Abstract
Objectives:
To examine plasma levels of dichlorodiphenyldichloroethene (DDE) and dichlorodiphenyltrichloroethane (DDT) in association with survival among women with breast cancer who participated in a population-based case-control study.
Methods:
Participants included 456 white and 292 black women from the Carolina Breast Cancer Study Phase I who were diagnosed with primary invasive breast cancer from 1993–1996, and who had available DDE/DDT and lipid measurements from blood samples obtained on average 4.1 months after diagnosis. Using the National Death Index, we identified 392 deaths (210 from breast cancer) over a median follow-up of 20.6 years. We used Cox regression to estimate covariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and breast cancer-specific 5-year mortality, and 20-year mortality conditional on 5-year survival, for lipid-standardized DDE and DDT levels. Associations stratified by race and estrogen receptor (ER) status were also examined.
Results:
The highest versus lowest DDE tertile and the highest vs non-detectable DDT quantile were associated with HRs of 1.95 (95%CI=1.31–2.92) and 1.64 (95%CI=1.10–2.44), respectively, for 20-year conditional all-cause mortality. DDE levels above versus below the median were associated with a HR of 1.69 (95%CI=1.06–2.68) for 20-year conditional breast cancer-specific mortality among women overall, and HRs were 2.36 (95%CI=1.03–5.42) among black women and 1.57 (95%CI=0.86–2.89) among white women (PInteraction=0.42), and 3.24 (95%CI=1.38–7.58) among women with ER− tumors and 1.29 (95%CI=0.73–2.28) among women with ER+ tumors (PInteraction=0.03).
Conclusion:
Exposure to DDE/DDT may adversely impact overall and breast cancer-specific survival. DDE exposure may contribute to the racial disparities in breast cancer survival.
Keywords: organochlorine compounds, DDT, DDE, pesticides, breast cancer, survival
Introduction
The broad-spectrum organochlorine insecticide dichlorodiphenyltrichloroethane (DDT) was introduced into the United States (US) in the 1940’s for agricultural and residential use (1). DDT was used widely and indiscriminately for decades; however, its use was restricted in the US in the late 1960’s and eventually banned in 1972 due to growing environmental and health concerns (1). DDT and its metabolite, dichlorodiphenyldichloroethene (DDE), are lipophilic and thus bioaccumulate in adipose tissues (2). Additionally, DDT is designated as a probable human carcinogen by the International Agency for Research on Cancer (IARC) based on evidence of association with non-Hodgkin lymphoma and testicular and liver cancers (3) while DDE is designated as a probable human carcinogen by the US Environmental Protection Agency (EPA) based on increased incidence of liver tumors in mice and hamsters and of thyroid tumors in female rats (4). Furthermore, DDT and DDE and are well-known endocrine disruptors (5). Given these characteristics, it was hypothesized that DDT and DDE exposure may impact risk of developing breast cancer (6), a hormone-dependent tumor and the most frequently diagnosed cancer among US women (7). While initial cohort studies reported elevated risks of breast cancer among women with high blood levels of DDT or DDE (8,9), subsequent studies generally failed to link these chemicals to the development of breast cancer (10,11). However, studies suggest that exposure to DDT and DDE during developmentally sensitive periods, including in utero (12) or early life (13), may increase the risk of developing breast cancer.
Tamoxifen, a selective estrogen receptor (ER) modulator, is a central component of ER+ breast cancer treatment as it inhibits cell growth by binding to and inhibiting the ER thereby reducing the likelihood of breast cancer progression and recurrence (14). It is therefore plausible that exposure to exogenous compounds that show hormonal activity including DDT and DDE (15), influences relevant pathways involved in breast cancer progression. The few studies conducted to date to test this hypothesis suggest that these and other organochlorine compounds (16–20) may impact recurrence or survival following breast cancer. Furthermore, despite consistently higher measured DDT/DDE levels among US black women as compared to white women (9,21), including a previous analysis of the Carolina Breast Cancer Study (CBCS) (22), to our knowledge, no prospective studies have considered whether differences in DDT/DDE exposure contributes to the US racial disparities in breast cancer survival. Today, five-year survival is estimated at 90% among white women, but only 81% among black women (23).
In this study, we examined the associations between plasma levels of organochlorine compounds DDE and DDT and all-cause and breast cancer-specific survival among white and black women who participated in a population-based North Carolina study. Given our previous work identifying higher organochlorine levels (22) and higher mortality rates (24) among black women as compared to white women the CBCS, we hypothesized that higher levels of DDT/DDE would be associated with worse survival following breast cancer diagnosis.
Methods
Study population
This study included 456 white women, 98% of whom were Caucasian, and 292 black women diagnosed with first primary invasive breast cancer from 1993–1996 with available DDT and DDE and lipid measurements available who participated in the CBCS Phase I. The CBCS Phase I was a population-based case-control study of breast cancer with follow-up for mortality among women diagnosed with breast cancer, as previously reported (25). During May 1993–December 1996, the CBCS enrolled and interviewed 861 women with (and 790 women without) breast cancer in 24 counties of North Carolina. Approximately 98% of participants who were interviewed in-person by nurses using a standardized questionnaire provided, on average within 4.1 months of diagnosis (range=0.8–19.2 months), three 10 mL blood samples for laboratory analyses, including analyses of organochlorine compounds DDE and DDT. All procedures performed in the CBCS involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of North Carolina at Chapel Hill.
Follow-up for Mortality
Date and cause of death were ascertained by linking participants to the National Death Index (NDI), a centralized database of death record information compiled from state vital statistics records (26). Breast cancer-related deaths were identified using International Statistical Classification of Diseases (ICD) codes ICD-9–174.9 and ICD-10-C-50.9 listed on the death certificate. Follow-up for mortality occurred from date of diagnosis in 1993–1996 until December 31, 2016. The median survival time for the 748 women included in this study was 20.6 years (min=0.4, max=23.7 years). Over the 20 years of follow-up, 392 women died from any cause, and of these, 210 died from breast cancer.
Laboratory Assessment
DDE and DDT levels were measured in plasma samples using gas chromatography/electron capture detection, as previously described (22). In brief, 2.0 mL plasma samples were treated with 1.0 mL methanol, spiked with a surrogate standard, and then extracted with three 2.5 mL portions of hexane:diethyl ether (1:1). The extract was fractionated using Florisil (R) open-column chromatography and the fraction was eluted with 35 mL of hexane, which contained p,p’-DDE, o,p’-DDE, and DDT. This fraction was concentrated to 0.5 mL and spiked with octachloronaphthalene as an external quantitation standard. The extract was analyzed by gas chromatography/electron capture detection using a DB-5 column and Hewlett-Packard Model 6890 instrument. Individual compounds were identified based upon chromatographic retention times relative to the internal standards and pattern recognition in the sample extract. Values below the limit of detection (LOD=0.0625 ng/mL), one p-p’-DDE value (0.1%) and 473 DDT values (63.2%), were imputed as the LOD divided by the square root of two (27). Because only 1% of participants had detectable levels of o,p’-DDE, this isomer was not considered further.
