Abstract
Background:
Postoperative bloodstream infection (BSI) is the most important determinant of recipient morbidity and mortality after liver transplantation (LT). Children who underwent LT are at the highest risk of developing BSI because of the significant surgical intervention, use of multiple devices, and administration of immunosuppressive agents. However, information regarding the risk factors for BSI in children after LT is limited.
Methods:
We retrospectively reviewed 210 children who underwent living-donor LT at the largest pediatric LT center in Japan. Patients’ characteristics, blood culture results and clinical outcomes were extracted from electronic medical records. Univariate and multivariate analyses were performed to identify the risk factors for BSI.
Results:
Among the 210 LT recipients, 53 (25%) recipients experienced 86 episodes of BSI during the observational period. The source of the BSI was identified only in 38%: catheter-related BSI (27%) peritonitis (7%), urinary tract infection (2%), pneumonia (1%) and infectious endocarditis (1%). A multivariate analysis demonstrated that body weight (P = 0.03), volume of blood loss during LT (P < 0.001) and cytomegalovirus (CMV) antigenemia positivity (P = 0.04) were independently associated with the development of BSI. The risk factors for BSI differed when we analyzed the subjects according to age (≤24 months and >24 months), blood loss and pediatric end-stage liver disease/model for end-stage liver disease versus positive CMV antigenemia.
Conclusions:
The volume of blood loss, postoperative CMV antigenemia positivity and body weight were associated with the development of BSI after LT in pediatric living-donor recipients. To identify the age-specific predictors of BSI in children who underwent LT, age-specific analyses are crucial.
Keywords: liver transplantation, bacteremia, children, age
Liver transplantation (LT) is the most effective procedure for patients with irreversible liver failure. Although the perioperative management of LT has advanced during the last few decades, morbidity and mortality because of serious infection after transplantation, including LT, are still major issues. Especially, bacterial infection is the most common cause of death in recipients after LT.1 Bloodstream infection (BSI) has been known as a major determinant for recipient morbidity and mortality after LT.2–7 In adults, several risk factors are known to be associated with BSI after LT. Preoperative factors for developing BSI after LT include severity of liver diseases [Child-Pugh class C, higher model for end-stage liver disease (MELD) score and united network of organ sharing class IIA], underlying diseases (posthepatitis B or C cirrhosis), massive pleural effusion or ascites requiring drainage, diabetes mellitus, low serum albumin level, older donor or recipient age and ABO incompatibility.2,3,5,8–10 Similarly, operative blood loss, positive bile culture, surgery after LT (including retransplantation), postoperative cytomegalovirus (CMV) infection, higher acute physiology and chronic health evaluation (APACHE) II score after LT and longer catheterization after LT have been reported as intraoperative or postoperative risk factors.3,5,7–9
In children, bacterial infection after LT is also an important factor in determining the morbidity and mortality of recipients. However, studies of infectious complications after LT in children are scarce, and previous studies were performed with limited number of study population and age-specific analyses.11–13 The purpose of this study is to investigate the risk factors of BSI and mortality in children after living-donor LT.
PATIENTS AND METHODS
Study Subjects
We retrospectively reviewed recipients who underwent LT at the National Center for Child Health and Development, the largest pediatric LT Center in Japan, between November 2005 and February 2013. A total of 232 LTs were performed for 227 recipients during the study period. Among them, adult cases (>19 years; n = 6), deceased donor cases (n = 10) and recipients who received retrans-plantation because of graft failure (n = 5, and 4 of them received deceased donor LT) were excluded. Finally, a total of 210 pediatric living-donor cases were included in the study.
The following information was extracted from electronic medical records: preoperative variables including age, gender, weight, underlying diseases, ABO incompatibility, CMV serostatus of donors and recipients, donor age and pediatric end-stage liver disease (PELD)/MELD score; intraoperative variables including operating time, blood volume loss during LT, graft-to-recipient body weight ratio, cold ischemic time, warm ischemic time and biliary complication and postoperative variables including acute rejection, positivity of CMV antigenemia after LT, blood culture results, clinical course and mortality. This study was approved by the Institutional Board of Privacy and Security at the National Center for Child Health and Development.
