Abstract
Obesity is characterized by enhanced mineralocorticoid receptor (MR) activation, vascular stiffness and associated cardiovascular and kidney disease. Consumption of a western style diet (WD), high in saturated fat and refined carbohydrates, by female mice, leads to obesity and vascular stiffening. Use of endothelial cell-specific MR (ECMR) knockout mice support that ECMR activation is critical for development of vascular and cardiac fibrosis and stiffening. However, the role of ECMR activation in kidney inflammation and fibrosis remains unknown. We hypothesized that cell-specific deletion of ECMR would prevent WD-induced central aortic stiffness and protect the kidney from endothelial dysfunction and vascular stiffening. Four-week-old female ECMR KO and wild-type mice were fed either mouse chow or WD for 16 weeks. WD-feeding increased body weight and fat mass, proteinuria as well as vascular stiffness indices (pulse wave velocity and kidney artery stiffening) and impaired endothelial-dependent vasodilatation without blood pressure changes. The WD-induced kidney arterial stiffening was associated with attenuated endothelial NO synthase (NOS) activation, increased oxidative stress, pro-inflammatory immune responses, alterations in extracellular matrix degradation pathways and fibrosis. ECMR deletion prevented these abnormalities by improving endothelial NOS activation and reducing macrophage pro-inflammatory M1 polarization, expression of transglutaminase 2 and matrix metalloproteinase 9. Our data support the concept that ECMR activation contributes to endothelial dysfunction, increased kidney artery fibrosis/stiffening and impaired NOS activation, processes associated with macrophage infiltration and polarization, inflammation and oxidative stress, collectively resulting in tubulointerstitial fibrosis in females consuming a WD.
Keywords: western diet, eNOS, TG2, RECK, MMP9
Summary:
Activation of endothelial specific mineralocorticoid receptor promotes macro- and microvascular stiffening, enhanced kidney inflammation and oxidative stress and reductions in bioavailable NO associated with increased transglutaminase activation, decreased levels of protein RECK and enhanced macrophage pro-inflammatory response.
INTRODUCTION
Obesity is promoted by a western style diet (WD), high in saturated fat and refined carbohydrates,1,2 that is associated with vascular stiffening and the development of cardiovascular and kidney disease1–4. In this regard, kidney disease in obesity has been attributed to the impact that visceral adiposity has on physical compression of the kidney and/or alteration of glomerular hyperfiltration that leads to development of low-level albuminuria (e.g. microalbuminuria)1,4,5. However, recent population level data support the notion that obesity, independent of the presence of diabetes or hypertension, contributes to progressive kidney disease that extends beyond incident albuminuria, a process that is characterized by advancing tubulointerstitial fibrosis1,4–6. The mechanisms driving early tubulointerstitial fibrosis in obesity remain unknown.
Increases in body weight and the presence of kidney disease have been associated with vascular stiffness regardless of age and race, particularly in women7–9. In this regard, obesity is associated with increased central aortic stiffness in preclinical and clinical models10. The presence of central aortic stiffening results in propagation of an excessive pulsatile (kinetic) energy into peripheral organs, such as the kidney, where there is high flow but low precapillary resistance or impedance. Over time, this persistent excessive pulsatile wave force contributes to alterations in auto-regulatory mechanisms through chronic increases in myogenic tone and consequent wall remodeling and strain that eventually leads to perivascular and end-organ fibrosis8,11,12. In addition to central aortic stiffening, intrinsic kidney fibrosis also involves microvascular stiffness in the kidney,8,12–14 but the mechanisms involved in these processes are poorly understood.
The development of fibrosis in a target organ such as the kidney can be initiated by a number of mechanisms including; interstitial myofibroblast activation, epithelial cell and/or extracellular matrix stiffening15–17. Recent studies support a role for endothelial stiffness and impaired endothelial NO production in endothelium-mediated vascular and kidney inflammatory responses as well as the extracellular matrix remodeling that contribute to vascular and kidney fibrosis18–20. Our recent work indicates that stiffening initially occurs at the level of the endothelium and that these changes precede the development of perivascular fibrosis and myocardial tissue interstitial fibrosis.21–23 This process occurs more rapidly in females than males after consumption of a WD leading to obesity21–23. Recent work also supports an enhanced activation of cardiovascular endothelial cell-specific mineralocorticoid receptor (ECMR) signaling in female mice compared to males24–27. Further work in this area suggests that enhanced ECMR signaling promotes vascular endothelial cell stiffening, which leads to vascular fibrosis and increased central aortic stiffness25–26. How these changes in central aortic stiffness impact a target organ like the kidney is not clear.
In this context, MR antagonism is known to ameliorate kidney inflammatory responses and fibrosis;1,28 however, the precise mechanisms by which cell specific MR activation promotes kidney fibrosis in the setting of obesity are unknown. Therefore, we hypothesized that WD-induced obesity in females would promote kidney peri-arteriolar oxidant, inflammatory and fibrotic responses dependent on activation of the ECMR. To this end, we evaluated the role that alterations in endothelial NO synthase, vascular extracellular matrix metalloproteinases (MMPs) and cross-linking enzymes such as transglutaminase 2 (TG2) as well as the anti-fibrotic RECK protein had in regulating kidney fibrosis.
METHODS
Animals and Treatments
The data that support the findings in this study are available from the corresponding author on reasonable request. ECMR−/− (ECMR KO) mice were generated by crossing MRf/f mice with VE-Cad-Cre+ mice as previously described25,29. All procedures were approved in advance by the Institutional Animal Care and Use Committee of the University of Missouri and the Harry S. Truman Memorial Veterans Hospital Subcommittee for Animal Studies and mice were cared for according to National Institutes of Health guidelines. Groups of 4 week old female mice were fed a WD consisting of high-fat (46%) and a high-carbohydrate component as constituted with sucrose (17.5%) and high-fructose corn syrup (17.5%) and water for 16 weeks (supplemental Table S1)24–26. A parallel group of age-matched female controls (ECMR+/+) were fed regular mouse chow (CD) for the same period of time.