As lipid adjustment of organochlorine levels is recommended to account for non-fasting variation (27) and to more closely approximate adipose tissue levels (28), lipid profiles were measured at the Core Laboratory for Clinical Studies at Washington University School of Medicine (St. Louis, Missouri). As previously described (22), automated enzymatic assays were performed on a Technicon RA-1000 analyzer using cholesterol esterase and lipoprotein lipase. Organochlorine and lipid measurements were available on 748 (84%) women with breast cancer; reasons for failure to obtain laboratory measurements included insufficient plasma and interference in the sample.
Interview and Medical Record Data
As part of the case-control interview, participants were asked about known and suspected breast cancer risk and prognostic factors and demographic characteristics including self-reported race, education, smoking status, and parity and lactation history. At the time of the in-person interview, trained nurses collected anthropometric measurements in duplicate including height (inches) and weight (pounds) to determine body mass index (BMI in kg/m2). Breast cancer disease characteristics including stage at diagnosis, grade, tumor size, nodal status, and ER status, were obtained from medical records.
Statistical Analysis
For our primary analyses, we categorized levels of lipid-standardized DDE into tertiles (≤0.36, 0.37–1.00, and ≥1.01 μg/g lipid) and detectable levels of DDT at the median (<LOD, 0.01–0.03, and ≥0.04 μg/g lipid). We first used Kaplan-Meier survival curves to examine the unadjusted associations between DDE and DDT and all-cause and breast cancer-specific survival and to visually examine the proportional hazards assumption. We also evaluated the proportional hazards assumption by testing interaction terms of the exposure variables with dichotomized time (at 5 years) and by Schoenfeld residuals. We observed a divergence in the survival curves five years following breast cancer diagnosis. This interaction with time was supported by time-by-exposure interactions (all P<0.01) and by the Schoenfeld residuals; residuals were significantly correlated with time, ln-time, or time2 for DDE and DDT and all-cause mortality (all P<0.05), but not breast cancer-specific mortality (all P>0.10). Given the violations in the proportional hazards assumption, in multivariable models, examined associations within five years of diagnosis, as well as 20 years after diagnosis conditional on 5-year survival using Cox regression with a Heaviside function. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between tertiles of lipid-standardized DDE levels and quantiles of DDT and all-cause and breast cancer-specific mortality. We first adjusted the Cox models for age at diagnosis (continuous in years) and race (black vs. white). We then also included adjustment for smoking status (never, former, and current smoker), education (less than high school, high school, and college or greater), BMI (<25.0, 25.0–29.9, ≥30.0 kg/m2), parity/lactation history (nulliparous, parous/never lactated, parous/ever lactated), stage (III/IV vs. I/II), and ER status (ER+ vs. ER−). We used Efron’s approximation for tied event times (29). Estimates for linear trends (i.e., HRs and corresponding 95%CIs) used continuous natural log-transformed and log2-transformed lipid-standardized DDE/DDT levels in age- and race-adjusted and in full covariate-adjusted regression models. For full covariate-adjusted models, we also report the P-values for linear trend (PTrend). In sensitivity analyses, we examined associations between wet weight concentrations with and without adjustment for lipids in the regression models given that all three approaches may vary depending on the assumptions of the underlying causal structure (30).
For our secondary analyses in which we examined effect measure modification by race and ER status, DDE and DDT levels were categorized at the median (>0.58 vs. ≤0.58 μg/g lipid) and at the LOD, respectively. We first adjusted the Cox models for age at diagnosis and race, as appropriate. We then included adjustment for smoking status, education, BMI, parity/lactation history, stage, and ER status, as appropriate. For DDT models stratified by race, we also compared models with and without adjustment for ER status, as ER status may mediate the association between DDT and breast cancer-specific mortality (31). Effect modification was evaluated by stratifying the Cox models by race (black vs. white) and ER status (ER− vs. ER+). We also evaluated in full covariate-adjusted regression models, continuous log2-transformed lipid-standardized DDE/DDT levels-by covariate interactions (i.e., PInteraction). Last, using results of the Cox model we estimated the partial attributable fractions (PARp) and their corresponding 95% CIs for DDE among black women and white women separately for 20-year breast cancer-specific mortality using the publicly available SAS PAR Macro (https://www.hsph.harvard.edu/donna-spiegelman/software/par/) as outlined by Hertzmark, Wand, and Spiegelman (32,33).
All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) and were based on participants with complete data (i.e., complete-case analysis).
Results
Participant characteristics for the 748 women with invasive breast cancer included in this study, overall and by DDE tertiles, are reported in Table 1. Black women had mean DDE levels that were three times higher than those of white women (1.96 vs. 0.66 μg/g lipid), as previously reported for this group of women (22). Mean DDE levels were also higher among women aged ≥50 years than those <50 at diagnosis (1.71 vs. 0.78 μg/g lipid), among never smokers than among former smokers (1.28 vs 0.93 μg/g lipid), among women with a less than high school than some college or greater education (2.37 vs. 0.76 μg/g lipid), and among women with breast cancer stages III/IV than stages I/II (1.57 vs. 1.11 μg/g lipid). Patterns for lipid-standardized DDT quantiles by participant characteristics were similar to those observed for DDE (Supplemental Table 1).
Table 1.
Characteristics of CBCS Phase I women with invasive breast cancer and with available plasma DDE and lipid data (n=748).
