Functional Brain Signature of the Posture First Hypothesis, Brain Topography 29(2) (2016) 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Holtzer R, Yuan J, Verghese J, Mahoney JR, Izzetoglu M, Wang C, Interactions of Subjective and Objective Measures of Fatigue Defined in the Context of Brain Control of Locomotion, The journals of gerontology. Series A, Biological sciences and medical sciences 72(3) (2017) 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Maidan I, Bernad-Elazari H, Gazit E, Giladi N, Hausdorff JM, Mirelman A, Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures, Journal of Neurology 262(4) (2015) 899–908. [DOI] [PubMed] [Google Scholar]
- [48].Maidan I, Bernad-Elazari H, Giladi N, Hausdorff JM, Mirelman A, When is Higher Level Cognitive Control Needed for Locomotor Tasks Among Patients with Parkinson’s Disease?, Brain Topography (2017) 1–8. [DOI] [PubMed]
- [49].Maidan I, Nieuwhof F, Bernad-Elazari H, Reelick MF, Bloem BR, Giladi N, Deutsch JE, Hausdorff JM, Claassen JA, Mirelman A, The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson’s Disease and Healthy Older Adults: An fNIRS Study, Neurorehabilitation & Neural Repair 30(10) (2016) 963–971. [DOI] [PubMed] [Google Scholar]
- [50].Mirelman A, Maidan I, Bernad-Elazari H, Shustack S, Giladi N, Hausdorff JM, Effects of aging on prefrontal brain activation during challenging walking conditions, Brain and Cognition 115 (2017) 41–46. [DOI] [PubMed] [Google Scholar]
- [51].Nieuwhof F, Reelick MF, Maidan I, Mirelman A, Hausdorff JM, Rikkert MGO, Bloem BR, Muthalib M, Claassen JA, Measuring prefrontal cortical activity during dual task walking in patients with Parkinson’s disease: feasibility of using a new portable fNIRS device, Pilot and feasibility studies 2(1) (2016) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Osofundiya O, Benden ME, Dowdy D, Mehta RK, Obesity-specific neural cost of maintaining gait performance under complex conditions in community-dwelling older adults, Clinical Biomechanics 35 (2016) 42–48. [DOI] [PubMed] [Google Scholar]
- [53].Rosso AL, Cenciarini M, Sparto PJ, Loughlin PJ, Furman JM, Huppert TJ, Neuroimaging of an attention demanding dual-task during dynamic postural control, Gait and Posture 57 (2017) 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Takeuchi N, Mori T, Suzukamo Y, Tanaka N, Izumi SI, Parallel processing of cognitive and physical demands in left and right prefrontal cortices during smartphone use while walking, BMC Neuroscience 17(1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Verghese J, Wang C, Ayers E, Izzetoglu M, Holtzer R, Brain activation in high-functioning older adults and falls: Prospective cohort study, Neurology 88(2) (2017) 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang B, Zhang M, Bu L, Xu L, Wang W, Li Z, Posture-related changes in brain functional connectivity as assessed by wavelet phase coherence of NIRS signals in elderly subjects, Behavioural Brain Research 312 (2016) 238–245. [DOI] [PubMed] [Google Scholar]
- [57].Chang C-J, Yang T-F, Yang S-W, Chern J-S, Cortical Modulation of Motor Control Biofeedback among the Elderly with High Fall Risk during a Posture Perturbation Task with Augmented Reality, Frontiers in Aging Neuroscience 8 (2016) 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fujiwara K, Maekawa M, Kiyota N, Yaguchi C, Adaptation changes in dynamic postural control and contingent negative variation during backward disturbance by transient floor translation in the elderly, Journal of physiological anthropology 31 (2012) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Huang CY, Lin LL, Hwang IS, Age-related differences in reorganization of functional connectivity for a dual task with increasing postural destabilization, Frontiers in Aging Neuroscience 9(APR) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Maekawa M, Fujiwara K, Kiyota N, Yaguchi C, Adaptation changes in dynamic postural control and contingent negative variation during repeated transient forward translation in the elderly, Journal of Physiological Anthropology 32(1) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Malcolm BR, Foxe JJ, Butler JS, De Sanctis P, The aging brain shows less flexible reallocation of cognitive resources during dual-task walking: A mobile brain/body imaging (MoBI) study, NeuroImage 117 (2015) 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Marcar VL, Bridenbaugh SA, Kool J, Niedermann K, Kressig RW, A simple procedure to synchronize concurrent measurements of gait and brain electrical activity and preliminary results from a pilot measurement involving motor-cognitive dual-tasking in healthy older and young volunteers, Journal of Neuroscience Methods 228 (2014) 46–49. [DOI] [PubMed] [Google Scholar]
- [63].Ozdemir RA, Contreras-Vidal JL, Lee BC, Paloski WH, Cortical activity modulations underlying age-related performance differences during posture–cognition dual tasking, Experimental Brain Research 234(11) (2016) 3321–3334. [DOI] [PubMed] [Google Scholar]