Body Composition, Blood Pressure and Proteinuria
After 16 weeks of feeding, mice underwent EchoMRI500 for quantitative magnetic resonance analysis24–25 of whole body fat mass and lean mass. Average systolic, diastolic, and pulse pressure (PP) were determined by catheterization of the right carotid artery under isoflurane anesthesia as previously described24,25. Blood samples were collected at the time of sacrifice, plasma was separated and stored at – 80 °C for further analysis. Urine samples collected into chilled 4°C tubes from mice placed in metabolic cages for 24 h, within one week of sacrifice, and were subsequently stored at −80 °C for further analysisof total protein and creatinine.
Vascular stiffness and reactivity
In vivo aortic stiffness as evaluated by pulse wave velocity was determined by Doppler ultrasound (Indus Mouse Doppler System, Webster, TX)24,26. EX vivo renal vascular stiffness was determined by evaluation of stiffness of endothelial cells within intact vessel explants measured at room temperature using a cell nano-indentation protocol with atomic force microscopy24. Mechanical and elastic characteristics of mesenteric arteries (e.g. second order vessels with 185-215 μm diameter) and reactivity studies were determined ex vivo as a surrogate for renal vessels as described26.
Immunoblotting and immunohistochemistry
Preparation of whole cell homogenates, immunoblotting, detection of the immuno-reactive bands by enhanced chemiluminescence, and quantification by densitometry were all described previously and are described in detail under supplemental information 24–26.
Immunohistochemistry:
Fluorescent and bright-field immunohistochemistry were used to quantify protein expression in the different components of the aorta in different groups as previously described24–26.
RNA extraction and quantitative real-time RT-PCR
Total RNA was isolated using the TRIzol reagent (Sigma) method as previously described26. First-strand cDNA synthesis was done using Improm II reverse transcription kit (Promega, Madison, WI). After the first strand synthesis, real-time PCR (qRT PCR) was done using a real-time PCR system (CFX96; Bio-Rad Laboratories). Primers used and conditions used for PCR are described under supplemental information.
Statistical analysis
Imaging and histological data were collected by genotype- and treatment-blinded investigators. Data are reported as means±SEM. Differences in outcomes were determined using ANOVA multiple comparison analysis and Gabriel Students-Newman-Keuls post-test and paired t tests (two groups) and were considered significant when P<0.05. All statistical analyses were performed using Sigma Plot (version 12) software (Systat Software, Point Richmond, CA).
Expanded methods and supplemental data in this study are available in the online-only Data Supplement
RESULTS
WD-induces proteinuria independent of blood pressure changes that is dependent on the ECMR
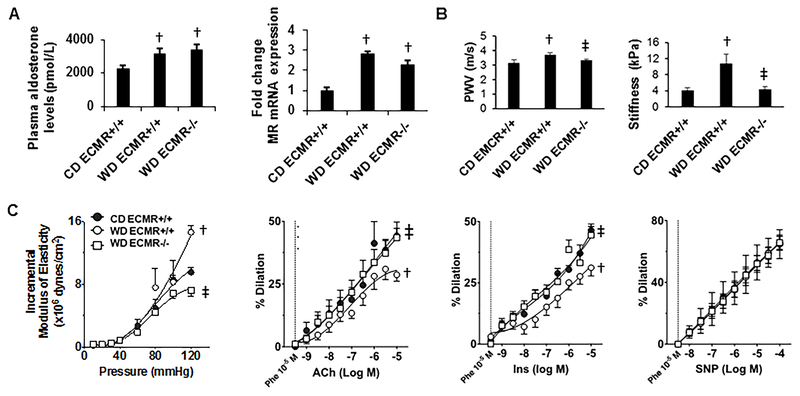
As we have previously reported, there were expected weight gains over 16 weeks of WD feeding (supplemental Table S2).21–24 Mice fed a WD had a 39% increase in body weight and a 220% increase in fat mass without change in lean body mass. In parallel with these changes, there were increases in proteinuria and fasting blood glucose but no elevations in blood pressure with WD feeding (supplemental Table S2). The levels of plasma urea nitrogen or urine creatinine were not significantly different between the groups (supplemental Table S2). ECMR deletion (e.g. ECMR−/−) in WD fed mice had no impact on body weight, fat mass, fasting blood glucose or blood pressure indices; however, ECMR deletion prevented WD-induced proteinuria (p<0.05) (supplemental Table S2). Additionally, plasma aldosterone levels and ex vivo kidney tissue MR expression were higher in both WD fed ECMR+/+ and ECMR−/− compared to CD fed mice (Fig. 1A). In this regard, mice that were fed a CD were phenotypically not different in ECMR+/+ versus ECMR−/− for whole body fat mass, fat content, lean body mass, insulin sensitivity, proteinuria, and aortic stiffening as evaluated by pulse wave velocity (PWV) and atomic force microscopy of ex vivo aortic explants25,26.
Figure 1: WD induces aortic, kidney artery, and microvessel endothelial stiffening as well as impairs endothelial relaxation dependent on the ECMR.
A. Plasma aldosterone levels and kidney expression of MR. Aldosterone: N=6 for CD ECMR+/+, 7 for WD ECMR+/+ and 6 for WD ECMR−/−. Expression of MR: N=4 for CD ECMR+/+, 7 for WD ECMR+/+.and 6 for WD ECMR−/−. B. Pulse wave velocity and renal endothelial stiffness evaluated by atomic force microscopy using kidney vessel explants. Pulse wave velocity: N=6 for CD ECMR+/+, 8 for WD ECMR+/+and 11 for WD ECMR−/−. Endothelial stiffness: N= 3 for CD ECMR+/+, 3 for WD ECMR+/+ and 4 for WD ECMR−/−. C. Microvessel mechanical and functional studies. Microvessel stiffness evaluated with incremental moduli of elasticity using pressure myography in mesenteric vessels. Relaxation studies included vasorelaxation response to the endothelium-dependent dilator acetylcholine (ACh), vasorelaxation response to the endothelium-dependent dilator insulin (Ins) and vasorelaxation response to the vascular smooth muscle-dependent dilator sodium nitroprusside (SNP). N=6 for CD ECMR+/+, 7 for WD ECMR+/+ and 7 for WD ECMR−/−. Control diet (CD), Western Diet (WD). Data are expressed as means ± SEM. † P<0.05 vs CD; ‡ p<0.05 vs WD ECMR+/+.