| Characteristic | n (%) | DDE | DDE Tertiles (μg/g lipid) | ||
|---|---|---|---|---|---|
| μg/g lipid | ≤0.36 | 0.37–1.00 | ≥1.01 | ||
| Mean (SD) | n (%) | n (%) | n (%) | ||
| Race | |||||
| White | 456 (61.0) | 0.66 (0.77) | 209 (83.9) | 162 (65.1) | 85 (34.0) |
| Black | 292 (39.0) | 1.96 (2.24) | 40 (16.1) | 87 (34.9) | 165 (66.0) |
| Age at diagnosis (years) | |||||
| <50 | 438 (58.6) | 0.78 (0.93) | 197 (79.1) | 143 (57.4) | 98 (39.2) |
| ≥50 | 310 (41.4) | 1.71 (2.20) | 52 (20.9) | 106 (42.6) | 152 (60.8) |
| Education | |||||
| Less than high school | 137 (18.3) | 2.37 (2.85) | 25 (10.0) | 33 (13.2) | 79 (31.6) |
| High school | 281 (37.6) | 1.06 (1.18) | 74 (29.7) | 107 (43.0) | 100 (40.0) |
| Some college or greater | 330 (44.1) | 0.76 (0.93) | 150 (60.2) | 109 (43.8) | 71 (28.4) |
| Body mass index (kg/m2) | |||||
| <25.0 | 221 (30.1) | 1.22 (1.72) | 63 (26.0) | 82 (33.5) | 76 (30.8) |
| 25.0–29.9 | 229 (31.2) | 1.51 (1.55) | 46 (19.0) | 75 (30.6) | 108 (43.7) |
| ≥30.0 | 284 (38.7) | 0.85 (1.61) | 133 (55.0) | 88 (35.9) | 63 (25.5) |
| Missing | 14 | 7 | 4 | 3 | |
| Smoking status | |||||
| Never | 392 (52.4) | 1.28 (1.83) | 131 (52.6) | 120 (48.2) | 141 (56.4) |
| Former | 183 (24.5) | 0.93 (1.04) | 63 (25.3) | 62 (24.9) | 58 (23.2) |
| Current | 173 (23.1) | 1.16 (1.74) | 55 (22.1) | 67 (26.9) | 51 (20.4) |
| Parity/Lactation history | |||||
| Nulliparous | 112 (15.0) | 1.05 (1.49) | 41 (16.5) | 35 (14.1) | 36 (14.4) |
| Parous/never lactated | 387 (51.7) | 1.11 (1.28) | 115 (46.2) | 134 (53.8) | 138 (55.2) |
| Parous/ever lactated | 249 (33.3) | 1.30 (2.15) | 93 (37.3) | 80 (32.1) | 76 (30.4) |
| Stage | |||||
| I/II | 613 (88.2) | 1.11 (1.63) | 213 (92.2) | 206 (88.4) | 194 (84.0) |
| III/IV | 82 (11.8) | 1.57 (1.98) | 18 (7.8) | 27 (11.6) | 37 (16.0) |
| Missing | 53 | 18 | 16 | 19 | |
| Grade | |||||
| I/II | 376 (57.7) | 1.27 (1.94) | 132 (60.6) | 115 (54.5) | 129 (51.9) |
| III | 276 (42.3) | 1.10 (1.34) | 86 (39.4) | 96 (45.5) | 94 (42.1) |
| Missing | 96 | 31 | 38 | 27 | |
| Tumor size (cm) | |||||
| ≤2.0 | 396 (56.3) | 1.14 (1.73) | 130 (55.8) | 143 (61.1) | 123 (51.9) |
| >2.0 | 308 (43.8) | 1.22 (1.60) | 103 (44.2) | 91 (38.9) | 114 (48.1) |
| Missing | 44 | 16 | 15 | 13 | |
| Node status | |||||
| Negative | 442 (62.3) | 1.10 (1.64) | 148 (62.7) | 157 (66.5) | 137 (57.6) |
| Positive | 268 (37.7) | 1.30 (1.73) | 88 (37.3) | 79 (33.5) | 101 (42.4) |
| Missing | 38 | 13 | 13 | 12 | |
| Estrogen receptor (ER) status | |||||
| ER+ | 404 (58.7) | 1.30 (1.98) | 128 (55.4) | 134 (59.8) | 142 (60.9) |
| ER− | 284 (41.3) | 1.00 (1.13) | 103 (44.6) | 90 (40.2) | 91 (39.1) |
| Missing | 60 | 18 | 25 | 17 | |
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993–1996 (Phase I) and followed-up for vital status through December 31, 2016.
Breast Cancer-Specific Mortality
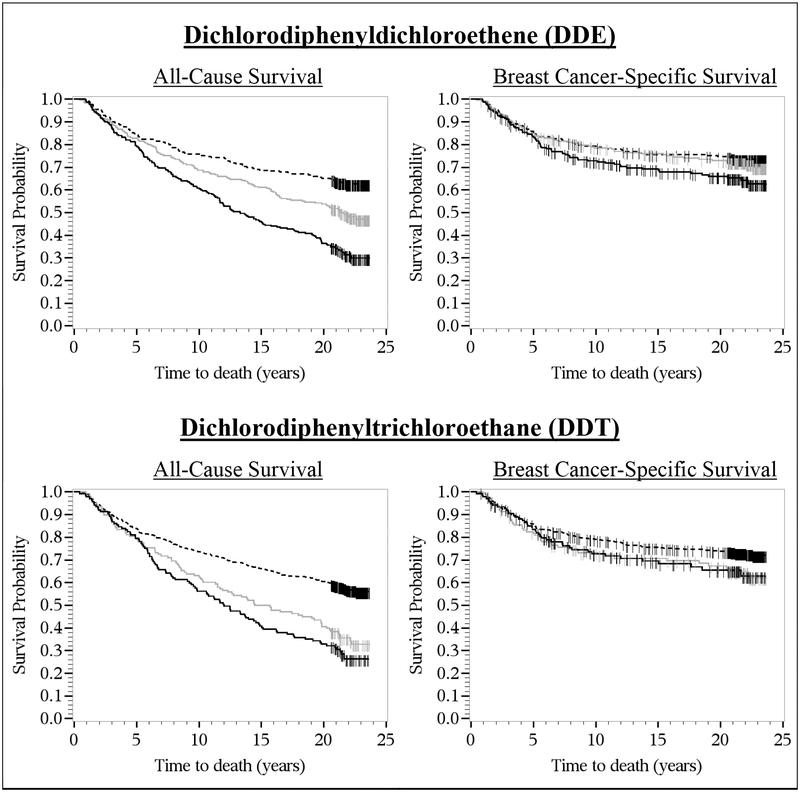
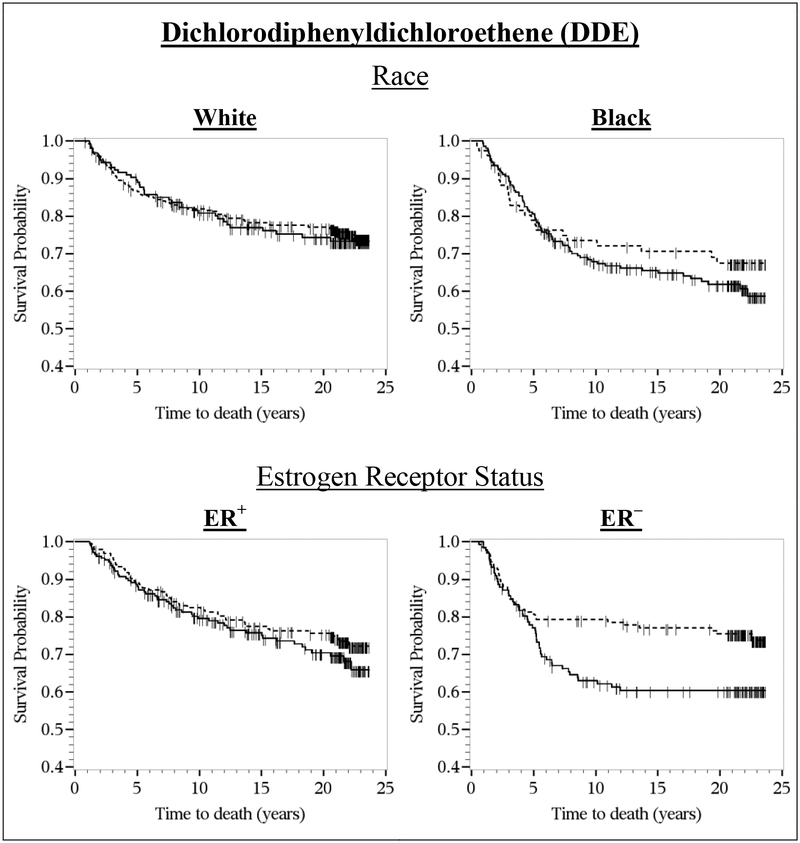
In the Kaplan-Meier survival curves, there was no difference in the breast cancer survival rates by DDE tertiles or DDT quantiles within the first five years of diagnosis (Figure 1). After five years of diagnosis, however, the survival curves diverged, with lower survival rates among women with the highest (≥1.01 μg/g lipid) versus lowest (≤0.36 μg/g lipid) DDE tertiles and among women with the highest (≥0.04 μg/g lipid) versus non-detectable DDT quantiles. In the covariate-stratified Kaplan-Meier survival curves, DDE levels above versus below the median were associated with lower survival rates among black women, but not among white women, and DDE-survival associations were more pronounced among women with ER– tumors than among women with ER+ tumors (Figure 2).