Antimicrobial Prophylaxis
The regular perioperative prophylaxis consisted of ampicillin (120 mg/kg/day, q6hrs) and cefotaxime (120 mg/kg/day, q6hrs) administered intravenously within 1 hour before the LT and continued for 48 hours. We did not routinely check for methicillin-resistant Staphylococcus aureus (MRSA) colonization; however, if the patient had a history of MRSA infection or colonization, alternative regimen including vancomycin was considered. In addition, the physician may modify the prophylactic regimen according to the recipient’s history of infectious diseases. Oral kanamycin and miconazole were used for selective decontamination of digestive tract for 3 days before LT. Sulfamethoxazole/trimethoprim (trimethoprim 4 mg/kg/day, orally, q24hrs) was prescribed for Pneumocystis jirovecii infection prophylaxis for the first 3 months after LT. CMV was monitored weekly by CMV antigenemia for the first 3 months after LT, and ganciclovir was initiated preemptively if CMV antigenemia reached over 5/50,000.14 Routine antiviral or antifungal prophylaxis was not performed.
Immunosuppression
Standard immunosuppression consisted of corticosteroids and tacrolimus. Methylprednisolone was started intraoperatively (10 mg/kg/dose) and continued with tapering for the first 3 months after LT. Tacrolimus was also started 1 day after LT, and the dose was adjusted to maintain a trough level of 10–15 mg/L for the first 2 weeks, followed by 8–10 mg/L (day 15–28 after LT), 6–8 mg/L (day 29–90) and 4–6 mg/L (after day 91).14
Definition of BSI
All blood cultures collected after LT were included in the study. Skin contaminants such as coagulase-negative staphylococci, Bacillus spp., Propionibacterium spp. and Micrococcus spp. when positive in blood culture were considered as pathogens causing BSI only if the organisms were isolated from 2 separate blood cultures accompanied with clinical signs of infection. We also considered an isolate as a pathogen if it was isolated as a part of polymicrobial bacteremia. Other organisms were considered significant when a single blood culture became positive with signs of infection.
Fever Workup and Source of BSI
When recipients developed fever or abnormal vital signs suggesting BSI, blood cultures were performed routinely. We usually performed blood cultures from central venous catheter (CVC; if in place) and other sites (arterial line or venipuncture) simultaneously. Other investigations for medical device–related infection (sputum, urine and ascites cultures and/or imaging studies such as chest and abdominal radiographs or a computed tomography scans) were performed as indicated to detect the focus of infection. In addition, exploratory laparotomy was performed when necessary.
Catheter-related BSI was defined as the same pathogens being identified from both catheter tip and blood cultures, or the same pathogens were identified from 2 blood cultures taken from the central line and peripheral blood.15 Similarly, urinary tract infection, pneumonia and peritonitis were considered as foci of BSI if the same pathogens were identified from both blood culture and urine, sputum or ascites cultures, respectively, with clinical signs compatible for these infections. CVCs were inserted under maximal barrier precaution to minimize the risk of catheter-related BSI. For maintenance, we used either gauze or a sterile, semipermeable dressing to cover the catheter insertion site. The catheter site dressing was replaced when it became damp or loose, or when observation of the catheter-inserted site was necessary. We also removed any intravascular catheter that was no longer essential.
Statistical Analysis
Demographic and clinical differences between those with and without BSI were evaluated using the Mann-Whitney U test for continuous variables and Fisher exact test for categorical and binary variables; 95% confidence intervals for the effect of demographic and clinical predictors on BSI were evaluated using logistic regression. The distributions of blood loss during LT and operative time were highly skewed, so natural log transformation was applied to blood loss during LT and to the operative time before analyses. The effects of predictors of BSI, adjusted for other covariates, were evaluated using multiple logistic regression. Variables that showed a P value ≤0.20 in univariate analyses were introduced into a multivariate model. Backward selection logistic regression analysis was performed, with an inclusion threshold of 0.20 to identify the risk factors for BSI after LT. Several variables were included in the multiple logistic regression model, regardless of statistical significance, because of their predictive importance based on the existing literature: donor age, ABO compatibility, blood loss during LT and CMV antigenemia positivity. This approach ensures that the final multivariable model achieves a balance between parsimony and including important covariates.16 Thus, each potentially important predictor will be considered as candidate in the multivariable model, while accounting for the effect of the other predictors; the larger 0.20 threshold for inclusion insures that important predictors will be accounted for in the model even though they may not reach statistical significance at the 0.05 level, possibly because of the limited sample size. Because the data of PELD/MELD scores had several missing values, multiple imputation was used throughout the multiple logistic regression analysis.17 The strength and shape of association between several clinical predictors and BSI was modified by age; therefore, separate univariable and multivariable analyses were performed for recipients ≤24 and >24 months old. Overall survival distributions for the children with and without BSI were calculated using the Kaplan-Meier method and compared using the log-rank test. All analyses were performed by SPSS version 22.0 software package (SPSS, Inc., Chicago, IL).