WD induces aortic, kidney artery and microvessel stiffening and promotes impaired endothelial NO-mediated microvessel relaxation dependent on ECMR activation.
There has been increasing interest in exploring the link between aortic vascular stiffness and microcirculatory changes that can influence kidney function over time20, 30. In this regard, WD feeding over 16 weeks induced increases in aortic stiffness as determined by PWV (Fig. 1B) and significant changes in kidney artery stiffness (Fig. 1B). WD feeding led to significant increases in microvessel stiffness as evaluated by incremental modulus of elasticity of mesenteric resistance arteries (Fig. 1C). Further, there were impairments in endothelium NO-dependent vasodilatory responses including acetylcholine- and insulin-dependent relaxation (Fig. 1C) and no changes in vascular responses to sodium nitroprusside (Fig. 1C) in mesenteric arteries. Importantly, each of these findings were prevented in the ECMR−/− mice fed WD. These data suggest macro and microvascular stiffening and impaired endothelial NO-dependent microvascular relaxation caused by consumption of WD is dependent on the ECMR.
WD attenuates kidney eNOS activation and enhances kidney oxidative stress dependent on ECMR activation.
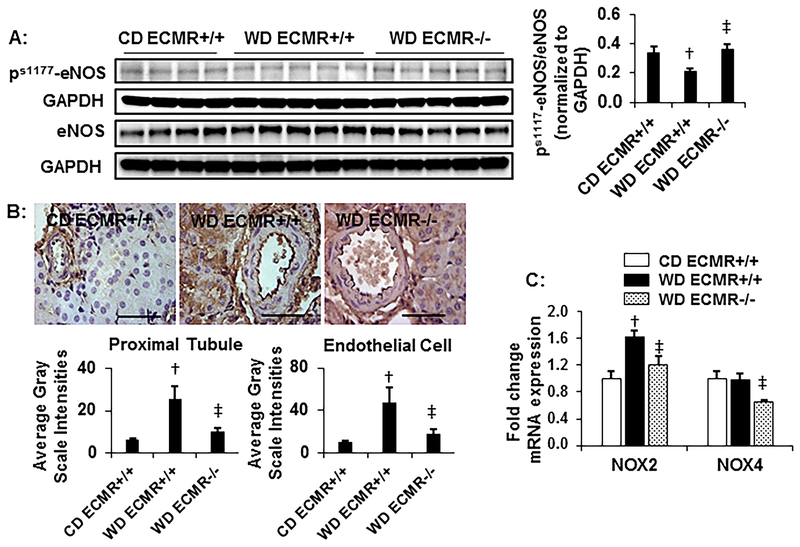
Impaired NO signaling and enhanced oxidative stress has been implicated in obesity-associated nephropathy1,30–32. Therefore, we examined the effects of ECMR deletion on WD impairment of eNOS activation and enhancement of oxidative stress in kidney tissue. There were reductions in Ser1177 phosphorylation of eNOS coupled with accumulation of 3-nitrotyrosine (3-NT) with WD feeding (Fig 2A and B, respectively). There were no differences in kidney eNOS phosphorylation in CD fed ECMR+/+ and ECMR−/− mice (supplemental Fig.S1). The increases in 3-NT were associated with increased NADPH oxidase (NOX) subunit 2 (Fig 2C) as well as plasma malondialdehyde (MDA) (supplemental Table S2). Deletion of the ECMR significantly improved the S1177phosphorylation of eNOS (Fig 2A), 3-NT content (Fig 2B) as well as expression signals for NOX2 and NOX4 subunits (Fig 2C) but not circulating MDA.
Figure 2: WD attenuates eNOS activation and promotes nitroso- and oxidative stress dependent on the ECMR.
A. Western blots of serine1177 phospho(p)-eNOS and total eNOS normalized to GAPDH with representative quantitative analyses of protein expression to the right. N=4 for CD ECMR+/+, 5 for WD ECMR+/+ and 5 for WD ECMR−/−. B. Representative images of 3-NT by immunostaining with quantification in the vascular endothelium and proximal tubule below. N=5 for CD ECMR+/+, 6 for WD ECMR+/+ and 6 for WD ECMR−/−. C. mRNA expression of the NADPH oxidase subunits NOX2 and 4 N=4 for CD ECMR+/+, 7=for WD ECMR+/+ and 6=for WD ECMR−/−. Kidney tissue from upper pole was used for immunostaining. Kidney tissue from lower pole was used for western blots and RNA isolation. Control diet (CD), Western Diet (WD). Data are expressed as means ± SEM. † P<0.05 vs CD; ‡ p<0.05 vs WD ECMR+/+.