Figure 1.
Kaplan-Meier survival curves for DDE (dashed line, ≤0.36 μg/g lipid; solid gray line, 0.37–1.00 μg/g lipid; solid black line, ≥1.01 μg/g lipid) and DDT (dashed line, <LOD; solid gray line, 0.01–0.03 μg/g lipid; solid black line, ≥0.04 μg/g lipid) and all-cause and breast cancer-specific survival. CBCS women were diagnosed with invasive breast cancer from 1993–1996 and followed-up for vital status through December 31, 2016. The x-axis shows times to death in years; the y-axis shows proportion of participants alive.
Figure 2.
Kaplan-Meier survival curves for DDE (dashed line, ≤0.58 μg/g lipid; solid line, >0.58 μg/g lipid) and breast cancer-specific survival, overall and by race and estrogen receptor (ER) status. CBCS women were diagnosed with invasive breast cancer from 1993–1996 (Phase I) and followed-up for vital status through December 31, 2016. The x-axis shows times to death in years; the y-axis shows proportion of participants alive.
Detectable versus non-detectable DDT levels were associated with lower survival among white women, but not among black women, and DDT-survival associations were more pronounced among women with ER− tumors than among women with ER+ tumors (Supplemental Figure 1).
Consistent with the Kaplan-Meier survival curves, in the Cox regression analyses, lipid-standardized DDE and DDT levels were not associated with 5-year breast cancer-specific mortality. The highest (vs. lowest) tertile of DDE was associated with a HR of 1.79 (95%CI=1.06–3.03) for 20-year conditional breast cancer-specific mortality, in age and race-adjusted models (Table 2). However, this association was attenuated (HR=1.50, 95%CI=0.85–2.65; PTrend=0.17) after further adjustment for demographic and tumor characteristics. When categorized at the median, DDE levels above versus below the median were associated with a HR of breast cancer-specific mortality of 1.69 (95% CI=1.06–2.68) (Table 3). HRs for 20-year conditional breast cancer-specific mortality for DDE levels above versus below the median were elevated more so among black women (HR=2.36, 95%CI=1.03–5.42) than among white women (HR=1.57, 95%CI-0.86–2.89; PInteraction=0.42), and among women with ER− tumors (HR=3.24, 95%CI=1.38–7.58) than among women with ER+ tumors (HR=1.29, 95%CI=0.73–2.28; PInteraction=0.03) (Table 3). Furthermore, among black women the attributable fraction (PARp) for DDE for 20-year conditional breast cancer-specific mortality was 0.304 (95%CI= −0.103 to 0.624) whereas it was 0.162 (95%CI= −0.142 to 0.439) among white women. That is, among black women and white women, 30.4% and 16.2% of breast cancer-specific deaths, respectively, can be prevented if DDE exposure above versus below the median is eliminated from the population, while the distribution of other modifiable and non-modifiable risk factors is unchanged.
Table 2.
Cox regression hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between plasma levels of lipid-standardized DDE and DDT levels and mortality in the CBCS Phase I women diagnosed with invasive breast cancer in 1993–1996 (n=748).
| 5-Year All-Cause Mortality | 5-Year Breast Cancer Specific-Mortality | |||||||||
|
Analyte (μ/g lipid) |
Deaths (n=133) |
Censored (n=615) |
Model 1a | Model 2b |
Deaths (n=111) |
Censored (n=637) |
Model 1a | Model 2b | ||
| HR (95% CI) | HR (95% CI)c | PTrend | HR (95% CI) | HR (95% CI)d | PTrend | |||||
| DDE | ||||||||||
| ≤0.36 | 38 | 211 | 1 (Reference) | 1 (Reference) | 35 | 214 | 1 (Reference) | 1 (Reference) | ||
| 0.37–1.00 | 43 | 206 | 0.93 (0.60–1.45) | 0.85 (0.52–1.39) | 37 | 212 | 1.15 (0.71–1.85) | 1.00 (0.59–1.70) | ||
| ≥1.01 | 52 | 198 | 0.93 (0.60–1.47) | 0.86 (0.53–1.41) | 39 | 211 | 1.29 (0.78–2.13) | 1.01 (0.58–1.77) | ||
| Ln DDE | 0.98 (0.84–1.15) | 0.94 (0.79–1.11) | 1.13 (0.94–1.36) | 1.02 (0.84–1.25) | ||||||
| Log2(DDE) | 0.99 (0.88–1.10) | 0.96 (0.85–1.07) | 0.45 | 1.09 (0.96–1.23) | 1.01 (0.88–1.16) | 0.84 | ||||
| DDT | ||||||||||
| <LOD | 76 | 397 | 1 (Reference) | 1 (Reference) | 66 | 407 | 1 (Reference) | 1 (Reference) | ||
| 0.01–0.03 | 29 | 109 | 1.05 (0.68–1.62) | 1.03 (0.65–1.65) | 23 | 115 | 1.39 (0.86–2.24) | 1.27 (0.75–2.15) | ||
| ≥0.04 | 28 | 109 | 0.85 (0.53–1.36) | 0.72 (0.43–1.21) | 22 | 115 | 1.19 (0.70–2.03) | 0.80 (0.44–1.46) | ||
| Ln(DDT) | 0.91 (0.76–1.10) | 0.89 (0.73–1.09) | 1.04 (0.84–1.29) | 0.94 (0.74–1.20) | ||||||
| Log2(DDT) | 0.94 (0.83–1.07) | 0.92 (0.80–1.06) | 0.26 | 1.03 (0.89–1.19) | 0.96 (0.81–1.14) | 0.63 | ||||
| 20-year Conditional All-Cause Mortality | 20-year Conditional Breast Cancer-Specific Mortality | |||||||||
|
Analyte (μ/g lipid) |
Deaths (n=259) |
Censored (n=356) |
Model 1a | Model 2b |
Deaths (n=97) |
Censored (n=518) |
Model 1a | Model 2b | ||
| HR (95% CI) | HR (95% CI)c | PTrend | HR (95% CI) | HR (95% CI)d | PTrend | |||||
| DDE | ||||||||||
| ≤0.36 | 54 | 157 | 1 (Reference) | 1 (Reference) | 29 | 182 | 1 (Reference) | 1 (Reference) | ||
| 0.37–1.00 | 85 | 121 | 1.44 (1.02–2.05) | 1.33 (0.90–1.96) | 29 | 177 | 1.14 (0.67–1.92) | 0.99 (0.56–1.74) | ||
| ≥1.01 | 120 | 78 | 2.09 (1.46–2.99) | 1.95 (1.31–2.92) | 39 | 159 | 1.79 (1.06–3.03) | 1.50 (0.85–2.65) | ||
| Ln DDE | 1.25 (1.10–1.42) | 1.18 (1.03–1.36) | 1.27 (1.04–1.56) | 1.16 (0.94–1.43) | ||||||
| Log2(DDE) | 1.17 (1.07–1.28) | 1.12 (1.02–1.24) | 0.02 | 1.18 (1.03–1.36) | 1.11 (0.96–1.28) | 0.17 | ||||
| DDT | ||||||||||
| <LOD | 127 | 270 | 1 (Reference) | 1 (Reference) | 57 | 340 | 1 (Reference) | 1 (Reference) | ||
| 0.01–0.03 | 61 | 48 | 1.67 (1.21–2.29) | 1.61 (1.13–2.28) | 20 | 89 | 1.45 (0.86–2.45) | 1.28 (0.73–2.24) | ||
| ≥0.04 | 71 | 38 | 1.86 (1.32–2.61) | 1.64 (1.10–2.44) | 20 | 89 | 1.59 (0.91–2.78) | 1.07 (0.56–2.01) | ||
| Ln(DDT) | 1.19 (1.05–1.36) | 1.19 (1.02–1.39) | 1.26 (1.01–1.56) | 1.17 (0.91–1.51) | ||||||
| Log2(DDT) | 1.13 (1.03–1.24) | 1.13 (1.01–1.25) | 0.03 | 1.17 (1.01–1.36) | 1.12 (0.94–1.33) | 0.22 | ||||
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993–1996 (Phase I) and followed-up for vital status through December 31, 2016. Conditional analyses are among women who survived >5 years.