RESULTS
Pathogens and Focus of BSI
The characteristics of the 210 recipients who received living-donor LT are summarized in Table 1. We observed 86 posttransplant BSI episodes in 53 (25.2%) recipients. The most common pathogen was S. aureus [n = 17 (19%), MRSA = 12 (71%), Methicillin-sensitive Staphylococcus aureus = 5 (29%)], followed by Klebsiella spp. [n = 17 [19%]), coagulase-negative staphylococci [n = 9 (10%)], Enterobacter spp. [n = 9 (10%), Escherichia coli [n = 6 (7%)] and Enterococcus spp. [n = 5 (6%)]. Gram-negative rods (GNRs) were more common than Gram-positive coccis (GPCs; 53% and 37%, respectively); however, Candida spp. [n = 4 (4%)] was rare even with a lack of routine antifungal prophylaxis after LT. In spite of extensive fever workup, 62% of recipients did not demonstrate clear focus of infection. Among the identified sources, catheter-related BSI (27%) was the most common followed by peritonitis (7%), urinary tract infection (2%), pneumonia (1%) and infectious endocarditis (1%).
TABLE 1.
Patients’ Characteristics
| Variables | n |
|---|---|
| Total number of patients | 210 |
| Age (mo) | 14 (7–65) |
| Gender, male | 87 (41.4) |
| Body weight (kg) | 9.1 (6.7–16.8) |
| History of preoperative bacteremia* | 27 (12.9) |
| Underlying diseases | |
| Biliary atresia | 104 (49.5) |
| Metabolic diseases | 40 (19.0) |
| Acute liver failure | 35 (16.7) |
| Liver fibrosis | 12 (5.7) |
| Liver cirrhosis | 8 (3.8) |
| Vascular abnormalities | 6 (2.9) |
| Hepatic tumor | 5 (2.4) |
| PELD/MELD score (IQR)† | 13.0 (6.0–22.8) |
| Number of cases with postoperative bacteremia | 53 (25.2) |
| Number of episodes of postoperative bacteremia | 86 (1.6/case) |
| Death within 1 yr after liver transplantation | 20 (9.5) |
Preoperative bacteremia indicates a previous history of bacteremia that occurred at any period before liver transplantation.
In total, 164 cases (78%) were available for PELD/MELD data.
IQR indicates interquartile range; MELD, model for end-stage liver disease; PELD, pediatric end-stage liver disease.
Data are represented as n (%) and median (IQR).
Timing of BSI
Among the 53 recipients with BSI, the majority of the first episode of BSI occurred within 28 days after LT [n = 47 (89%)]. When we analyzed the timing of the first BSI episode by GPC and GNR, the median onset of BSI by GPC [6 days; interquartile range, 4–11 days] was shorter than that of BSI by GNR (14 days; IQR: 7–26 days; P = 0.003).
Predictors of BSI
The perioperative clinical variables of posttransplant BSI were compared between BSI (n = 53) and non-BSI group (n = 157). In preoperative variables, univariate analyses showed significant differences in ABO compatibility (P = 0.045) and PELD/MELD score (P = 0.006). In contrast, no significant differences were noted in other variables, such as age (P = 0.11), sex (P = 0.63), underlying diseases (P = 0.76) and CMV serostatus before LT (P = 0.22) and donor age (P = 0.48; Table 2, preoperative variables).
TABLE 2.