WD induces TG2 activation and macrophage polarization dependent on the ECMR
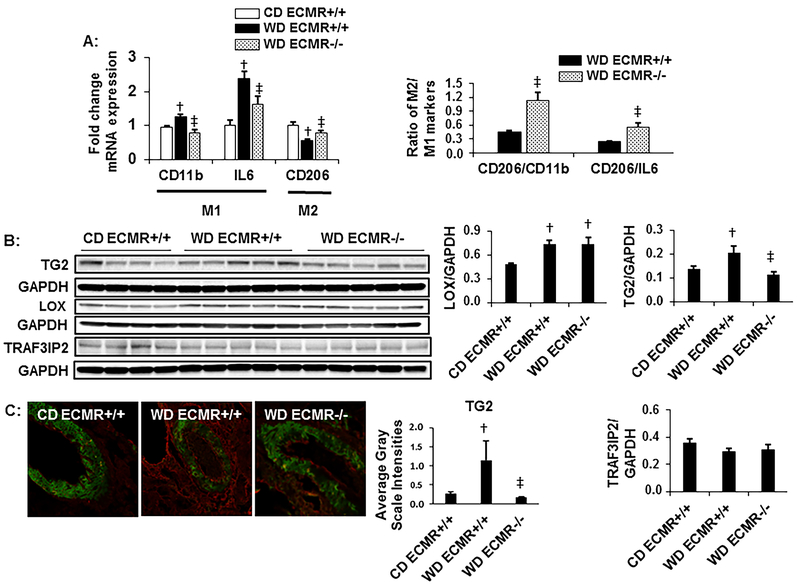
Reductions in bioavailable NO have been implicated with inflammatory responses31,32. In this study, WD feeding led to accumulation of macrophages (Cd11b), increased M1 pro-inflammatory cytokine responses (IL-6) and decreased expression of the M2 macrophage marker (CD206) together with increases in CD206 negative CD11b levels suggesting an inflammatory M1 macrophage polarization (Fig. 3A). These changes were attenuated with deletion of the ECMR resulting in increases in anti-inflammatory M2 macrophage phenotype changes (Fig 3A). Reductions in bioavailable NO also increases intracellular activation, translocation, and extracellular release of TG233. TG2 is a multifunctional protein that causes intrinsic cell stiffness through its effect on cytoskeletal remodeling. It also crosslinks collagen and favors vascular stiffening,34 macrophage infiltration and polarization,35 and kidney fibrosis36. Herein, we found that vascular TG2 expression was increased in WD-fed mice (Fig 3B) which was confirmed by immunostaining (Fig 3C). Similar to eNOS phosphorylation, there were no differences in TG2 in CD fed ECMR+/+ and ECMR−/− mice (supplemental Fig.S1). ECMR deletion decreased TG2 protein expression (Fig 3B and 3C). We also examined the expression of another cross-linking enzyme lysyl oxidase (LOX) in kidney32, which was increased in WD fed mice but was not decreased by ECMR deletion (Fig. 3B). These data suggest ECMR regulation of TG2 plays a role in the development of kidney arterial wall remodeling and fibrosis in the setting of WD feeding. However, in our current work, TRAF3IP2, a downstream target of MR activation and regulator of immune and inflammatory response, is not altered either in kidney extracts from WD fed mice or ECMR−/− mice fed WD (Fig.3B) suggesting that inflammation in WD model is not mediated by TRAF3IP2.
Figure 3: WD alters macrophage polarization and induces extracellular matrix cross-linking enzymes dependent on the ECMR.
A. Expression of macrophage markers CD11b, IL-6 and CD 206. N=4 for CD ECMR+/+, 7 for WD ECMR+/+ and 6 for WD ECMR−/−. B. Western blot analysis of lysyl oxidase (LOX), transglutaminase (TG)2, and TRAF3IP2 normalized to GAPDH with quantitative analysis to the right. N=4 for CD ECMR+/+, 5 for WD ECMR+/+ and 5 for WD ECMR−/−. C. Representative images for quantification of endothelial TG2 (red) by immunostaining with quantification to the right. N=5 for CD ECMR+/+, 6 for WD ECMR+/+ and 6 for WD ECMR−/−. Kidney tissue from upper pole was used for immunostaining. Kidney tissue from lower pole was used for western blots and RNA isolation. Control diet (CD), Western Diet (WD). Data are expressed as means ± SEM. † P<0.05 vs CD; ‡ p<0.05 vs WD ECMR+/+.
WD suppresses anti-fibrotic RECK, induces extracellular MMP-9 as well as peri-arteriolar and interstitial fibrosis dependent on the ECMR
MMP-2 and -9 have both been implicated in tubulointerstitial fibrosis37. Recent work in endothelial and tumor tissue suggest the Reversion-inducing, cysteine-rich protein with Kazal motif (RECK) protein negatively regulates extracellular matrix remodeling and fibrosis through modulation of MMP-2, -9 or both in a context dependent manner38. In this study, WD feeding resulted in significant reductions in RECK and increases in the MMP-9 without changing the activation of MMP-2 (Fig 4A and B). ECMR deletion improved RECK2 protein expression and MMP9 activation. The alterations in the extracellular cross-linking enzyme and metalloproteases in the endothelium occurred in conjunction with increases in peri-arteriolar fibrosis (Fig 5A). These findings correlate with early morphologic changes of interstitial fibrosis after WD feeding, with consistent findings of peri-arteriolar fibrosis extending into the interstitium (Fig 5A, top middle panel, arrow). Further, WD induced increases in peri-arteriolar and interstitial fibrosis in parallel with collagen type 1 but not collagen 3 accumulation (Fig 5B), findings that were reversed with ECMR deletion. There were no differences in CD fed ECMR+/+ and ECMR−/− mice (supplemental Fig.S1).
Figure 4: WD regulates extracellular metalloproteinases as well as transcription of fibrotic products dependent on the ECMR.
A. Western blot analysis of RECK N=4 for CD ECMR+/+, 5 for WD ECMR+/+ and 5 for WD ECMR−/−. B. Zymographic analysis of MMP-9 and -2 with quantification of the results below. N=4 for CD ECMR+/+, 5 for WD ECMR+/+ and 5 for WD ECMR−/− Kidney tissue from lower pole was used for western blots and zymographic analysis. Control diet (CD), Western Diet (WD). Data are expressed as means ± SEM. † P<0.05 vs CD; ‡ p<0.05 vs WD ECMR+/+.
Figure 5: WD induces peri-arteriolar fibrosis, collagen accumulation and interstitial fibrosis dependent on the ECMR.
A. Quantification of peri-arteriolar and interstitial fibrosis with representative images to the right (peri-arteriolar, top panel; interstitial fibrosis, bottom panel). Note the peri-arteriolar fibrosis extending into the interstitium (top middle panel). N=5 for CD ECMR+/+, 6 for WD ECMR+/+ and 6 for WD ECMR −/−. B. Western blot analysis of collagen 1 and collagen 3 expression normalized to GAPDH with quantification below. N=4 for CD ECMR+/+, 5 for WD ECMR+/+ and 5 for WD ECMR−/−. Kidney tissue from upper pole was used for immunostaining. Kidney tissue from lower pole was used for western blots. Control diet (CD), Western Diet (WD). Data are expressed as means ± SEM. † P<0.05 vs CD; ‡ p<0.05 vs WD ECMR+/+.