Adjusted for age at diagnosis (continuous in years) and race (black vs. white).
Adjusted for age at diagnosis (continuous in years), race (black vs. white), smoking status (never, former, and current smoker), education (less than high school, high school, and college or greater), BMI (<25.0, 25.0–29.9, ≥30.0 kg/m2), parity/lactation history (nulliparous, parous/never lactated, parous/ever lactated), stage (III/IV vs. I/II), and ER status (ER+ vs. ER−).
Cox models based on 333 deaths and 313 censored observations due to missing covariate data.
Cox models based on 183 deaths and 463 censored observations due to missing covariate data.
Table 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between plasma levels of lipid-standardized DDE and DDT levels and 20-year conditional breast cancer-specific mortality in the CBCS Phase I women diagnosed with invasive breast cancer in 1993–1996 (n=615), stratified by race and ER status.
| DDE (μg/g lipid) |
Median | 20-year Conditional Breast Cancer-Specific Mortality | DDT (μg/g lipid) |
20-year Conditional Breast Cancer-Specific Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths (n=97) |
Censored (n=518) |
Model 1a | Model 2b | Deaths (n=97) |
Censored (n=518) |
Model 1a | Model 2b | |||||
| HR (95% CI) | HR (95% CI)c | PInt.d | HR (95% CI) | HR (95% CI)c | PInt.d | |||||||
| Overall | Overall | |||||||||||
| ≤0.58 | 0.284 | 40 | 273 | 1 (Reference) | 1 (Reference) | <LOD | 57 | 340 | 1 (Reference) | 1 (Reference) | ||
| >0.58 | 1.41 | 57 | 245 | 1.77 (1.15–2.72) | 1.69 (1.06–2.68) | ≥LOD | 40 | 178 | 1.52 (0.98–2.36) | 1.22 (0.75–1.98) | ||
| Ln(DDE) | 1.27 (1.04–1.56) | 1.16 (0.94–1.43) | Ln(DDT) | 1.26 (1.01–1.56) | 1.17 (0.91–1.51) | |||||||
| Log2(DDE) | 1.18 (1.03–1.36) | 1.11 (0.96–1.28) | Log2(DDT) | 1.17 (1.01–1.36) | 1.12 (0.94–1.33) | |||||||
| Race | 0.42 | Race | 0.70 | |||||||||
| White | White | |||||||||||
| ≤0.58 | 0.254 | 32 | 222 | 1 (Reference) | 1 (Reference) | <LOD | 41 | 269 | 1 (Reference) | 1 (Reference) | ||
| >0.58 | 1.03 | 21 | 113 | 1.56 (0.89–2.74) | 1.57 (0.86–2.89) | ≥LOD | 12 | 66 | 1.67 (0.87–3.21) | 1.37 (0.66–2.83) | ||
| Ln(DDE) | 1.16 (0.87–1.55) | 1.10 (0.81–1.51) | Ln(DDT) | 1.42 (0.92–2.18) | 1.33 (0.84–2.12) | |||||||
| Log2(DDE) | 1.11 (0.91–1.35) | 1.07 (0.86–1.33) | Log2(DDT) | 1.27 (0.94–1.71) | 1.22 (0.88–1.68) | |||||||
| Black | Black | |||||||||||
| ≤0.58 | 0.366 | 8 | 51 | 1 (Reference) | 1 (Reference) | <LOD | 16 | 71 | 1 (Reference) | 1 (Reference) | ||
| >0.58 | 1.89 | 36 | 132 | 2.49 (1.12–5.51) | 2.36 (1.03–5.42) | ≥LOD | 28 | 112 | 1.46 (0.77–2.77) | 1.31 (0.62–2.73) | ||
| Ln(DDE) | 1.49 (1.08–2.06) | 1.30 (0.93–1.82) | Ln(DDT) | 1.24 (0.94–1.65) | 1.20 (0.85–1.69) | |||||||
| Log2(DDE) | 1.32 (1.06–1.65) | 1.20 (0.95–1.51) | Log2(DDT) | 1.16 (0.96–1.41) | 1.13 (0.89–1.44) | |||||||
| ER Status | 0.03 | ER Status | 0.76 | |||||||||
| ER+ | ER+ | |||||||||||
| ≤0.58 | 0.284 | 29 | 144 | 1 (Reference) | 1 (Reference) | <LOD | 37 | 180 | 1 (Reference) | 1 (Reference) | ||
| >0.58 | 1.45 | 32 | 142 | 1.29 (0.75–2.21) | 1.29 (0.73–2.28) | ≥LOD | 24 | 106 | 1.31 (0.74–2.32) | 1.18 (0.62–2.26) | ||
| Ln(DDE) | 1.12 (0.87–1.45) | 1.07 (0.83–1.38) | Ln(DDT) | 1.26 (0.94–1.69) | 1.11 (0.81–1.53) | |||||||
| Log2(DDE) | 1.08 (0.91–1.29) | 1.05 (0.88–1.25) | Log2(DDT) | 1.17 (0.96–1.44) | 1.07 (0.86–1.34) | |||||||
| ER− | ER− | |||||||||||
| ≤0.58 | 0.287 | 9 | 110 | 1 (Reference) | 1 (Reference) | <LOD | 16 | 131 | 1 (Reference) | 1 (Reference) | ||
| >0.58 | 1.51 | 21 | 79 | 3.25 (1.43–7.38) | 3.24 (1.38–7.58) | ≥LOD | 14 | 58 | 1.96 (0.91–4.18) | 1.43 (0.64–3.19) | ||
| Ln(DDE) | 1.66 (1.16–2.39) | 1.55 (1.04–2.30) | Ln(DDT) | 1.30 (0.91–1.87) | 1.30 (0.86–1.97) | |||||||
| Log2(DDE) | 1.42 (1.11–1.83) | 1.35 (1.03–1.78) | Log2(DDT) | 1.20 (0.94–1.54) | 1.20 (0.90–1.60) | |||||||
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993–1996 (Phase I) and followed-up for vital status through December 31, 2016. Conditional analyses are among women who survived >5 years.