Univariate and Multivariate Analyses of Predictors of Blood Stream Infections After Liver Transplantation
| Variables | BSIs (n = 53) |
Non-BSIs (n = 157) |
OR (95% CI)* | OR (95% CI)† | ||
|---|---|---|---|---|---|---|
| Unadjusted | P Value* | Adjusted | P value† | |||
| Preoperative variables‡ | ||||||
| Age (mo), median (IQR) | 8.0 (6.0–56.5) | 16.0 (7.5–66.0) | 1.00 (0.99–1.01) | 0.11 | ||
| Body weight (kg), median (IQR) | 8.0 (5.7–17.2) | 9.6 (7.0–16.5) | 1.00 (0.98–1.03) | 0.20 | 1.04 (1.00–1.08) | 0.03 |
| Gender, male | 20 (37.7%) | 67 (42.7%) | 0.81 (0.43–1.54) | 0.63 | ||
| Underlying diseases, n (%) | ||||||
| Biliary atresia | 25 (47.2) | 79 (50.3) | Reference | 0.76 | ||
| Metabolic diseases | 11 (20.8) | 29 (18.5) | 1.20 (0.52–2.74) | |||
| Acute liver failure | 11 (20.8) | 24 (15.3) | 1.45 (0.62–3.37) | |||
| Liver fibrosis | 2 (3.8) | 10 (6.4) | 0.63 (0.13–3.08) | |||
| Liver cirrhosis | 3 (5.7) | 5 (3.2) | 1.90 (0.42–8.50) | |||
| Vascular abnormalities | 1 (1.9) | 5 (3.2) | 0.63 (0.07–5.67) | |||
| Hepatic tumor | 0 (0) | 5 (3.2) | 0 | |||
| Number of operation before LT, median (IQR) | 1 (0–1) | 1 (0–1) | 0.75 (0.53–1.06) | 0.14 | 0.67 (0.43–1.02) | 0.06 |
| CMV serostatus, n (%) | ||||||
| Donor +/recipient + | 19 (35.8) | 72 (45.9) | Reference | 0.22 | ||
| Donor +/ Recipient − | 19 (35.8) | 44 (28.0) | 1.64 (0.78–3.42) | |||
| Donor −/ Recipient + | 10 (18.9) | 16 (10.2) | 2.37 (0.93–6.05) | |||
| Donor −/ Recipient − | 5 (9.4) | 20 (12.7) | 0.95 (0.32–2.85) | |||
| Unknown | 0 (0.0) | 5 (3.2) | 0 | |||
| Preoperative status, n (%) | ||||||
| At home | 6 (11.3) | 26 (16.6) | Reference | 0.19 | ||
| Hospitalized (general ward) | 29 (54.7) | 97 (61.8) | 1.30 (0.49–3.45) | |||
| Hospitalized (ICU) | 18 (34.0) | 34 (21.7) | 2.29 (0.80–6.60) | |||
| ABO compatibility, n (%) | ||||||
| Identical/compatible | 40 (75.5) | 138 (87.9) | Reference | |||
| Incompatible | 13 (24.5) | 19 (12.1) | 2.36 (1.07–5.19) | 0.045 | 1.75 (0.73–4.21) | 0.21 |
| Donor age (yr) | 35.0 (31.5–41.5) | 35.0 (31.0–39.0) | 1.01 (0.97–1.06) | 0.48 | 1.01 (0.95–1.06) | 0.84 |
| PELD/MELD score§ | 19.0 (9.8–35.3) | 12.5 (3.5–21.0) | 1.04 (1.01–1.07) | 0.006 | 1.03 (1.00–1.06) | 0.07 |
| Intraoperative and postoperative variables‡ | ||||||
| Reconstruction of the biliary duct, n (%) | ||||||
| Roux-en Y | 48 (90.6) | 147 (93.6) | Reference | 0.54 | ||
| Choledococholedocostomy | 5 (9.4) | 10 (6.4) | 1.53 (0.50–4.70) | |||
| Log-operative time (min), median (IQR) | 6.32 (6.05–6.52) | 6.21 (6.08–6.36) | 3.21 (1.05–9.85) | 0.10 | ||
| Log-blood volume loss during LT (mL/kg), | 4.48 (4.02–5.46) | 4.11 (3.56–4.68) | 1.80 (1.28–2.55) | 0.001 | 2.23 (1.43–3.46) | <0.001 |
| median (IQR) | ||||||
| GRWR (%) | 2.4 (1.5–3.2) | 2.3 (1.6–3.2) | 1.00 (0.72–1.38) | 0.79 | ||
| CIT (min) | 35.0 (24.0–65.5) | 37.0 (22.5–71.5) | 1.00 (1.00–1.01) | 0.74 | ||
| WIT (min) | 32 (27.5–36.5) | 29 (25.0–36.0) | 1.01 (0.98–1.05) | 0.23 | ||
| Positivity of CMV antigenemia after LT | 22 (41.5%) | 38 (24.2%) | 2.22 (1.15–4.29) | 0.02 | 2.24 (1.05–4.77) | 0.04 |
| Acute rejection | 24 (45.3%) | 56 (35.7%) | 1.49 (0.79–2.81) | 0.25 | ||
| Biliary complication | 8 (15.1%) | 13 (8.3%) | 1.97 (0.77–5.05) | 0.19 | ||
P value from the Fisher exact test or Mann-Whitney U nonparametric test; 95% CIs are based on the logistic regression.