Discussion
Progressive loss of kidney function is histologically characterized by advancing fibrosis, the final common pathway leading to end stage kidney disease1. Although the role of epithelial cells and immune cells in kidney fibrosis has been well examined, the role of endothelium in regulation of kidney vascular stiffness and development of kidney fibrosis are just emerging1,15–20. This investigation demonstrates a novel mechanism by which obesity contributes to progressive kidney disease through impairments in macro- and microvessel stiffening and inflammation (Fig. 6). Here we demonstrate that WD feeding for 16 weeks in female mice leads to central aortic stiffness with increases in PWV, impaired microvessel endothelial-dependent vasodilatation, arterial wall remodeling and endothelial stiffening. The WD-induced microvascular changes were associated with reductions in bioavailable NO, endothelial nitroso and oxidant stress, pro-inflammatory M1 macrophage polarization, and alterations in extracellular matrix degradation that propagate fibrosis. Indeed, these data support the concept the persistent excessive pulsatile wave force induced by a western diet contributes to altered end organ auto-regulatory mechanisms, consequent wall strain, remodeling and fibrosis8,11,12. In this context, WD feeding led to alterations in the extracellular matrix through modulation of pro-fibrotic and anti-fibrotic responses characterized by increases in TG2 and decreases in RECK protein. We further demonstrate these responses were attenuated with cell-specific deletion of the endothelial MR, supporting a direct role for the vascular endothelium in the regulation of fibrosis by ECMR signaling in the setting of obesity in females. To the best of our knowledge, these are the first data to support a direct role for obesity in the development of fibrosis as mediated through the vascular endothelium and the first to demonstrate a role for endothelial cell specific MR activation in obesity mediated progressive kidney injury.
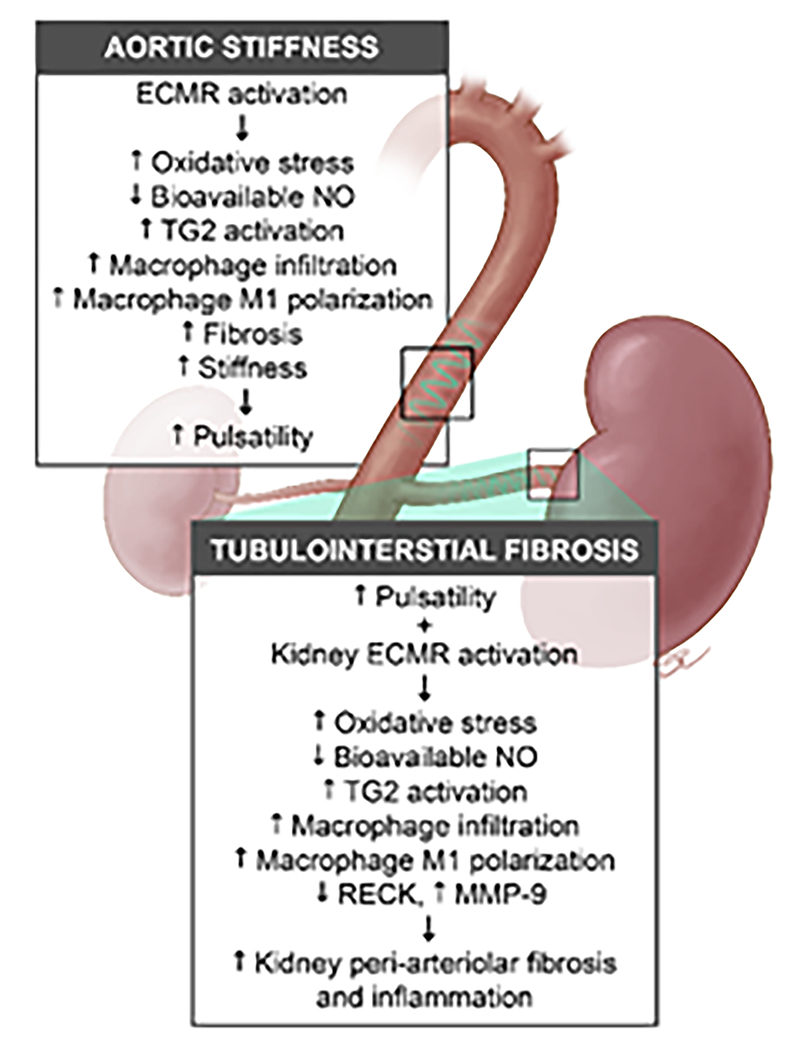
Figure 6: Hypothetical model showing the contribution of the endothelial mineralocorticoid receptor to central aortic stiffening and kidney tubulointerstitial fibrosis.
Activation of the endothelial cell mineralocorticoid receptor (ECMR) in the aorta contributes to reductions in bioavailable nitric oxide (NO), enhanced oxidant, and inflammatory pathways that lead to central aortic perivascular fibrosis and stiffening. The increased central aortic stiffness contributes to an excessive pulsatile wave (e.g., pulsatility) that in combination with kidney ECMR activation, leads to reductions in endothelial NO synthase and bioavailable NO with downstream activation of oxidant and inflammatory pathways in the kidney. The net result in the kidney is endothelial regulation of macrophage infiltration and M1 polarization, disruption of extracellular adhesion through cross-linking enzymes like transglutaminase 2, and matrix metalloproteinase (MMP-9). These changes result in peri-arteriolar inflammation, fibrosis, stiffness and downstream tubulointerstitial fibrosis.
There is a clear and direct relationship between obesity and aortic vascular stiffness as evaluated by PWV as well as incident chronic kidney disease1–4,7–10. Furthermore, population level studies indicate that premenopausal women are predisposed to vascular stiffening in the presence of obesity39. In this regard, our previous work suggested that female mice display higher circulating levels of aldosterone compared to males with increased expression of MR in the aorta of WD fed mice. These increases in aldosterone were accompanied by central aortic stiffness, and impairment in mesenteric vessel vasodilation in response to diet-induced obesity compared to non-obese male counterparts23,27. Thereby, our current finding that WD-induced increases in body weight and fat mass in female mice were associated with increases in PWV as a measure of central aortic stiffness in concert with increased plasma aldosterone levels in mice fed WD for 16 weeks are consistent with prior observations.