- [64].Shine JM, Handojoseno AMA, Nguyen TN, Tran Y, Naismith SL, Nguyen H, Lewis SJG, Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in parkinson’s disease, Clinical Neurophysiology 125(3) (2014) 569–576. [DOI] [PubMed] [Google Scholar]
- [65].Shoushtarian M, Murphy A, Iansek R, Examination of central gait control mechanisms in Parkinson’s disease using movement-related potentials, Movement Disorders 26(13) (2011) 2347–53. [DOI] [PubMed] [Google Scholar]
- [66].Tilley S, Neale C, Patuano A, Cinderby S, Older people’s experiences of mobility and mood in an urban environment: A mixed methods approach using electroencephalography (EEG) and interviews, International Journal of Environmental Research and Public Health 14(2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Plichta MM, Herrmann MJ, Baehne CG, Ehlis AC, Richter MM, Pauli P, Fallgatter AJ, Event-related functional near-infrared spectroscopy (fNIRS): Are the measurements reliable?, NeuroImage 31(1) (2006) 116–124. [DOI] [PubMed] [Google Scholar]
- [68].Kline JE, Huang HJ, Snyder KL, Ferris DP, Isolating gait-related movement artifacts in electroencephalography during human walking, Journal of neural engineering 12(4) (2015) 046022–046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cooper RJ, Selb J, Gagnon L, Phillip D, Schytz HW, Iversen HK, Ashina M, Boas DA, A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy, Frontiers in neuroscience 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vitorio R, Stuart S, Rochester L, Alcock L, Pantall A, fNIRS response during walking -Artefact or cortical activity? A systematic review, Neuroscience and biobehavioral reviews 83 (2017) 160–172. [DOI] [PubMed] [Google Scholar]
- [71].Nathan K, Contreras-Vidal JL, Negligible Motion Artifacts in Scalp Electroencephalography (EEG) During Treadmill Walking, Frontiers in Human Neuroscience 9(708) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Melnik A, Legkov P, Izdebski K, Kärcher SM, Hairston WD, Ferris DP, König P, Systems, Subjects, Sessions: To What Extent Do These Factors Influence EEG Data?, Frontiers in Human Neuroscience 11(150) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Oliveira AS, Schlink BR, Hairston WD, Konig P, Ferris DP, Induction and separation of motion artifacts in EEG data using a mobile phantom head device, Journal of neural engineering 13(3) (2016) 036014. [DOI] [PubMed] [Google Scholar]
- [74].Lau TM, Gwin JT, Ferris DP, How Many Electrodes Are Really Needed for EEG-Based Mobile Brain Imaging?, Journal of Behavioral and Brain Science 2(3) (2012) 4. [Google Scholar]
- [75].Oliveira AS, Schlink BR, Hairston WD, König P, Ferris DP, Proposing Metrics for Benchmarking Novel EEG Technologies Towards Real-World Measurements, Frontiers in Human Neuroscience 10(188) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Slobounov S, Cao C, Jaiswal N, Newell KM, Neural basis of postural instability identified by VTC and EEG, Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale 199(1) (2009) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Slobounov S, Sebastianelli W, Moss R, Alteration of posture-related cortical potentials in mild traumatic brain injury, Neuroscience letters 383(3) (2005) 251–5. [DOI] [PubMed] [Google Scholar]
- [78].Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL, Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging, Neuron 33(5) (2002) 827–40. [DOI] [PubMed] [Google Scholar]
- [79].Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB, Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity, Journal of neurophysiology 93(3) (2005) 1209–1222. [DOI] [PubMed] [Google Scholar]
- [80].Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC, A Parametric Study of Prefrontal Cortex Involvement in Human Working Memory, NeuroImage 5(1) (1997) 49–62. [DOI] [PubMed] [Google Scholar]
- [81].Asaad WF, Rainer G, Miller EK, Task-specific neural activity in the primate prefrontal cortex, Journal of Neurophysiology 84(1) (2000) 451–459. [DOI] [PubMed] [Google Scholar]
- [82].Lewis SJG, Barker RA, A pathophysiological model of freezing of gait in Parkinson’s disease, Parkinsonism & Related Disorders 15(5) (2009) 333–338. [DOI] [PubMed] [Google Scholar]
- [83].Vandenbossche J, Deroost N, Soetens E, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E, Freezing of Gait in Parkinson Disease Is Associated With Impaired Conflict Resolution, Neurorehabilitation and Neural Repair 25(8) (2011) 765–773. [DOI] [PubMed] [Google Scholar]
- [84].Sigman M, Dehaene S, Dynamics of the central bottleneck: Dual-task and task uncertainty, PLoS biology 4(7) (2006) e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pashler H, Dual-task interference in simple tasks: data and theory, Psychological bulletin 116(2) (1994) 220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


