Abstract
Objective
Overall survival (OS) for advanced stage (IIIA-IV) non-small cell lung cancer is highly variable, and retrospective data show a survival advantage for patients receiving therapeutic intent pulmonary resection. We hypothesized that this variability in OS can be modeled separately by stage to allow a personalized estimate of OS.
Methods
In a cohort of advanced stage NSCLC patients from the National Cancer Database, we assessed the accuracy of Surgical Selection Score (SSS) to predict OS using Cox proportional hazards models, and determined by stage, the effect of surgery on survival among people with similar high levels of SSS.
Results
300,572 patients were identified; 18,701 (6%) had surgery. The SSS was a strong predictor of OS (C-index, 0.89; 95% CI, 0.89-0.90). We observed significantly higher OS (p<0.001) among patients who had surgery. The hazard of death was at least 2 times higher for patients in the upper quartile of SSS that did not receive surgery compared to surgical patients even when adjusting for the SSS (Stage IIIA: Hazard Ratio (HR) 2.1, 95% CI: 2.0-2.2, Stage IIIB: HR 2.3, 95% CI: 2.2-2.5, Stage IV: HR 2.3, 95% CI: 2.2-2.4).
Conclusions
The SSS is highly predictive of individual OS and can be used as a risk assessment tool. These findings are important for a more robust evaluation of the likely benefits of surgical resection for these patients. After further prospective validation, the SSS can be used during treatment decision-making for advanced stage NSCLC patients.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related mortality in the United States.1 Overall 5-year survival is approximately 18%, but for patients with advanced stage (stages IIIA, IIIB or IV) NSCLC, it is particularly poor. Importantly, however, survival rates for these subgroups are quite variable. Depending on factors such as stage of disease and treatment modality, 5-year survival ranges from 4-28%.2 The heterogeneity of the clinical, pathologic, and facility characteristics of these patients likely accounts, in part, for the variable survival outcomes among these patients, but these same variable characteristics have also been implicated as the foundation of widely disparate treatment approaches.2,3 Moreover, it is these advanced stage patients who make up the vast majority of NSCLC patients, representing roughly 79% of newly diagnosed patients, so reduction in treatment variation and/or risk stratification is anticipated to translate to significant therapeutic gains.1
Current NCCN guidelines only recommend therapeutic intent surgical treatment for patients with stage IIIA disease who have single station nodal disease or stage IV patients with oligometastatic disease to the brain or adrenal glands.4 Despite this limited recommendation for surgical treatment, several series have demonstrated a clinically and statistically significant survival advantage for patients treated surgically compared to patients treated non-surgically.3,5,6 However, uniformly, the analyses demonstrating a survival advantage with surgery are retrospective in nature, and the survival advantages observed are typically attributed to selection bias in the surgical cohorts.3,5,6 An accepted approach to control for selection bias in retrospective analyses is to use statistical methodology such as propensity matching to control for confounding variables. However, this technique is limited by the inability to assess unmeasured confounders like severity of comorbidities and disease burden.7 Additionally, these techniques are typically limited to retrospective analyses of outcomes and do not directly assist with prospective treatment decision-making.5,6
In an effort to improve data-driven treatment decision making for these patients, we recently developed a Surgical Selection Score (SSS) to explore factors inherent in the selection for surgical treatment among advanced stage NSCLC.8 One distinct advantage of the SSS over propensity matching is that it can be used in a prospective manner when treatment decisions are being made. The SSS was created in a population cohort from advanced stage NSCLC patients in the National Cancer Database (NCDB) using a logistic regression model that predicts likelihood of selection for surgery based on clinical factors available at the time of treatment decisions. The factors included in the SSS are all clinical including: histology, tumor size, AJCC clinical T status, AJCC clinical N status, AJCC clinical M status, Charlson comorbidity index, age, race, facility type, insurance and income, with AJCC clinical M status, AJCC clinical N status and age having the strongest influence on selection for surgery. In the current analysis, our objectives were to define the stage-specific ability of the SSS to predict OS for advanced stage NSCLC patients, to assess the impact of surgery in patients at same stage and comparable SSS, and to provide a risk assessment tool.
Methods
Using the NCDB Participant User File from 1998-2012, we identified cases of biopsy-proven NSCLC. The NCDB is a joint program of the Commission on Cancer (CoC) and the American Cancer Society (ACS).9 Data from the NCDB represent 1,500 CoC- accredited facilities including over 70% of all newly diagnosed cancer cases in the United States.9
Patients with clinical stage IIIA, IIIB and IV NSCLC per the 7th edition of the International Association for the Study of Lung Cancer with histologic data available were included (Figure 1). Patients with an additional cancer diagnosis, missing clinical TNM stage group, clinical stage I or II, or missing Charlson index or other demographic data were excluded. Standard patient, tumor, and treatment data were extracted and categorized as appropriate. Patients were categorized into surgical and non-surgical groups. Non-surgical treatments included: chemotherapy only, chemotherapy and radiation, radiation only, and no treatment. Surgical treatments included: surgery only; chemotherapy and surgery; chemotherapy, radiation and surgery; and surgery and radiation. Surgical operations included wedge resection, sublobar resection, lobectomy, bilobectomy, and pneumonectomy in an attempt to focus our analysis on therapeutic intent procedures aimed at resection of the primary tumor.
Figure 1.