Studies in humans have focused on the relationship between central aortic stiffness and alterations in microvessel hemodynamics in highly susceptible target organs such as the kidney17,18. The propagation of an excessive pulsatile (kinetic) energy caused by central aortic stiffening contributes to alterations in auto-regulatory mechanisms through chronic increases in myogenic tone and consequent wall strain and remodeling that eventually leads to increased renal vascular stiffness associated with perivascular and end-organ fibrosis8,11,12. Current data supports the notion that obesity increases central aortic stiffness that extends to kidney artery stiffening and microvessel impairment in endothelial-dependent relaxation, changes that are related to cortical tissue peri-arterial fibrosis. Further, our observation that ECMR deletion ameliorated kidney fibrosis and stiffness without substantial changes in blood pressure support the notion that kidney microcirculation can be regulated by forces independent of systemic hemodynamics. It should be noted blood pressure in our current studies was assessed in a smaller cohort and meant to corroborate previous observations24–26, yet are inadequately powered to assess between group differences. Our finding that WD-induced kidney artery stiffening occurs in conjunction with central aortic stiffness is novel as it has not previously been demonstrated in a preclinical model of obesity.
Impairments in endothelial-dependent relaxation are directly related to reductions in endothelial NOS phosphorylation/activation and resultant bioavailable NO. There has been work done to uncover MR regulation of eNOS in explanted aortic, mesenteric, and cardiac tissues and in culture models where the role of MR activation is especially prominent in females compared to males26,27,32. However, in kidney studies much of the work has been confined to cultured epithelial cells,26,27,32,40 and there has been little work done on in vivo/ex vivo kidney function. The in vivo work to date is confined to the impact of pharmacologic MR antagonism on NO-dependent by-products and inflammatory pathways32.40. Resistance mesenteric arteries are often used as a surrogate for other vessel beds such as the kidney microcirculation. The magnitude of the relaxation response of the microvessel preparation to acetylcholine was somewhat lower than other studes,27 which may be due to high concentration of vasoconstrictor used to constrict the vessels in this study, ECMR KO deletion prevented WD-induced increases in stiffness as well as WD-induced impairments in endothelium-dependent vasorelaxation. These finding suggest that ECMR promotes WD-induced reductions in bioavailable NO and endothelial stiffening. This finding is consistent with the prior observation that the MR regulates mesenteric endothelial function in female mice to a greater extent than their male counterparts27. Our additional finding that ECMR deletion rescued phosphorylation of eNOS and suppressed oxidative stress support a role for endothelial regulation of kidney tissue oxidant stress. In this context, we have previously shown that activation of the ECMR regulates vascular NOX as well as 3-NT which in turn regulated through eNOS24,26. In this study, we further observed that ECMR deletion lessened 3-NT content, a marker for peroxynitrite formation, as well as NOX subunit 2 and 4 expression. These data support the concept that ECMR regulates NADPH oxidase activation in the kidney suggest that ECMR-mediated dysregulation of NO bioavailability associated with oxidant stress as one of the possible mechanisms for micro-vessel dysfunction and stiffness in the kidney.
Alterations in eNOS activation and bioavailable NO not only mediate tissue injury through oxidant pathways, but also regulate MΦ polarization in tissue injury. Recent data using a model of high fat diet feeding support the notion that endothelial NO signaling mediated increases in a pro-inflammatory MΦ M1 phenotype in hepatic tissue fibrosis under conditions of obesity31. The MΦ M1 proinflammatory phenotype change in endothelial stiffness and tissue injury is thought to be a prototypical, pro-inflammatory phenotypic alteration of MΦs associated with increases of ROS and reactive nitrogen species and pro-inflammatory cytokines. These changes enhance monocyte adhesion, endothelial permeability and trans-endothelial migration of monocytes25,26,28. The alterations in endothelial permeability associated with macrophage polarization contribute to alterations in endothelial function and stiffness. Similar to these findings we have also observed that rescue of eNOS activation in aortic tissue improved the anti-inflammatory M2 MΦ polarization that was dependent on the ECMR activation26. Our finding then that WD enhanced the MΦ pro-inflammatory M1 phenotype markers CD11b and IL6 and ECMR deletion rescued the anti-inflammatory M2 phenotype through CD206 expression in kidney tissue is noteworthy. Although 1L-6 is one of the secretory cytokines of pro-inflammatory M1 macrophages, it may have sources other cells besides macrophages.41 However, the decrease in the M2 marker CD206 expression with concomitant increase in CD11b also favors an enhanced proinflammatory response. Both findings support an important role for obesity-dependent ECMR-dependent reductions in bioavailable NO and related MΦ polarization in tissue fibrosis. These data would extend prior findings on the importance of the ECMR and MΦ polarization on vascular and tubulointerstitial fibrotic responses into the kidney42.
In addition to oxidant and inflammatory pathways that regulate vessel stiffness, there has been recent interest in exploring the relationship between vessel stiffness and histologic changes such as tissue fibrosis1,12,13,25. This relationship is thought to be dictated by applied tension resulting from alterations in tissue extracellular matrix via actin-myosin cytoskeletal rearrangement, derangements in cell-cell adhesions and increases in fibrillary collagens40. The net result of these changes dictate vessel fibrosis, which in the kidney can lead to increased interstitial fibrosis. Our observation that the WD-induced peri-arteriolar fibrosis emanated into the interstitial space leading to tubulointerstitial fibrosis suggest an endothelial mesenchymal transition mechanism18–20.
As previously noted the mechanisms that influence perivascular fibrosis extend beyond stiffening of the vessel to alterations in tissue extracellular matrix via actin-myosin cytoskeletal rearrangement to derangements in cell-cell adhesions and increases in fibrillary collagens. In this regard, the role of TG2 and MMP-9 in renal extracellular matrix stiffness and fibrotic remodeling has been increasingly recognized. TG2 is a multifunctional protein that favors vascular stiffening34 and fibrosis 36 though collagen cross linking and macrophage infiltration and polarization35. In this regard, eNOS activation /NO production has been shown to regulate TG2 in cultured endothelial cells and in aorta35,36. Specifically, bioavailable NO reduces TG2 activation which involves nitrosylation, translocation, and then extracellular release of TG2. This, in turn, promotes actin cross-linking and G-actin polymerization causing cell cortical stiffness, increased cell permeability, and synthesis of collagen33–35. In this study, we observed that WD reductions in eNOS and increases in TG2 activity were prevented with ECMR deletion. There is limited information on TG2 in the kidney largely confined to a model of unilateral ureteral obstruction and aging supporting that TG2 regulates the ECM remodeling in promotion of tubulointerstitial fibrosis36,43,44. Our data support a novel role for ECMR activation of TG2 in promotion of peri-arterial fibrosis and in promotion of endothelial stiffening and tubulointerstitial fibrosis in the kidney that is dependent on the ECMR. Another collagen cross-linking enzyme LOX also contributes to vascular stiffness32. However, the increases in LOX expression in the WD-fed model is not under the control of the ECMR, thus supporting a TG2-dependent mechanism in ECMR mediated fibrosis.