P value and 95% CIs from multiple logistic regression.
Univariate (unadjusted) and multivariate (adjusted) ORs are presented.
PELD/MELD score was calculated only cholestatic liver disease (42 in BSIs group and 122 in non-BSIs group).
BSIs indicates blood stream infections; CI, confidence interval; CMV, cytomegalovirus; CIT, cold ischemic time; GRWR, graft-to-recipient body weight ratio; ICU, intensive care unit; IQR, interquartile range; LT, liver transplantation; MELD, model for end-stage liver disease; OR, odds ratio; PELD, pediatric end-stage liver disease; WIT, warm ischemic time.
The intraoperative and postoperative variables of the recipients between these 2 groups are compared in Table 2. Univariate analyses revealed that both log-blood volume loss during LT (4.48 vs. 4.11 mL/kg, P = 0.001) and positive CMV antigenemia after LT (42% vs. 24%, P = 0.02) were higher in those who experienced BSI compared with those who did not experience BSI. The rate of Rouxen Y as a biliary reconstruction was similar in both groups (91% vs. 94%, P = 0.54). A logistic regression analysis demonstrated that the body weight, blood volume loss during LT and positive CMV anti-genemia after LT were independently associated with the development of BSI (P = 0.03, P <0.001 and P = 0.04, respectively).
Next, to identify the predictors of BSI in different age groups given their different clinical background, we analyzed the risk factors of BSI for recipients aged ≤24 months and >24 months (Table, Supplemental Digital Content 1, http://links.lww.com/INF/C186). For those ≤24 months, univariate analyses demonstrated that the following factors were associated with a higher risk of BSI: younger age, lower body weight, fewer operations before LT, higher PELD/MELD score and larger blood loss during LT. In multivariable analyses, only higher PELD/MELD score and larger blood loss were associated with a higher risk of BSI. In contrast, in those >24 months, univariable predictors of BSI were longer operative time, larger blood loss, lower graft-to-recipient body weight ratio and positive CMV antigenemia, whereas in multivariable analyses, only positive CMV antigenemia remained a significant predictor of BSI. Identified risk factors developing BSI in total study subjects and in subgroups stratified by age by univariate and multivariate analyses are summarized in Table, Supplemental Digital Content 2, http://links.lww.com/INF/C187.
Prognosis
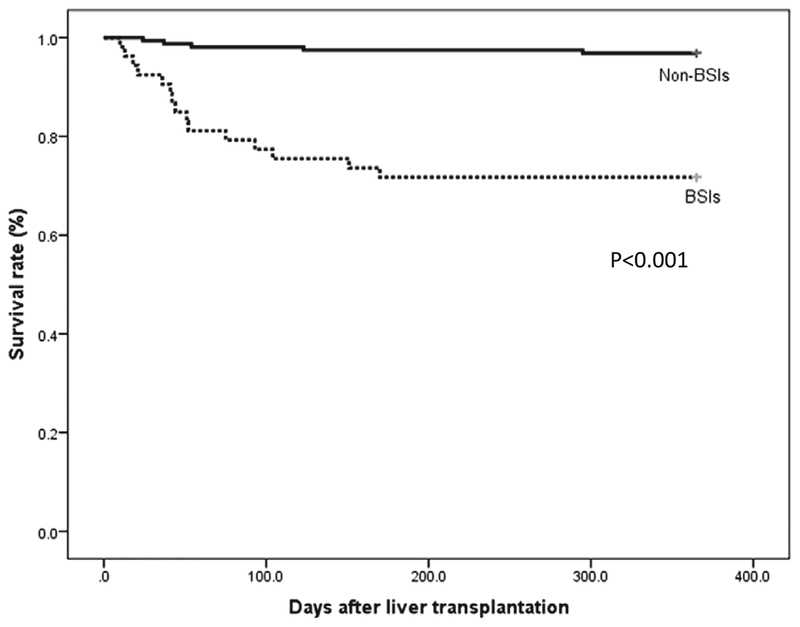
The survival curves of the 2 groups are shown in Figure 1. Overall, 20 of 210 (9.5%) recipients died within 1 year after living-donor LT. The causes of death were sepsis [including both micro-biologically proven and clinical sepsis; n = 14 (70%)], graft failure [n = 3 (15%)] and others [n = 3 (15%)]. One-year mortality rate after LT was higher in those who experienced BSI (28.3%, 15/53) compared with those who did not (3.2%, 5/157; P < 0.001).