Adjusted for age at diagnosis (continuous in years) and race (black vs. white), as appropriate.
Adjusted for age at diagnosis (continuous in years), race (black vs. white), smoking status (never, former, and current smoker), education (less than high school, high school, and college or greater), BMI (<25.0, 25.0–29.9, ≥30.0 kg/m2), parity/lactation history (nulliparous, parous/never lactated, parous/ever lactated), stage (III/IV vs. I/II), and ER status (ER+ vs. ER−), as appropriate.
Cox models based on 183 deaths and 463 censored observations due to missing covariate data.
P for multiplicative interaction from Cox regression models using continuous log2-transformed lipid-standardized DDE/DDT levels.
The highest (vs. non-detectable) quantile of DDT was associated with a HR of 1.59 (95%CI=0.91–2.78) for 20-year conditional breast cancer-specific mortality in models adjusted for age and race, but this association was attenuated (HR=1.07, 95%CI=0.56–2.01) after full covariate adjustment. We observed little evidence of effect measure modification for DDT by race and ER status in full covariate-adjusted models (Table 3). However, the HRs adjusted for all covariates except ER status were slightly more elevated among black women (HR=1.48, 95%CI=0.73–2.99) than among white women (HR=1.43, 95%CI=0.71–2.86), though not after adjustment for ER status (HR among black women=1.31, 95%CI=0.62–2.73 vs. HR among white women=1.37, 95%CI=0.66–2.83) (PInteraction=0.70). Furthermore, HRs were slightly more elevated among women with ER− tumors (HR=1.43, 95%CI=0.64–3.19) than among women with ER+ tumors (HR=1.18, 95%CI=0.62–2.26) in full covariate-adjusted models (PInteraction=0.76).
Results using wet weight concentrations with and without covariate adjustment for lipids were similar to those in which we used lipid-standardized concentrations (Supplemental Table 2) with two notable exceptions. First, the HRs for 5-year breast cancer-specific mortality for the highest (versus lowest) tertiles of DDE were 1.19 (95%CI=0.67–2.12) in lipid-adjusted models, 1.13 (95%CI=0.64–1.99) in lipid-unadjusted models, and 1.01 (95%CI=0.58–1.77) in lipid-standardized models. Second, the HRs for 20-year breast cancer-specific mortality for the highest quantile (vs. non-detectable) of DDT were associated with HRs of 1.35 (95%CI=0.72–2.51) in lipid-adjusted models, 1.30 (95%CI=0.70–2.41) in lipid-unadjusted models, and 1.07 (95%CI=0.56–2.01) in lipid-standardized models.
All-Cause Mortality
From the Kaplan-Meier curves, increasing DDE and DDT levels were associated with worse overall survival (Figure 1). In the Cox regression analyses lipid-standardized DDE and DDT levels were not associated with 5-year all-cause mortality; however, the highest (vs. lowest) DDE tertile and the highest detectable (vs. undetectable) DDT quantile were associated with HRs for 20-year conditional all-cause mortality of 1.95 (95%CI=1.31–2.92) and 1.64 (95%CI=1.10–2.44), respectively, after full covariate adjustment (Table 2). These associations exhibited dose-response relationships as supported by the trend analyses. A one-unit increase in ln-transformed DDE levels was associated with an 18% increase in the rate of all-cause mortality [ln(DDE) HR=1.18, 95%CI=1.03–1.36], while a doubling of levels was associated with an 12% increase in the rate of all-cause mortality [log2(DDE) HR=1.12, 95%CI=1.02–1.24] (PTrend=0.02). A one-unit increase in ln-transformed DDT levels was associated with an 19% increase in the rate of all-cause mortality [ln(DDT) HR=1.19, 95%CI=1.02–1.39], while a doubling of levels was associated with an 13% increase in the rate of all-cause mortality [log2(DDT) HR=1.13, 95%CI=1.01–1.25] (PTrend=0.03). As shown in Supplemental Table 3, patterns of effect measure modification by race for all-cause mortality were similar to those for breast cancer-specific mortality. However, there was little evidence of effect measure modification by race for DDT and all-cause mortality.
Discussion
In this study of organochlorine compounds DDE and DDT and survival following breast cancer, we observed higher rates of long-term (i.e., 20 years post-diagnosis) all-cause and breast cancer-specific mortality among women with high lipid-standardized plasma DDE levels compared to women with low levels. Furthermore, in stratified analyses, breast cancer-specific mortality rates were elevated 136% among black women, but only elevated 57% among white women, and elevated 224% among with ER− tumors, but elevated only 29% among women with ER+ tumors. Detectable versus undetectable levels of DDT were also associated with increased rates of long-term all-cause mortality, but not breast cancer-specific mortality.
Few studies have prospectively investigated organochlorine pesticides in association with all-cause or breast cancer-specific mortality following breast cancer (16,17,20), and to our knowledge, no studies have examined these exposures within the context of racial breast cancer survival disparities. In the study by Høyer et al. of overall, but not breast cancer-specific survival among 195 Danish women with breast cancer, women with the highest versus lowest DDE quartile had a two-fold increase in overall mortality risk over a median six year follow-up (17). However, this association was evident only in models in which a second blood sample collected five years after enrollment was considered, and the association was attenuated in models adjusted for tumor characteristics. In a follow-up to their study, Høyer et al. examined organochlorine compounds and overall survival according to ER status (34). The association between DDE and overall breast cancer survival did not vary by ER status; however, their follow-up study was underpowered to examine effect modification by ER status as only 161 (n=116 ER+ and n=45 ER−) women were included. In a second study of 633 predominantly Caucasian US women with breast cancer, the highest versus lowest DDT tertile was associated with HRs for all-cause and breast cancer-specific mortality of 2.19 and 2.72, respectively, at five years post-diagnosis; however, the associations were attenuated 15 years post-diagnosis (16), which was not consistent with the pattern for DDT in our study. Also, in contrast to our findings reported here, in that study, the third versus first DDE tertile was inversely associated with 15-year all-cause and breast cancer-specific mortality (16). A recently published study of organochlorine compounds measured in adipose tissue from 399 postmenopausal Danish women and breast cancer survival reported inverse association between increasing DDE/DDT levels and overall mortality over a median follow-up of eight years (20), also in contrast to our findings reported. Reasons for the inconsistencies between previous studies and our results reported here may be due in part to the differences in the exposure distributions and to the type of blood sample used. In the study by Parada et al., 92% of women had detectable DDT levels (16), whereas only 37% of women in our study had detectable DDT levels. Furthermore, women in our study had the highest mean lipid-standardized DDE levels (mean=1.96 μg/lipid) as compared to women in the studies by Parada et al. (mean=0.672 μg/lipid) (16) and Høyer et al. (mean=1.38 μg/lipid) (17). However, we measured DDE and DDT in plasma, whereas Parada et al. and Høyer et al. used serum samples. A previous study comparing organic compound concentrations measured in various types of blood samples from adult volunteers reported consistently higher compound concentrations in plasma as compared to whole blood and dried blood spot samples (35).