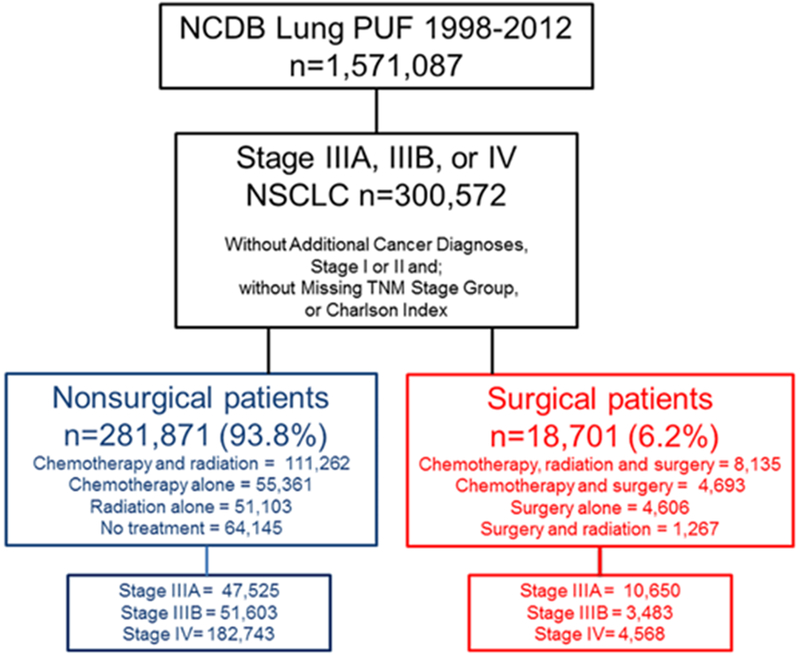
Cohort of Stage IIIA, IIIB and IV NSCLC patients from the NCDB
Patient comorbidities were assessed using the Charlson comorbidity index, described by Deyo et al.10 Additional categorical variables examined included clinical tumor group, clinical T group, clinical N group, clinical M group, histology, age, gender, race, income, education, insurance status (Medicaid, Medicare, other government, private health insurance, no insurance), and treatment facility. Age was categorized by percentile (10th, 25th, 50th, 75th, 90th). Income categories were defined as follows: low ≤$38,000; middle >$38,000-47,999, and high ≥$48,000.
Statistical Analyses
As we have previously described, logistic regression was used to create the surgical selection score (SSS) with the outcome variable representing inclusion of surgery in the treatment regimen.8 The SSS was created by a linear rescaling of the logarithm of the odds ratio of inclusion of surgery in the treatment regimen, to generate a single numeric value between 0 and 1200 for each patient depending on the value of the clinical factors for each patient. In the current analysis the ability of the SSS to predict OS was assessed using the Cox proportional hazards model with SSS as the only explanatory variable, for the entire cohort and separately by stage. Discrimination ability was measured by calculating an overall C–index (concordance index) for the Cox model.11,12 The C-index was introduced by Harrell as a natural extension of the Area Under the Receiver Operator Curve (AUROC) from logistic regression.13 The C-index is defined as the proportion of all usable patient pairs in which the predictions and outcomes are concordant. The overall C-index and confidence interval hereof for survival analysis where censoring is an issue has been calculated as proposed by Pencina and D’Agostino. 11,12
OS were illustrated and median survival times (MST) calculated using a Kaplan-Meier life table approach and compared by log-rank test. For comparison of which variables are most important in terms of predicting surgical treatment versus survival a Cox Model was fitted using all the predictors used to create the SSS but not the SSS itself.
Distribution of the SSS and the Kaplan-Meier estimate of 3-year survival were visually illustrated for each stage by treatment category (surgical vs non-surgical) and within each stage the mean values of SSS were statistically compared for the two treatment categories. To assess the potential benefit of surgery for patients with high SSS (above approximately the 75% percentile), the overall effect of surgery on the hazard of death was estimated for these patients by the Cox model separately by stage. These models were further adjusted for SSS by categorizing into small subgroups each representing 5% of the top quartile. P-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute Cary, NC). This study received a determination letter from the University of California, Davis Institutional Review Board.
Results
We identified 300,572 patients with biopsy-proven stage IIIA, IIIB or IV NSCLC from the NCDB (Figure 1). Notably, 18,701 (6.2%) of these patients had surgery as part of their treatment regimen. 265,183 patients had survival data available for follow up for use in Cox proportional hazards model. The patients with missing survival information were evenly distributed across the surgery and non-surgery groups and SSS. As previously reported, the strongest predictors of selection for surgery were AJCC clinical metastatic group, AJCC clinical nodal group, and age.8 The strongest predictors affecting the hazard of death were AJCC clinical metastatic group and age (Table 1).
Table 1.