FIGURE 1.
Survival rate of recipients with blood stream infection (BSI; n = 53) and without blood stream infection (n = 157).
DISCUSSION
This is a large-scale study with extensive statistical analyses evaluating the risk factors for BSI after living-donor LT in pediatric recipients. We found that the volume of blood loss, postoperative CMV antigenemia positivity and body weight of recipients were independently associated with the development of BSI in pediatric LT recipients. Notably, these risk factors differed with age.
To the best of our knowledge, this is the first report that demonstrated the relationship between the volume of blood loss during LT and frequency of BSI after pediatric liver LT. In adults, operative blood loss was reported as an independent risk factor for posttransplant bacteremia in living-donor LT recipients.9 In addition, some investigators reported that blood transfusion, which is indirectly correlated with blood loss, increased the risk of bacterial infection after certain surgical interventions.18–20 The reason for the relationship is still unclear; however, it could be because the volume of blood loss is a surrogate marker for technical difficulty of the operation, or poor preoperative condition, which can predis-pose to postoperative infection. Several investigators reported that immunosuppression, such as low CD4:CD8 T-lymphocyte ratio or natural killer T-cell activity, may alter consequently after blood transfusion.21–23 This study did not evaluate the amount of blood transfused. Therefore, it is difficult to evaluate the impact of blood transfusion on the rate of BSI.
CMV infection is one of the most common viral infections after LT.24 There are a few reports that describe the causal relationship between CMV infection and bacterial infection in adult LT recipients,25 stem cell transplant recipients26 and animal models.27 Potential mechanisms for CMV-induced immunosuppression have been proposed, including suppression of CD4 T-lymphocyte activation and proliferation,28 as well as inhibition of alveolar macrophages expression of surface-soluble CD14, which impairs responsiveness to GNRs infection.29 In contrast, bacterial infection may further compromise and predispose recipients to CMV infection. The onset of CMV antigenemia was generally later than the onset of BSI, and patients were treated preemptively. Notably, some articles reported no significant differences in the rate of bacterial infection between universal prophylaxis and preemptive therapy in solid-organ transplant recipients.30,31 A further prospective study is necessary to clarify this issue for pediatric LT recipients.
In this study, 1 in 4 recipients (25.2%) developed BSI after LT. Among the recipients who developed BSI, the focus of fever was unclear in 62% of the cases in spite of detailed workup. A similar rate of BSI without an apparent focus (50%, 18/36) was also reported in pediatric living-donor LT recipients.13 In contrast, the rates of BSI and unknown focus of infection among those who developed BSI were apparently lower in living-donor LT adult recipients.3,10 One study demonstrated that 8.6% (21/242) of recipients developed bacteremia, whereas 14% (3/21) were diagnosed with bacteremia with unknown source.10 Similarly, a BSI rate of 34.1% (62/181) with the focus unknown was reported in 43% (27/62) of recipients.3 Although the reason for the higher rates of BSI and unknown focus of infection among pediatric patients who developed BSI without an apparent focus of fever is unclear, one possible explanation is the difference in bile duct reconstruction procedure between pediatric and adult recipients. In pediatric recipients, the Roux-en Y method, which connects the bile duct to the intestine, is generally chosen because of the small size of bile ducts or the requirements related to technical variants of LT.32 Several studies demonstrated that LT recipients who underwent the Roux-en Y method experienced more infectious episodes, including BSI, than those who underwent choledochocholedochostomy (CDCD).33–35 The Roux-en Y method might give rise to bacterial translocation or cholangitis, which can in turn cause BSI without an apparent focus of fever. In this study, we compared the frequency of bacteremia in those who received the Roux-en Y method (n = 195) and CDCD (n = 15), but no significant difference was observed (24.6% and 33.3%, P = 0.54). In view of the limited number of cases who underwent CDCD, further collection of cases are warranted to investigate the impact of surgical procedures on the rate of BSI.