Our results for DDE of higher rates of breast cancer-specific mortality and a larger attributable fraction for DDE among black women than among white women may in part be explained by differences in the prevalence or magnitude of exposure or to differences in dietary sources of exposure to DDE/DDT. We observed higher and more extreme DDE levels in black women than among white women, which were associated with worse mortality in black women than among white women thus providing evidence that the excess for breast cancer mortality among black women may have a contribution from DDT/DDE exposure. Furthermore, our race-stratified models, which were adjusted for ER status, suggest that racial differences in mortality may not be driven entirely by ER status, and vice versa. However, we also note that the ER− basal-like intrinsic subtype is more prevalent among black women compared to white women (24). Thus, the impact of DDE and ER− disease on breast cancer mortality may be synergistic, providing further evidence that DDE exposure contributes to poor breast cancer survival among black women.
As DDT and DDE are endocrine disrupting chemicals (5) and probable human carcinogens (3,4), several biological mechanisms may explain the associations observed here. In vivo and in vitro laboratory evidence shows that p,p’-DDE is not estrogenic (36), but is able to bind to and inhibit androgen binding to the androgen receptor (AR) (37). Previous studies have hypothesized that anti-androgenic chemicals may inhibit breast cancer progression (38), as the AR dimerizes and translocates to the nucleus upon binding of androgen ligands where it binds to androgen response elements (AREs) to promote target gene transcription (39). In the CBCS, we do not have information on AR status; future studies should consider the emerging role of the androgen receptor in the DDE-breast cancer-specific mortality association. Our findings for DDE by ER status are not consistent with mechanisms of endocrine disruption; however, it is possible that DDE/DDT may impact prognosis via other mechanisms including oxidative stress, inflammation, and immune suppression (2). However, in this observational study, we cannot rule out non-causal explanations including random error and bias due to uncontrolled confounding and other sources of error.
Previous studies of women with breast cancer (16,17), as well one study of a national representative sample of US adults 60 years or older (40), have reported increased rates of overall mortality among women with high DDT levels, consistent with our findings reported here. While it is well-established that p-p’-DDT is able to bind to the ER (36,41,42), and thus stimulate tumor growth and metastasis in breast cancer, in this study, we observed only modest increases in risk of breast cancer-specific mortality in association with dichotomized DDT. One possible reason for these discrepancies is that most women in the CBCS had non-detectable DDT levels and thus exposure misclassification for DDT, if non-differential with respect to mortality, could have resulted in estimates that were biased towards the null for women of both races. While women in our study were born from 1918–1971, and thus were likely to have been exposed to DDT directly, blood samples were collected in the 1990’s. Thus, it is likely that most of the measured DDE was ingested as DDE rather than DDT.
Ours is the largest study to date to prospectively examine the association between DDE/DDT and survival following breast cancer. Additionally, our follow-up of 20+ years allowed us to examine associations with long-term survival, and while previous studies (16,17) have focused on Caucasian women, by design, the CBCS oversampled black women, which allowed us to examine associations by race. Furthermore, ours is the largest study to date to examine these associations by ER status. However, our study had several limitations. First, we relied on a single blood sample measurement taken shortly after diagnosis to examine associations with mortality up to 20+ years post-diagnosis and we only considered two organochlorine compounds. In the study by Høyer et al., repeated exposure assessment improved the accuracy of the measurement (17). However, DDT residues have biological half-lives ranging from 5–10 years (43,44) and therefore a single measurement may be sufficient to characterize these associations. Additionally, blood samples drawn after diagnosis may also be impacted by breast cancer treatment. However, in the CBCS case-control design, blood DDT/DDE levels were not different when stratified by weight loss or gain, as well as stage of disease (22). Second, although the NDI provides high ascertainment of mortality there is a potential for misclassification of deaths and cause of death. This is likely to be a minor limitation as the sensitivity and specificity of NDI search are estimated to be 98% and approximately 100%, respectively (45). Third, for breast cancer-specific survival estimates, we assumed that censoring was non-informative and we did not consider causes of death other than breast cancer (46). However, our approach accurately estimates the relative hazard (rate) of breast cancer-specific death and thus addresses how death from breast cancer is associated with DDE and DDT (46). Last, the production and use of DDT was banned in the US in 1972 and raises the question of whether these low-level exposures remain relevant to US women diagnosed with breast cancer, though high-level exposure is relevant to women who live in parts of the world where DDT is still actively used for vector control (47). While worldwide DDT and DDE levels have declined since the restriction of DDT production and use (48), diet remains an important source of exposure, albeit at low levels (49) and our results reported here indicate that even at low levels, exposure may be a cause for concern.
Conclusion
In conclusion, in this study of organochlorine compounds DDE and DDT and breast cancer survival among US white and black women, high versus low levels of DDE and DDT were associated with increased rates of long-term all-cause and breast cancer-specific mortality. Furthermore, results of our study indicate that exposure to DDE, but not DDT, may contribute to the racial disparities in breast cancer survival. Breast cancer-specific mortality rates comparing high versus low DDE levels were elevated more so among black women than among white women and among women with ER− tumors than among women with ER+ tumors. Because exposure to DDE/DDT may adversely impact overall and breast cancer-specific survival, future studies should consider the impact of other organochlorine compounds, and in particular more contemporary chemicals, alone or in combination, on survival following breast cancer.
Supplementary Material
Highlights.
Higher DDE and DDT levels were associated with worse overall survival
Higher DDE levels were associated with worse breast cancer-specific survival
Mortality rates were elevated more so among black women than among white women
Mortality rates were elevated more so among women with ER− than ER+ breast cancer
Funding:
This research was funded in part by the University Cancer Research Fund of North Carolina and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA 58223), and by the National Institute of Environmental Health Sciences (NIEHS T32 ES007018).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare they have no conflict of interest.
Ethical standards: All procedures performed in the Carolina Breast Cancer Study involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of North Carolina at Chapel Hill and were in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical approval: Informed consent was obtained from all individual participants included in the study.