Association Between Hazard of Death and the Patient Variables used to create the Surgical Selection Score
| Variable | Hazard Ratio (95% Confidence Interval) |
|---|---|
| Clinical T Status | |
| 1 | 0.77 (0.76-0.78) |
| 2 | 0.83 (0.82-0.84) |
| 3 | 0.87 (0.86-0.88) |
| 4 | Reference |
| X | 0.97 (0.96-0.99) |
| Tumor Size | |
| T1a | 0.72 (0.70-0.73) |
| T1b | 0.74 (0.73-0.75) |
| T2a | 0.79 (0.78-0.80) |
| T2b | 0.87 (0.86-0.89) |
| T3 | Reference |
| Clinical N Status | |
| 0 | 0.75 (0.74-0.77) |
| 1 | 0.84 (0.82-0.85) |
| 2 | 0.92 (0.91-0.93) |
| 3 | Reference |
| X | 1.00 (0.99-1.02) |
| Clinical M Status | |
| 0 | 0.46 (0.45-0.46) |
| 1 | 0.92 (0.91-0.94) |
| 1A | 0.68 (0.66-0.70) |
| 1B | Reference |
| Histology | |
| Squamous cell carcinoma | 0.91 (0.90-0.92) |
| Adenocarcinoma | 0.86 (0.85-0.87) |
| NSCLC, NOS | Reference |
| Age Group, y | |
| <52 | 0.55 (0.54-0.56) |
| 52-59 | 0.59 (0.58-0.60) |
| 59-67 | 0.63 (0.62-0.64) |
| 67-75 | 0.68 (0.68-0.69) |
| 75-81 | 0.81 (0.79-0.82) |
| >81 | Reference |
| Charlson-Deyo Index | |
| 0 | Reference |
| 1 | 1.20 (1.19-1.21) |
| 2 | 1.42 (1.41-1.44) |
| Race | |
| White | 1.04 (1.03-1.06) |
| Other | 0.82 (0.79-0.84) |
| Black | Reference |
| Insurance Status | |
| Private | 0.80 (0.79-0.82) |
| Medicare | 0.91 (0.89-0.93) |
| Medicaid | 0.96 (0.93-0.98) |
| Other government | 0.93 (0.89-0.97) |
| Not insured | Reference |
| Income | |
| High | 0.92 (0.91-0.93) |
| Middle | 0.97 (0.96-0.98) |
| Low | Reference |
| Facility Type | |
| Academic/research program | 0.87 (0.86-0.88) |
| Comprehensive community cancer program | 0.95 (0.94-0.97) |