We demonstrated that risk factors such as blood loss, age, body weight, duration of operation, positivity of CMV antigenemia and PELD/MELD score were associated with BSI. Interestingly, these risk factors differed when the study subjects were classified according to age. There are a few possible explanations for the differences. First, recipients ≤ 24 months and recipients > 24 months had essentially different baseline diseases for LT. Recipients ≤24 months required LT mainly because of severe biliary atresia (58%) and fulminant hepatic failure (21%), which were expected to have complicated postsurgical course. In contrast, recipients >24 months required LT because of milder form of biliary atresia (38%), metabolic diseases (22%) and liver fibrosis (14%). Second, before reaching 2 years of age, children grow more rapidly compared with those afterward.36 Therefore, the body weight of younger children may be a surrogate marker for age. A younger age and lower body weight were highly associated with BSI in recipients ≤24 months, which might be explained by their immature innate immune system. Finally, PELD score, calculated by serum albumin and bilirubin levels, INR, weight and height,37 reflects not only the severity of liver function but also the general condition including poor nutrition, which could lead to immunosuppression. Typically, immunity in infants and younger children is immature compared with that of older children; thus, the impact of immunosuppression because of poor general condition might be enhanced in infants and younger children.
This study was limited by its retrospective study nature. The information regarding the number of patients with medical devises including CVCs at the time of bacteremia, which was important to determine the focus of bacteremia, was not available in the database.
In conclusion, blood volume loss during LT and positive CMV antigenemia after LT were the risk factors for developing posttransplant BSI in pediatric living-donor LT recipients. Recognition of these factors is useful in identifying individuals who are at risks of developing BSI after LT. Age-specific analyses aided in obtaining better predictors of BSI after LT. Further strategies to prevent posttransplant BSI in children are needed to improve the outcome.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study participants and laboratory technicians at National Center for Child Health and Development to identify the pathogens causing BSI and health care professionals dedicating the care for the LT donors and recipients. They also thank Dr. Julian Tang of the Department of Education for Clinical Research, National Center for Child Health and Development, for proofreading and editing this article and Dr. Takanobu Shigeta at the Transplantation Center, National Center for Child Health and Development, for his input related to liver transplantation.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Presented in part at the 50th Infectious Disease Society of America, Boston, MA, September 2011.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pidj.com).
REFERENCES
- 1.Torbenson M, Wang J, Nichols L, et al. Causes of death in autopsied liver transplantation patients. Mod Pathol. 1998;11:37–46. [PubMed] [Google Scholar]
- 2.Singh N, Paterson DL, Gayowski T, et al. Predicting bacteremia and bacteremic mortality in liver transplant recipients. Liver Transpl. 2000;6:54–61. [DOI] [PubMed] [Google Scholar]
- 3.Iida T, Kaido T, Yagi S, et al. Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transpl. 2010;16:1379–1385. [DOI] [PubMed] [Google Scholar]
- 4.Wan QQ, Ye QF, Ming YZ, et al. The risk factors for mortality in deceased donor liver transplant recipients with bloodstream infections. Transplant Proc. 2013;45:305–307. [DOI] [PubMed] [Google Scholar]
- 5.Kim SI, Kim YJ, Jun YH, et al. Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med J. 2009;50:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bert F, Larroque B, Paugam-Burtz C, et al. Microbial epidemiology and outcome of bloodstream infections in liver transplant recipients: an analysis of 259 episodes. Liver Transpl. 2010;16:393–401. [DOI] [PubMed] [Google Scholar]
- 7.Karvellas CJ, McPhail M, Pink F, et al. Bloodstream infection after elective liver transplantation is associated with increased mortality in patients with cirrhosis. J Crit Care. 2011;26:468–474. [DOI] [PubMed] [Google Scholar]
- 8.Bellier C, Bert F, Durand F, et al. Risk factors for Enterobacteriaceae bacteremia after liver transplantation. Transpl Int. 2008;21:755–763. [DOI] [PubMed] [Google Scholar]