References
- 1.US EPA. DDT - A Brief History and Status. 2017;(https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status). (Accessed July 12, 2018)
- 2.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for DDT, DDE, and DDD. Atlanta, GA: 2002(http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=81&tid=20) [PubMed] [Google Scholar]
- 3.Loomis D, Guyton K, Grosse Y, et al. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet. 2015;16:891–2. [DOI] [PubMed] [Google Scholar]
- 4.US EPA. p,p’-Dichlorodiphenyldichloroethylene (DDE); CASRN 72– 55-9. 1988;(https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=328). (Accessed October 25, 2018)
- 5.Longnecker MP, Rogan WJ, Lucier G. The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu. Rev. Public Health. 1997;18:211–44. [DOI] [PubMed] [Google Scholar]
- 6.Unger M, Kiaer H, Blichert-Toft M, et al. Organochlorine compounds in human breast fat from deceased with and without breast cancer and in a biopsy material from newly diagnosed patients undergoing breast surgery. Env. Res 1984;34(1):24–8. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA. Cancer J. Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 8.Wolff MS, Paolo G, Lee EW, et al. Blood levels of organochlorine residues and risk of breast cancer. J. Natl. Cancer Inst 1993;85(8):648–652. [DOI] [PubMed] [Google Scholar]
- 9.Krieger N, Wolff MS, Hiatt RA, et al. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J. Natl. Cancer Inst 1994;86(8):589–99. [DOI] [PubMed] [Google Scholar]
- 10.López-Cervantes M, Torres-Sánchez L, Tobías A, et al. Dichlorodiphenyldichloroethane burden and breast cancer risk: a meta-analysis of the epidemiologic evidence. Environ. Health Perspect 2004;112(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingber SZ, Buser MC, Pohl HR, et al. DDT/DDE and breast cancer: a meta-analysis. Regul. Toxicol. Pharmacol 2013;67(3):421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn B a., Merrill M La, Krigbaum NY, et al. DDT Exposure in Utero and Breast Cancer. J. Clin. Endocrinol. Metab 2015;100(8):2865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohn BA, Wolff MS, Cirillo PM, et al. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ. Health Perspect 2007;115(10):1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. [DOI] [PubMed] [Google Scholar]
- 15.Danielsen PH, Møller P, Jensen KA, et al. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chem. Res. Toxicol 2011;24(2):168–84. [DOI] [PubMed] [Google Scholar]
- 16.Parada H, Wolff MS, Engel LS, et al. Organochlorine insecticides DDT and chlordane in relation to survival following breast cancer. Int. J. Cancer. 2016;138(3):565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Høyer AP, Jørgensen T, Brock JW, et al. Organochlorine exposure and breast cancer survival. J. Clin. Epidemiol 2000;53(3):323–330. [DOI] [PubMed] [Google Scholar]
- 18.Muscat JE, Britton JA, Djordjevic MV., et al. Adipose Concentrations of Organochlorine Compounds and Breast Cancer Recurrence in Long Island, New York. Cancer Epidemiol. Biomarkers Prev 2003;12(12):1474–1478. [PubMed] [Google Scholar]
- 19.Parada H, Wolff MS, Engel LS, et al. Polychlorinated biphenyls and their association with survival following breast cancer. Eur. J. Cancer. 2016;56:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roswall N, Sørensen M, Tjønneland A, et al. Organochlorine concentrations in adipose tissue and survival in postmenopausal, Danish breast cancer patients. Environ. Res 2018;163:237–48. [DOI] [PubMed] [Google Scholar]
- 21.Davies JE, Edmundson WF, Raffonelli A, et al. The role of social class in human pesticide pollution. Am. J. Epidemiol 1972;96(5):334–41. [DOI] [PubMed] [Google Scholar]
- 22.Millikan R, DeVoto E, Duell EJ, et al. Dichlorodiphenyldichloroethene, polychlorinated biphenyls, and breast cancer among African-American and white women in North Carolina. Cancer Epidemiol. Biomarkers Prev 2000;9(11):1233–40. [PubMed] [Google Scholar]
- 23.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015. (https://seer.cancer.gov/csr/1975_2015/,based on November 2017 SEER data submission, posted to the SEER web site, April 2018.). (Accessed July 8, 2018)
- 24.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502. [DOI] [PubMed] [Google Scholar]
- 25.Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res. Treat 1995;35(1):51–60. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. National Death Index. 2017;(http://www.cdc.gov/nchs/ndi.htm). (Accessed June 26, 2018)
- 27.Phillips DL, Pirkle JL, Burse VW, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol 1989;18(4):495–500. [DOI] [PubMed] [Google Scholar]
- 28.López-Carrillo L, Torres-Sánchez L, López-Cervantes M, et al. The adipose tissue to serum dichlorodiphenyldichloroethane (DDE) ratio: some methodological considerations. Environ. Res 1999;81(2):142–5. [DOI] [PubMed] [Google Scholar]
- 29.Efron B The efficiency of Cox’s likelihood function for censored data. J. Am. Stat. Assoc 1977;72(359):557–565. [Google Scholar]
- 30.Schisterman EF, Whitcomb BW, Buck Louis GM, et al. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ. Health Perspect. 2005;113(7):853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muñoz-de-Toro M, Durando M, Beldoménico PM, et al. Estrogenic microenvironment generated by organochlorine residues in adipose mammary tissue modulates biomarker expression in ERα-positive breast carcinomas. Breast Cancer Res 2006;8(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hertzmark E, Wand H, Spiegelman D. The SAS PAR Macro. 2012. 1–25 p.(https://www.hsph.harvard.edu/donna-spiegelman/software/par/)
- 33.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–579. [DOI] [PubMed] [Google Scholar]
- 34.Høyer AP, Jorgensen T, Rank F, et al. Organochlorine exposures influence on breast cancer risk and survival according to estrogen receptor status: a Danish cohort-nested case-control study. BMC Cancer. 2001;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batterman SA, Chernyak S, Su F. Measurement and comparison of organic compound concentrations in plasma, whole blood, and dried blood spot samples. Front. Genet 2016;7(64):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welch RM, Levin W, Conney AH. Estrogenic action of DDT and its analogs. Toxicol. Appl. Pharmacol 1969;14:358–367. [DOI] [PubMed] [Google Scholar]
- 37.Kelce WR, Stone CR, Laws SC, et al. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature. 1995;375(6532):581–5. [DOI] [PubMed] [Google Scholar]
- 38.Parada H, Gammon MD, Chen J, et al. Urinary phthalate metabolite concentrations and breast cancer incidence and survival following breast cancer: The Long Island Breast Cancer Study Project. Environ. Health Perspect 2018;126(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aleskandarany MA, Abduljabbar R, Ashankyty I, et al. Prognostic significance of androgen receptor expression in invasive breast cancer: transcriptomic and protein expression analysis. Breast Cancer Res. Treat 2016;159(2):215–27. [DOI] [PubMed] [Google Scholar]
- 40.Fry K, Power MC. Persistent organic pollutants and mortality in the United States, NHANES 1999–2011. Environ. Heal 2017;16(105):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bustos S, Denegri JC, Diaz F, et al. p,p’-DDT is an estrogenic compound. Bull. Environ. Contam. Toxicol 1988;41(4):496–501. [DOI] [PubMed] [Google Scholar]
- 42.Robison AK, Sirbasku DA, Stancel GM, et al. DDT supports the growth of an estrogen-responsive tumor. Toxicol. Lett [electronic article]. 1985;27(1):109–113. (http://www.sciencedirect.com/science/article/pii/0378427485901274). (Accessed January 20, 2014) [DOI] [PubMed] [Google Scholar]
- 43.Wolff MS. Half-lives of organochlorines (OCs) in humans. Arch. Environ. Contam. Toxicol 1999;36(4):504. [DOI] [PubMed] [Google Scholar]
- 44.Wolff MS, Britton JA, Teitelbaum SL, et al. Improving organochlorine biomarker models for cancer research. Cancer Epidemiol. biomarkers Prev 2005;14(9):2224–36. [DOI] [PubMed] [Google Scholar]
- 45.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax nationwide death search. Am. J. Epidemiol 1994;140(11):1016–9. [DOI] [PubMed] [Google Scholar]
- 46.Austin PC, Lee DS, Fine JP, et al. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. The use of DDT in malaria vector control. World Health Organization; 2011(http://www.who.int/malaria/publications/atoz/who_htm_gmp_2011/en/) [Google Scholar]
- 48.Smith D Worldwide trends in DDT levels in human breast milk. Int. J. Epidemiol 1999;28(2):179–188. [DOI] [PubMed] [Google Scholar]
- 49.Schafer KS, Kegley SE. Persistent toxic chemicals in the US food supply. J. Epidemiol. Community Health. 2002;56(11):813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.