| Community cancer program | Reference |
In Cox proportional hazards model, SSS was highly predictive of OS (Table 2). The C-index for a model based on the entire cohort was 0.89, and predictive ability increased when each stage group was considered separately. OS was also significantly higher in the surgically treated patients across all stage groups (p<0.001) (Figure 2), with MST for surgical patients about 3-fold longer (Table 3). Within each stage, surgical patients show both higher values of SSS (within each stage, mean values for SSS are significantly different between the surgical and non-surgical group with p<0.0001) and a higher probability of 3-year survival than their non-surgical counterparts (Figure 3.)
Table 2.
C-index for the Surgical Selection Score for prediction of Overall Survival
| Stage | C-index | 95% Confidence Interval |
|---|---|---|
| IIIA, IIIB, IV | 0.89 | 0.891-0.896 |
| IIIA | 0.91 | 0.908-0.919 |
| IIIB | 0.94 | 0.938-0.947 |
| IV | 0.94 | 0.941-0.946 |
Figure 2.
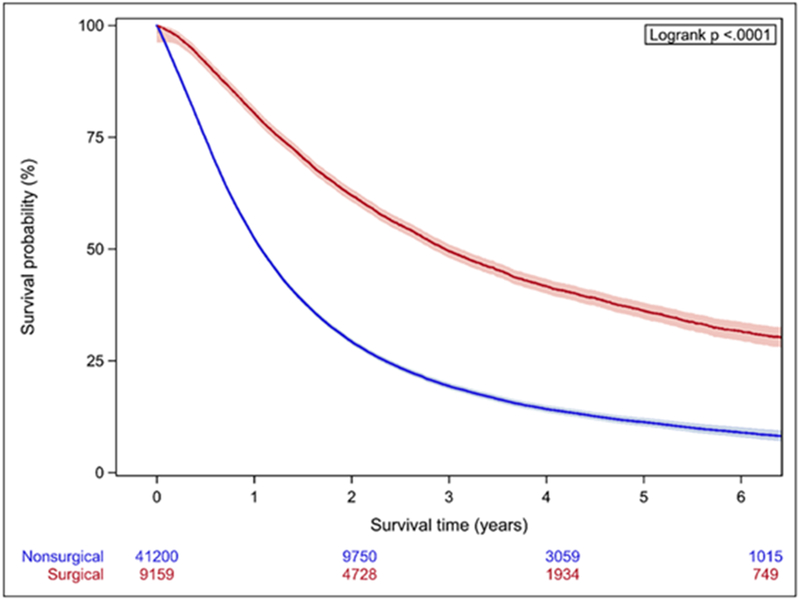
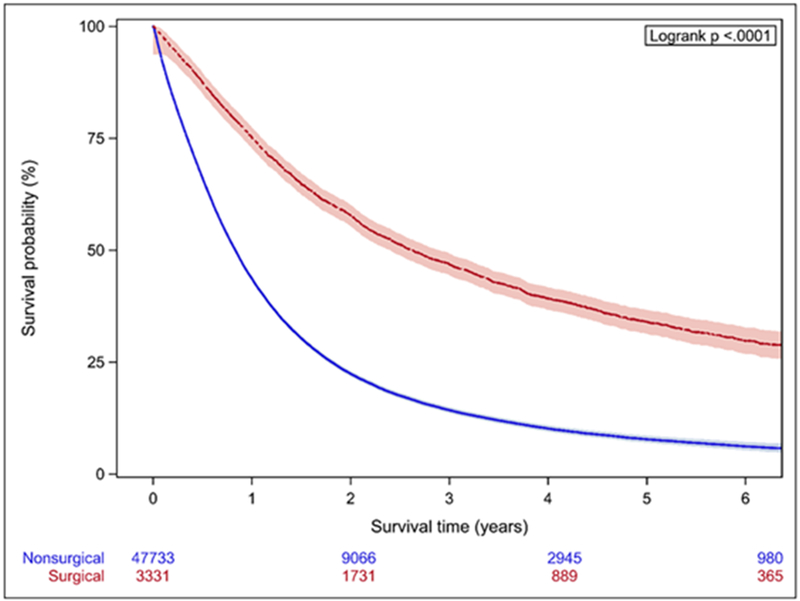
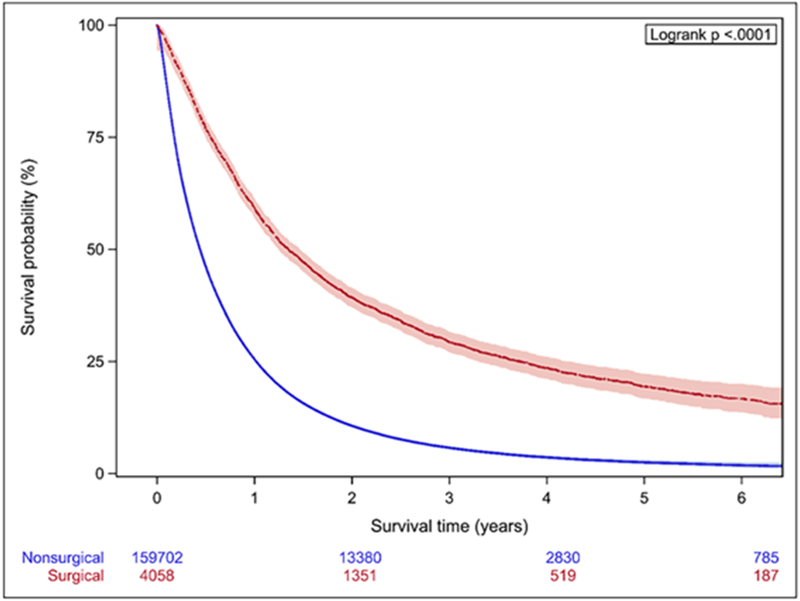
Kaplan-Meier analysis of patients stratified by stage. A. Stage IIIA B. Stage IIIB C. Stage IV Patients treated surgically have significantly longer OS across all stages.
Table 3.
Median Survival Time by Treatment Group
| MST (95% Confidence Interval) in Months | ||
|---|---|---|
| Surgical | Non-surgical | |
| Stage IIIA | 35.5 (34.3-36.9) | 12.9 (12.7-13.0) |
| Stage IIIB | 31.4 (29.4-33.6) | 10.0 (9.9-10.2) |
| Stage IV | 16.2 (15.2-17.3) | 5.3 (5.3-5.4) |
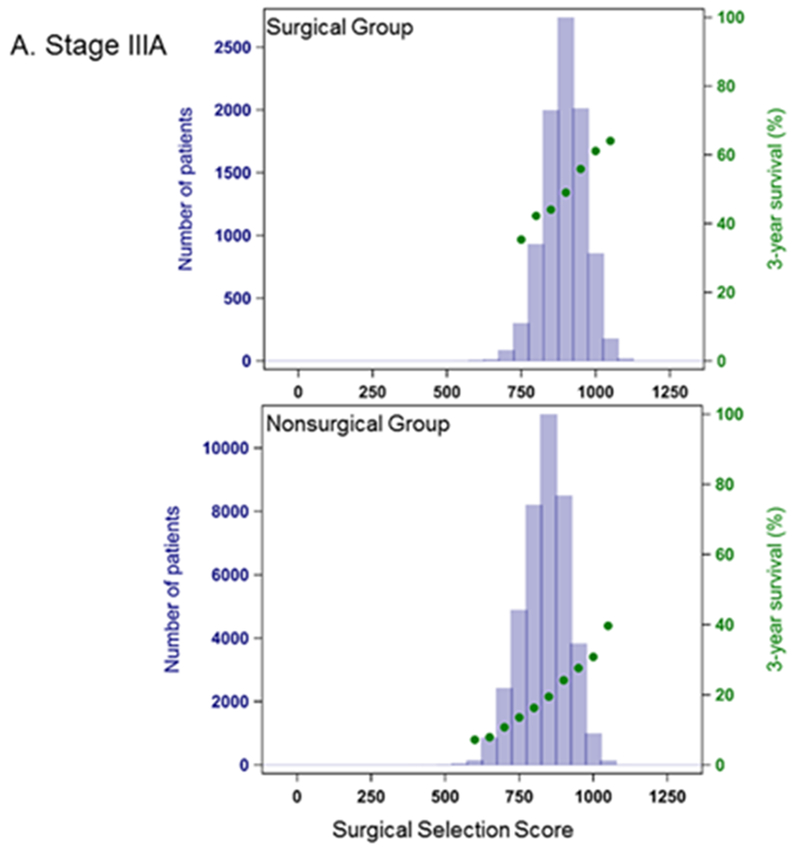
Figure 3.