Derangements in cell-cell adhesion through alterations in the activity of MMPs also play a role in the progression of kidney fibrosis37,45. Previous work in this area support the concept that reductions in bioavailable NO may regulate MMP-dependent tubulointerstitial fibrosis through increases in MMP-2 and -9 expression1,32,45. Recent work support a role for RECK in human and cell culture models as a significant endogenous inhibitor of MMP-2, -9 or both dependent on context38,46. RECK reductions are localized to both vasculature and proximal tubules in a renal cell carcinoma model of fibrosis38. In our current work WD-increases in peri-arteriolar and tubulointerstitial fibrosis, along with increases in MMP-9, were related to reductions in RECK and are consistent with RECK reductions are associated with renal fibrosis. The finding that RECK and MMP-9 were rescued by ECMR deletion support the concept that ECMR-dependent regulation of fibrosis.
It is important to note that a recent study demonstrated suppression of vascular injury by ECMR deletion without a significant impact on kidney function47–49. This observation is an important advance in our understanding of the ECMR, yet we suggest our unique findings are due to the use of the WD which enhances ECMR signaling. This would be consistent with other’s findings,47 and our previous work. Thus, upregulation of ECMR signaling under the setting of high consumption of fat and refined sugars, which may be the underlying factor that creates the milieu for endothelial and tissue injury. Although a cell specific role of macrophage MR has been shown to promote glomerular injury in a model of IgA nephropathy,48 the impact of macrophage MR deletion has not been examined in obesity-mediated nephropathy. In conclusion, significant suppression of obesity-mediated kidney fibrosis by ECMR deletion underscore the role of endothelium-dependent mechanisms in the development of obesity-mediated kidney fibrosis by MR activation.
Perspectives
This contribution provides a number of advances. Foremost, our work suggests that obesity impacts kidney function beyond development of glomerular permeability and glomerulosclerosis. Our data highlight that diet-induced obesity promotes central aortic stiffness, alterations in microvessel stiffening and impairments in endothelial-dependent vasorelaxation that are associated with end-organ kidney fibrosis. These vascular and kidney pathologies are associated with reductions in endothelial nitric oxide synthase, endothelial derived inflammatory, oxidant and fibrotic pathways that are dependent on endothelial mineralocorticoid receptor activation.
Supplementary Material
Novelty and Significance.
What is new?
Deletion of endothelium specific mineralocorticoid receptor prevents obesity-mediated alterations in microvessel stiffening as well as impairments in endothelial-dependent vasorelaxation associated with fibrosis in the kidney
What is relevant?
Our contribution provides a number of advances.
Foremost, our work suggests that obesity impacts kidney function beyond development of glomerular permeability and sclerosis.
Our data highlight that diet-induced obesity promotes central aortic stiffness, alterations in microvessel stiffening and impairments in endothelial-dependent vasorelaxation that is associated with end-organ kidney fibrosis.
Findings that are associated with reductions in endothelial nitric oxide synthase, endothelial derived inflammatory, oxidant and fibrotic pathways that are dependent on endothelial mineralocorticoid receptor activation.
Acknowledgements:
We acknowledge the work by Dr. Vincent G. DeMarco, who obtained and analyzed PWV data. We also acknowledge Matthew B. Martin and Dongqing Chen for their help in animal experiments. This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
Sources of Funding: Dr. Whaley-Connell receives funding from the Veterans Affairs Merit System (BX003391) as well as (BX001981). JRS also received funding from the Veterans Affairs Merit System (BX001981) and NIH (R01 HL73101-01A and R01 HL107910-01). Dr. Jia receives funding from American Diabetes Association (Innovative Basic Science Award #1-17-IBS-201). Dr. Martinez-Lemus receives funding from the National Institutes of Health (HL-088105). Dr. Jaffe receives funding from the National Institutes of Health (HL095590 and HL119290) and the American Heart Association (EIA18290005).
Footnotes
Disclosures: None.