- 9.Kaido T, Mori A, Ogura Y, et al. Pre-and perioperative factors affecting infection after living donor liver transplantation. Nutrition. 2012;28:1104–1108. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto M, Sugawara Y, Tamura S, et al. Bloodstream infection after living donor liver transplantation. Scand J Infect Dis. 2008;40:509–516. [DOI] [PubMed] [Google Scholar]
- 11.George DL, Arnow PM, Fox A, et al. Patterns of infection after pediatric liver transplantation. Am J Dis Child. 1992;146:924–929. [DOI] [PubMed] [Google Scholar]
- 12.Kim JE, Oh SH, Kim KM, et al. Infections after living donor liver transplantation in children. J Korean Med Sci. 2010;25:527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee KW, Oh SH, Kim KM, et al. Early bloodstream infection after pediatric living donor living transplantation. Transplant Proc. 2012;44:794–796. [DOI] [PubMed] [Google Scholar]
- 14.Saitoh A, Sakamoto S, Fukuda A, et al. A universal preemptive therapy for cytomegalovirus infections in children after live-donor liver transplantation. Transplantation. 2011;92:930–935. [DOI] [PubMed] [Google Scholar]
- 15.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vittinghof E, Gladden D, Shiboski S, McCulloch C. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer; 2012. [Google Scholar]
- 17.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chelemer SB, Prato BS, Cox PM Jr, et al. Association of bacterial infection and red blood cell transfusion after coronary artery bypass surgery. Ann Thorac Surg. 2002;73:138–142. [DOI] [PubMed] [Google Scholar]
- 19.Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–914. [DOI] [PubMed] [Google Scholar]
- 20.Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39:694–700. [DOI] [PubMed] [Google Scholar]
- 21.Triulzi DJ, Vanek K, Ryan DH, et al. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion. 1992;32:517–524. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan J, Sarnaik S, Gitlin J, et al. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308–310. [PubMed] [Google Scholar]
- 23.Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91:771–778. [PubMed] [Google Scholar]
- 24.Gao LH, Zheng SS. Cytomegalovirus and chronic allograft rejection in liver transplantation. World J Gastroenterol. 2004;10:1857–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Berg AP, Klompmaker IJ, Haagsma EB, et al. Evidence for an increased rate of bacterial infections in liver transplant patients with cytomegalovirus infection. Clin Transplant. 1996;10:224–231. [PubMed] [Google Scholar]
- 26.Nichols WG, Corey L, Gooley T, et al. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185:273–282. [DOI] [PubMed] [Google Scholar]
- 27.Erickson EJ, Saffle JR, Morris SE, et al. Cytomegalovirus infection promotes bacterial translocation in thermally injured mice. J Burn Care Rehabil. 1990;11:428–435. [DOI] [PubMed] [Google Scholar]
- 28.Fornara O, Odeberg J, Khan Z, et al. Human cytomegalovirus particles directly suppress CD4 T-lymphocyte activation and proliferation. Immunobiology. 2013;218:1034–1040. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins HA, Monick MM, Hunninghake GW. Cytomegalovirus inhibits CD14 expression on human alveolar macrophages. J Infect Dis. 1996;174:69–74. [DOI] [PubMed] [Google Scholar]
- 30.Owers DS, Webster AC, Strippoli GF, et al. Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2013;2:CD005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Florescu DF, Qiu F, Schmidt CM, et al. A direct and indirect comparison meta-analysis on the efficacy of cytomegalovirus preventive strategies in solid organ transplant. Clin Infect Dis. 2014;58:785–803. [DOI] [PubMed] [Google Scholar]
- 32.Halasa N, Green M. Immunizations and infectious diseases in pediatric liver transplantation. Liver Transpl. 2008;14:1389–1399. [DOI] [PubMed] [Google Scholar]
- 33.Kusne S, Dummer JS, Singh N, et al. Infections after liver transplantation. An analysis of 101 consecutive cases. Medicine (Baltimore). 1988;67:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel R, Badley AD, Larson-Keller J, et al. Relevance and risk factors of enterococcal bacteremia following liver transplantation. Transplantation. 1996;61:1192–1197. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S, Sato Y, Oya H, et al. Risk factors and prevention of biliary anastomotic complications in adult living donor liver transplantation. World J Gastroenterol. 2007;13:4236–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;11:19–20. [PubMed] [Google Scholar]
- 37.McDiarmid SV, Anand R, Lindblad AS; Principal Investigators and Institutions of the Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.