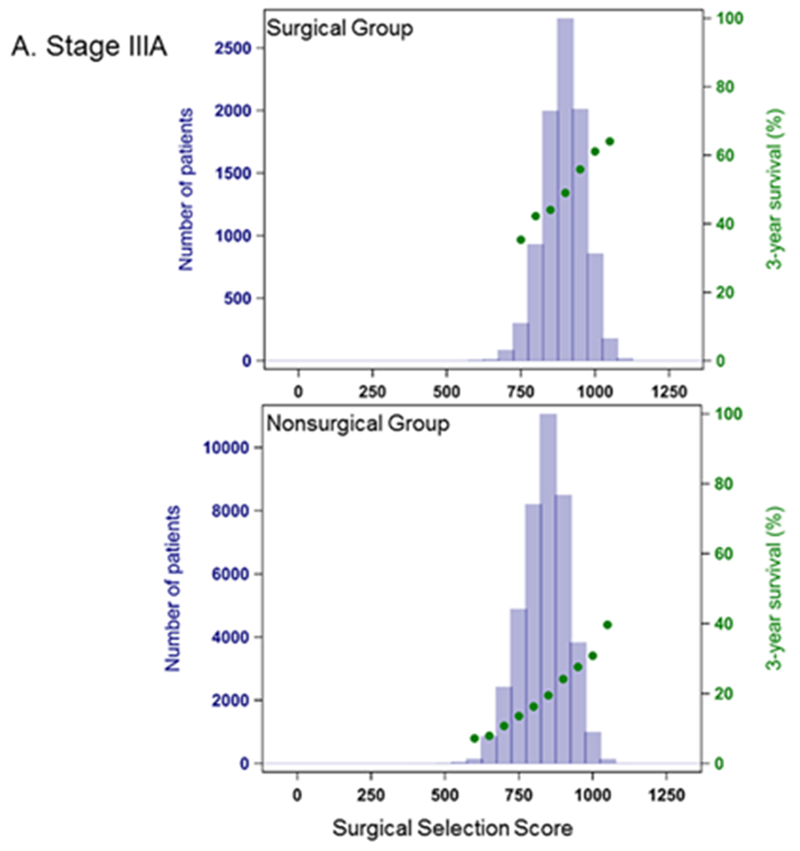
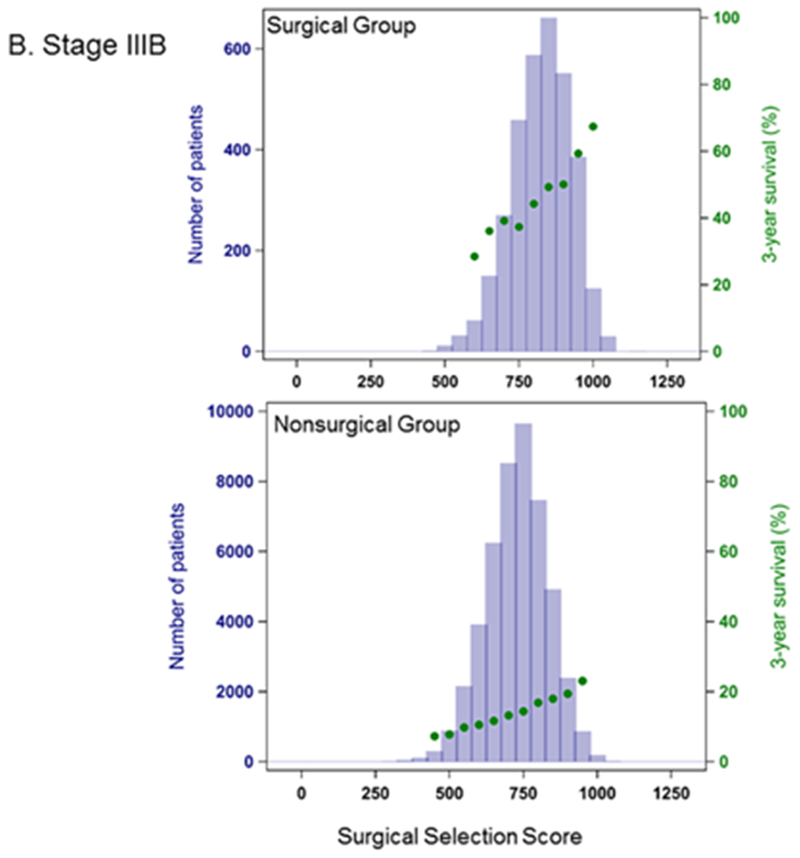
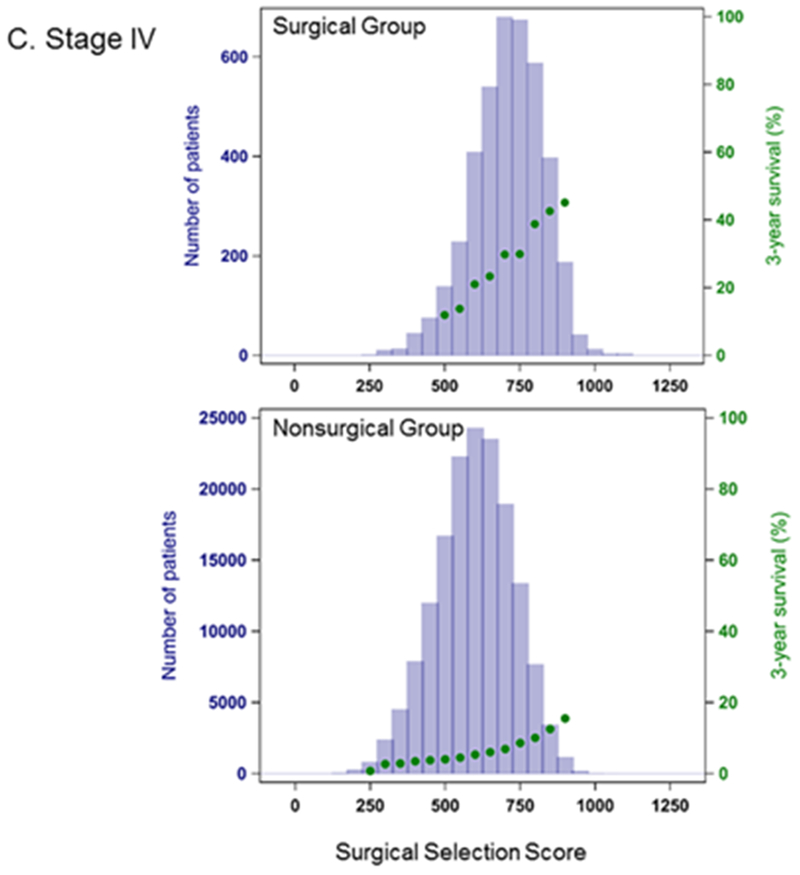
Distribution of Surgical Selection Score and Kaplan-Meier 3-year Survival Probability. A. Stage IIIA B. Stage IIIB C. Stage IV Patients have significantly longer survival when surgery is included in their treatment regimens.