REFERENCES
- 1.Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int 92(2): 313–323, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 315(21):2292–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73(1):19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol 17(6):1695–1702, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Virzì GM, Zhang J, Nalesso F, Ronco C, McCullough PA.The Role of Dendritic and Endothelial Cells in Cardiorenal Syndrome. Cardiorenal Med 8(2):92–104, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer HJ, Saranathan A, Luke A, Durazo-Arvizu RA, Guichan C, Hou S et al. Increasing body mass index and obesity in the incident ESRD population J Am Soc Nephrol, 17: 1453–1459, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L et al. CRIC Study Investigators. Association of Pulse Wave Velocity With Chronic Kidney Disease Progression and Mortality: Findings From the CRIC Study (Chronic Renal Insufficiency Cohort). Hypertension. 71(6):1101–1107, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Bottiglieri T, McCullough PA. The Central Role of Endothelial Dysfunction in Cardiorenal Syndrome. Cardiorenal Med 7(2):104–117, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai M, Kobayashi J, Takeda Y, Nagasawa SY, Yamakawa J, Moriya J et al. Sex Differences in Associations Among Obesity, Metabolic Abnormalities, and Chronic Kidney Disease in Japanese Men and Women. J Epidemiol 26(8):440–6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 42(4): 468–73, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 105(5): 1652–60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodard T, Sigurdsson S, Gotal JD, Torjesen AA, Inker LA, Aspelund T eta al. Mediation analysis of aortic stiffness and renal microvascular function. J Am Soc Nephrol 26:1181–1187, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce GL. Recent Advances: Mechanisms and Subclinical Consequences of Aortic Stiffness. Hypertension. 70(5):848–853, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabias BM, Konstantopoulos K. B The physical basis of renal fibrosis: effects of altered hydrodynamic forces on kidney homeostasis. Am J Physiol Renal Physiol 306(5):F473–85, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Humphreys BD. Mechanisms of Renal Fibrosis. Annu Rev Physiol 80:309–326.2018 [DOI] [PubMed] [Google Scholar]
- 16.Nastase MV, Zeng-Brouwers J, Wygrecka M, Schaefer L. Targeting renal fibrosis: Mechanisms and drug delivery systems. Adv Drug Deliv Rev 129:295–307, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med S0098-2997(18)30040-2, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Lee JP. Active maintenance of endothelial cells prevents kidney fibrosis. Kidney Res Clin Pract 36(4):329–341, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipphardt M, Song JW, Matsumoto K, Dadafarin S, Dihazi H, Müller G et al. The third path of tubulointerstitial fibrosis: aberrant endothelial secretome. Kidney Int 92(3):558–568, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry HM, Okusa MD. Endothelial Dysfunction in Renal Interstitial Fibrosis Nephron. 134(3):167–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR et al. Regular exercise reduces endothelial cortical stiffness in western diet-fed female mice. Hypertension. 68:1236–1244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foote CA, Castorena-Gonzalez JA, Ramirez-Perez FI, Jia G, Hill MA, Reyes-Aldasoro CC, Sowers JR, Martinez-Lemus LA. Arterial Stiffening in Western Diet-Fed Mice Is Associated with Increased Vascular Elastin, Transforming Growth Factor-β, and Plasma Neuraminidase. Front Physiol 7:285, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J et al. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 154(10):3632–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA et al. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension. 66:99–107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT et al. Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females. Hypertension. 66:1159–1167, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI et al. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res 118(6):935–943, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davel AP, Lu Q, Moss ME, Rao S, Anwar IJ, DuPont JJ et al. Sex-Specific Mechanisms of Resistance Vessel Endothelial Dysfunction Induced by Cardiometabolic Risk Factors. J Am Heart Assoc 7(4), 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol 9(8):459–69, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F et al. Endothelial Mineralocorticoid Receptors Differentially Contribute to Coronary and Mesenteric Vascular Function Without Modulating BloodPressure. Hypertension. 66(5):988–97, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgianos PI, Sarafidis PA, Lasaridis AN. Arterial stiffness: a novel cardiovascular risk factor in kidney disease patients. Curr Vasc Pharmacol 13(2):229–38, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Lee WJ, Tateya S, Cheng AM, Rizzo-DeLeon N, Wang NF, Handa P et al. M2 Macrophage Polarization Mediates Anti-inflammatory Effects of Endothelial Nitric Oxide Signaling. Diabetes. 64:2836–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aroor AR, Jia G, Sowers JR. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Regul Integr Comp Physiol 1;314(3):R387–R398, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jandu SK, Webb AK, Pak A, Sevinc B, Nyhan D, Belkin AM et al. Nitric oxide regulates tissue transglutaminase localization and function in the vasculature. Amino Acids. 44(1):261–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castorena-Gonzalez JA, Staiculescu MC, Foote CA, Polo-Parada L, Martinez-Lemus LA. The obligatory role of the actin cytoskeleton on inward remodeling induced by dithiothreitol activation of endogenous transglutaminase in isolated arterioles. Am J Physiol Heart Circ Physiol 306:H485–95, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Akker J, VanBavel E, van Geel R, et al. The redox state of transglutaminase 2 controls arterial remodeling. PLoS One. 6(8):e23067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC et al. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am J Pathol 173(3):631–42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan TK, Zheng G, Hsu TT, Lee SR, Zhang J, Zhao Y et al. Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 93(4):434–49. 2013 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Liu L, Liu J, Cheng Z, Wang Z, Shi C et al. Endothelial cells co-cultured with renal carcinoma cells significantly reduce RECK expression under chemical hypoxia. Cell Biol Int 41(8):922–927, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Sakurai M, Kobayashi J, Takeda Y, Nagasawa SY, Yamakawa J, Moriya J et al. Sex Differences in Associations Among Obesity, Metabolic Abnormalities, and Chronic Kidney Disease in Japanese Men and Women. J Epidemiol 26(8):440–6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 65(2):257–63, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Su H, Lei CT, Zhang C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front Immunol ;8:405.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 20(9):3350–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin CH, Chen J, Zhang Z, Johnson GV, Cooper AJ, Feola J et al. Endostatin and transglutaminase 2 are involved in fibrosis of the aging kidney. Kidney Int 89(6):1281–92, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatt MP, Lim YC, Hwang J, Na S, Kim YM, Ha KS. C-peptide prevents hyperglycemia-induced endothelial apoptosis through inhibition of reactive oxygen species-mediated transglutaminase 2 activation. Diabetes. 62(1):243–53, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steed MM, Tyagi N, Sen U, Schuschke DA, Joshua IG, Tyagi SC. Functional consequences of the collagen/elastin switch in vascular remodeling in hyperhomocysteinemic wild-type, eNOS−/−, and iNOS−/− mice. Am J Physiol Lung Cell Mol Physiol 299(3):L301–11.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabien A1, Stephan C, Kilic E, Weichert W, Kristiansen G, Miller K et al. Renal cell neoplasias: reversion-inducing cysteine-rich protein with Kazal motifs discriminates tumor subtypes, while extracellular matrix metalloproteinase inducer indicates prognosis. J Transl Med 11:258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laursen SB, Finsen S, Marcussen N, Quaggin SE, Hansen PBL, Dimke H. Endothelial mineralocorticoid receptor ablation does not alter blood pressure, kidney function or renal vessel contractility. PLoS One.13(2):e0193032,2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schäfer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos et al. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 34(45):3515–24, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang LL, Nikolic-Paterson DJ, Han Y, Ozols E, Ma FY, Young MJ et al. Myeloid mineralocorticoid receptor activation contributes to progressive kidney disease. J Am Soc Nephrol 25(10):2231–40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.