A substantial number of non-surgical patients, however, have high SSS values, comparable to the surgical group. We assessed the potential impact of surgery on the hazard of death using the Cox model separately by stage, restricting analysis to approximately the upper quartile of SSS (900 for Stage IIIA, 800 for Stage IIIB, 700 for Stage IV) and adjusting for narrowly defined SSS categories. Within this group, across all stages, the hazard rate was at least 2 times higher for patients not treated surgically compared to patients with similar SSS that received surgical treatment. The hazard ratios (HR) were Stage IIIA: HR 2.1, 95% CI: 2.0-2.2, Stage IIIB: HR 2.3, 95% CI: 2.2-2.5, Stage IV: HR 2.3, 95% CI: 2.2-2.4.
Discussion
Using clinical, demographic, and pathologic variables, we have created a novel prediction score that estimates the probability of surgical treatment and OS accurately in advanced stage NSCLC. Across all stages, there is a meaningful survival advantage for surgical patients. The SSS uses the AJCC staging system variables supplemented by selected clinical variables that are available at the time of treatment decisions to estimate probability of surgical treatment and survival in a stage-specific manner. It has excellent discriminatory capabilities as shown by C-indices approaching 1 for both the outcomes described in our previous publication and in the current analysis. In the current analysis, we defined the stage-specific ability of the SSS to predict OS for advanced stage NSCLC patients and assessed the impact of surgery in patients with same stage and very comparable high SSS in an attempt to mitigate the influence of selection bias.
Our data support a therapeutic effect of surgery for at least some of these patients beyond what can be explained by selection bias given the high precision of the SSS to predict surgical treatment and the improved OS in surgical patients at comparable high SSS. In our view, these findings warrant further investigation to understand the potential favorable effects of surgery in appropriately selected advanced stage NSCLC patients. As mentioned previously, there are data to suggest improved survival in patients with advanced stage NSCLC who undergo pulmonary resection. A recent study using NCDB data that reflected treatments given for stage IIIA NSCLC from 1998-2010 demonstrated a low incidence of pneumonectomy (16%) and superior survival outcomes for surgical patients who were matched to non-surgical patient using demographic variables (median survival 35.9 for trimodality patients vs. 19.7 months for chemotherapy and radiation patients, p <0.001).6 In a study of stage IIIB patients using data from the SEER database for 2004-2012, median OS was 29 months in patients treated with surgery and radiation compared to only 11 months in patients treated with radiation alone (p < 0.0001).14 Similarly, Bateni et al. found that lung resection in stage IV cancer patients is safe and associated with similar 30-day mortality and serious morbidity when compared to patients without disseminated malignancy in the American College of Surgeons National Surgical Quality Improvement Program dataset.15 Although these studies attempted to control for selection bias with statistical techniques, similar to ours, they are limited by their retrospective nature and inability to assess for confounding variables that are not captured in these large datasets.7 Despite these limitations, there are significant differences in the OS trends in these series compared to the earlier randomized trials, reinforcing the concept that improvements in staging and multimodality therapies, including surgical outcomes, may identify a small subset of advanced staged patients who may benefit from surgical management. In concert with these data, the SSS is applicable to each of stages IIIA, IIIB, and IV NSCLC patients and continues to highlight the potential benefit of including surgery in the treatment of advanced stage patients, including stage IIIB patients.
Importantly, although current NCCN guidelines endorse limited recommendations for surgical treatment for advanced stage NSCLC patients, these guidelines are drawn from studies that have not adequately evaluated the heterogeneity among advanced stage NSCLC patients. Moreover, these studies do not reflect contemporary surgical outcomes using current minimally invasive surgical techniques and strategies to minimize the use of highly morbid pneumonectomy.4,16,17 For example, in a randomized trial of induction chemotherapy followed by surgery or radiotherapy for stage IIIA-N2 NSCLC patients, representing treatments given from 1994-2002, van Meerbeeck et al. reported exploratory thoracotomy in 14%, pneumonectomy in 47% and incomplete resection in 50% of patients. Therefore, it is not surprising that the authors observed no differences in OS between the surgical and radiotherapy cohorts.17 Similarly, Albain et al. compared induction chemotherapy plus radiation followed by surgery to induction chemotherapy plus definitive radiotherapy in a randomized trial of stage IIIA-N2 patients. These authors also found no significant difference in OS between the surgical and non-surgical groups.16 However, this trial was also noted to have a high rate of pneumonectomy (34%) and 9% of patients underwent no or incomplete resection. Additionally, their patient sample was heterogeneous with 20% having 2 positive nodal stations and 2% having 3 positive nodal stations. Consequently, it is likely that patient heterogeneity and poor surgical outcomes did not allow for a fair comparison between surgically and nonsurgically treated groups as well as under-estimate survival outcomes observed with surgery in a more contemporaneous cohort such as ours. These data should raise questions about the role of thoracic surgery for carefully selected advanced stage NSCLC patients in the future and suggest the need for randomized trials applying contemporary surgical techniques.
Because of the heterogeneous nature of advanced stage NSCLC patients and multiplicity of treatment options, clinical trials to assess the true therapeutic impact of surgery for these patients are likely prohibitively challenging to design and accrue. Yet, delivery of an effective treatment modality, including surgery, to an appropriately selected patient subset who will derive benefit is the cornerstone of personalized medicine. Unlike retrospective database analyses which only allow for prediction of outcomes after treatment decisions have been made, we maintain that a significant strength of the SSS is its ability to be used prospectively to assess a patient’s probability of both receiving surgical treatment and deriving improved OS.18–20 Moreover, given its novel ability to be used prospectively, after further validation, the SSS may have a role in reducing treatment variation since it can be used by both surgeons and non-surgeons to identify patients who may benefit from consideration for surgical treatment and/or enrollment into prospective clinical trials stratified by SSS. We do not contend that the SSS should be used as a dichotomous tool to decide who can and cannot undergo surgery as part of multimodality treatment. Rather, we believe it may help non-surgeons identify additional patients who could be evaluated for surgery; and that surgeons would provide the appropriate evaluation of surgical candidacy to include a physiologic assessment, tumor resectability, and assessment of disease burden, as per guidelines.4 Multidisciplinary tumor boards and telehealth interventions could then be used to evaluate and extend surgical options to patients who might not otherwise be exposed.21,22
Despite its strengths, it is important to acknowledge the limitations of our analysis. The SSS is limited by the ongoing presence of unmeasured confounding variables. Because tumor resectability, pulmonary function, and smoking status are not captured in administrative datasets such as the NCDB, the SSS cannot definitively predict which patients will be appropriate for surgical resection. These factors may have biased our results in favor of surgery despite controlling for comorbidities using the Charlson index, which is the only available measure of performance status in the NCDB. Additionally, disease burden and intent of resection are not currently captured in the NCDB and would impact the decision to offer therapeutic surgical resection to a patient. It is also likely that response to systemic treatment and molecular status of tumors were considered in surgical decisions, but there is no large dataset that currently capture these data or allow it to be analyzed. Therefore, future analyses incorporating these data will be important to validate our findings and thereby optimize personalized treatment for these patients. In a manuscript that is heavily dependent on statistical analysis, we must acknowledge statistical limitations. Patients missing essential characteristics like the Charlson index or survival information (when needed) were omitted from the cohort prior to the statistical analysis and we did not attempt to impute missing values since we believe it is reasonable to assume these data were missing at random. It is a limitation that we had to omit incomplete data and we cannot be certain that data was missing at random even though patients with missing survival information were evenly distributed across the surgery and non-surgery groups and also SSS strata.
Although it is possible that there are patients with high SSS who are not candidates for surgical resection, it is also possible that the SSS will identify patients who will derive benefit from surgery, but were previously not offered this modality. We acknowledge that our analysis using the SSS cannot fully eliminate the impact of selection bias on the survival outcomes observed, but the magnitude of the clinically and statistically significant survival differences between the groups, particularly when controlling for SSS, suggest that further exploration of the association of surgery with improved survival is warranted. In the era of increasingly effective systemic therapies, the incorporation of surgery into a careful multimodality treatment approach may improve disease control and OS beyond what can be attributed to selection bias alone.
Conclusion
The management of advanced stage NSCLC is undergoing a paradigm shift, and patients whose prognosis was previously measured in months can now often be measured in years. We have derived a SSS, which identifies factors, which robustly predict selection for surgical management and OS in stage IIIA IIIR and IV NSCLC patients Therefore our data suggest that the SSS may prove to be a valuable tool for clinicians to help identify patients who could potentially benefit from surgical evaluation in an era where treatment options for advanced stage NSCLC are expanding, but overall treatment nihilism appears to persist for lung cancer patients across all stages 2,23–25
Central Picture Legend
Patients who undergo lung resection live longer than those treated nonsurgically.
Central Message
When patients with advanced stage NSCLC are treated with surgery, they live longer. This analysis demonstrates that this finding is not purely selection bias.
Perspective Statement
We used a prediction model to assess the therapeutic impact of lung resection on overall survival. We demonstrate that across all stages the hazard of death is twice as high in patients not treated surgically, compared to surgically treated patients. Our model can be used to identify patients who have the highest probability of benefiting from lung resection and to identify patients for whom lung resection has minimal opportunity to improve survival.
Acknowledgements:
The authors would like to thank Dr. Richard Bold for his assistance and support of this project.
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding sources:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This work was directly supported by the Department of Surgery Outcomes Research Group (EAD), an American Cancer Society Institutional Research Grant (EAD: ACS IRG-95-125-13) and the Christine and Helen Langraf Memorial Fund (EAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SEER Stat Fact Sheets: Lung and Bronchus Cancer. Surveillance, Epidemiology and End Results Program Cancer Statistics. http://seer.cancer.gov/statfacts/html/lungb.html Published 2016 Accessed February 28, 2018.
- 2.David EA, Daly ME, Li C-S, et al. Increasing Rates of No Treatment in Advanced Stage NSCLC Patients: A Propensity Matched Analysis. J Thorac Oncol. 2017;In Press. doi: 10.1016/j.jtho.2016.11.2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David EA, Canter RJ, Chen Y, et al. Surgical Management of Advanced Stage NSCLC is Decreasing but Remains Associated with Improved Survival. Ann Thorac Surg. 2016. doi: 10.1016/j.athoracsur.2016.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN Guidelines for NSCLC. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf Accessed January 1, 2018.
- 5.Bott MJ, Patel AP, Crabtree TD, et al. Role for Surgical Resection in the Multidisciplinary Treatment of Stage IIIB Non-Small Cell Lung Cancer. Ann Thorac Surg. 2015;99(6): 1921–1928. doi: 10.1016/j.athoracsur.2015.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel AP, Crabtree TD, Bell JM, et al. National Patterns of Care and Outcomes After Combined Modality Therapy for Stage IIIA Non-Small-Cell Lung Cancer. J Thorac Oncol. 2014;9(5):612–621. doi: 10.1097/JT0.0000000000000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research A Review. JAMA Oncol. 2017. doi: 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 8.David EA, Andersen SW, Beckett LA, et al. A Model to Predict the Use of Surgical Resection for Advanced-Stage Non-Small Cell Lung Cancer Patients. Ann Thorac Surg. 2017;104(5):1665–1672. doi: 10.1016/j.athoracsur.2017.05.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria K, Stewart A, Winchester D, Ko C. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 11.Penciana MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi: 10.1002/sim.1802 [DOI] [PubMed] [Google Scholar]
- 12.Pencina MJ, D’Agostino RB Sr. Evaluating discrimination of risk prediction models: The c statistic. Jama. 2015;314(10):1063–1064. doi: 10.1001/jama.2015.11082 [DOI] [PubMed] [Google Scholar]
- 13.Harrel F, Lee K, Mark D. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med. 1996;15(4):361–387. doi: [DOI] [PubMed] [Google Scholar]
- 14.Herskovic A, Chitti B, Christos P, Wernicke AG, Parashar B. Addition of Surgery After Radiation Significantly Improves Survival in Stage IIIB Non-small Cell Lung Cancer: A Population-Based Analysis. WorldJSurg. 2016. doi: 10.1007/s00268-016-3764-y [DOI] [PubMed] [Google Scholar]
- 15.Bateni SB, David EA, Bold RJ, Cooke DT, Meyers FJ, Canter RJ. Lung resection is safe and feasible among stage IV cancer patients: An American College of Surgeons National Surgical Quality Improvement Program analysis. Surg (UnitedStates). 2017; 161(5). doi: 10.1016/j.surg.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 16.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379–386. doi: 10.1016/S0140-6736(09)60737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Meerbeeck JP, Kramer GWPM, Van Schil PEY, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99(6):442–450. doi: 10.1093/jnci/djk093 [DOI] [PubMed] [Google Scholar]
- 18.Young KA, Efiong E, Dove JT, et al. External Validation of a Survival Nomogram for Non-Small Cell Lung Cancer Using the National Cancer Database. Ann Surg Oncol. 2017. doi: 10.1245/s10434-017-5795-5 [DOI] [PubMed] [Google Scholar]
- 19.Hui Z, Dai H, Liang J, et al. Selection of proper candidates with resected pathological stage IIIA-N2 non-small cell lung cancer for postoperative radiotherapy. Thorac Cancer. 2015;6(3):346–353. doi: 10.1111/1759-7714.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi: 10.1200/JCO.2014.56.6661 [DOI] [PubMed] [Google Scholar]
- 21.Clark JM, Heifetz LJ, Palmer D, Brown LM, Cooke DT, David EA. Telehealth allows for clinical trial participation and multimodality therapy in a rural patient with stage 4 non-small cell lung cancer. Cancer Treat Res Commun. 2016;9(September):139–142. doi: 10.1016/j.ctarc.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehl KL, Landrum MB, Kahn KL, Gray SW, Chen AB, Keating NL. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11(3):e267–78. doi: 10.1200/JOP.2015.003673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis PM. The importance of multidisciplinary team management of patients with non-small-cell lung cancer. Curr Oncol. 2012;19(Suppl 1):S7–S15. doi: 10.3747/co.19.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vest MT, Herrin J, Soulos PR, et al. Use of new treatment modalities for non-small cell lung cancer care in the medicare population. Chest. 2013;143(2):429–435. doi: 10.1378/chest.12-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawe DE, Pond GR, Ellis PM. Assessment of Referral and Chemotherapy Treatment Patterns for Elderly Patients With Non–small-Cell Lung Cancer. Clin Lung Cancer. 2016. doi: 10.1016/j.cllc.2016.05.012 [DOI] [PubMed] [Google Scholar]


