Watch a video presentation of this article
Watch the interview with the author
Alcohol consumption causes 3.8% mortality worldwide.1 Because alcoholic liver disease (ALD) is a leading cause of preventable death, it is imperative to detect moderate ALD and arrest its progression.
For the purposes of this article, we define patients with moderate ALD as those who have recovered from severe alcoholic hepatitis, have early signs of ALD, or have very early compensated cirrhosis. Multiple grading systems are available for determining the severity and prognosis of ALD, and patients with moderate ALD generally have a Maddrey discriminant function score <32 or a Model for End‐Stage Liver Disease score <21. Although they may not have immediately life‐threatening problems, a great opportunity exists to improve their modifiable risk factors, address their underdiagnosed malnutrition, and consider whether they would benefit from pharmacotherapy (Fig. 1).
Figure 1.
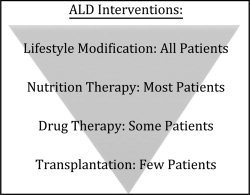
Interventions for moderate ALD patients lay on a continuum. All patients should control the modifiable risk factors of alcohol use, obesity, and smoking. Most patients will benefit from treatment for malnutrition. Some patients may need pharmacotherapy. The few patients who will be transplant candidates are those with severely decompensated ALD, which is not discussed here.
Many patients will be clinically evident with signs and symptoms of liver disease, whereas others will be asymptomatic at the time of diagnosis. Although not a prerequisite for the development of ALD, screening for alcohol abuse and/or dependency may detect an at‐risk population of patients. The Alcohol Use Disorders Identification Test (AUDIT) can identify risky alcohol use (score ≥8 for men up to age 60 or ≥4 for women, adolescents, or men over age 60) and alcohol dependence (score ≥20).1 Prior to complications of ALD, early ALD is clinically diagnosed in patients with a history of significant alcohol use combined with objective findings. Physical examination findings can be nonspecific, but most commonly hepatomegaly is present. Likewise, laboratory findings can be insensitive, but up to 80% of patients with ALD will have an aspartate aminotransferase/alanine aminotransferase ratio of >2.2 Other indicative laboratory findings include leukocytosis, thrombocytopenia, prolongation of prothrombin time, and hypoalbuminemia.
Extrahepatic manifestations of more advanced ALD include spider angiomata, latent portosystemic encephalopathy, hepatopulmonary syndrome, hypertriglyceridemia, and electrolyte abnormalities such as hypokalemia and hypomagnesemia. A complete physical examination and basic laboratory evaluation to screen for these common derangements should be part of routine ALD care.
Abbreviations.
ALD, alcoholic liver disease; MUST, Malnutrition Universal Screening Tool; SAMe, S‐adenosylmethionine.
Lifestyle Modification
When managing a patient with ALD, steps should be taken to achieve alcohol cessation. Many studies have shown that patients who quit drinking have improved survival; moreover, even cutting back on alcohol consumption can lead to some improvement in liver disease.3 Brief interventions, during which a patient has regular conversations with a nurse or physician focusing on feedback, responsibility, advice, empathy, and optimism, have been shown to reduce drinking.4 Patients should be encouraged to consider behavioral programs such as Alcoholics Anonymous. Although it is difficult to obtain controlled outcome data for organizations whose members remain anonymous, the Alcoholics Anonymous 2007 self‐reported membership survey reported that 33% of their members had been sober for more than 10 years, and the average sobriety of their members is more than 8 years.5 For patients who continue to crave alcohol despite undergoing brief interventions and attending behavioral programs, pharmacologic adjuncts can be offered.
Trials examining the effectiveness of medical therapy for alcohol abuse have lacked consistent endpoints, and the results have not always been reproducible.3 Naltrexone and baclofen both have shown benefit and failure in different clinical trials. Baclofen is the only drug for alcohol dependence currently under investigation that has good safety data in patients with cirrhosis, making it a reasonable first‐line choice in this patient population.6 Naltrexone has a black box warning for hepatotoxicity and is contraindicated in acute hepatitis or hepatic failure. Topiramate is a relatively new therapy for alcohol abuse and has not yet been tested in a double‐blind randomized trial with alcohol abstinence as an endpoint in patients with moderate ALD.7
The COMBINE Study showed that a multifaceted approach results in a higher number of days of abstinence from alcohol. Patients who met with a physician and also either took naltrexone or underwent combined behavioral intervention did better than those who only met with a physician and received a placebo.8
Cigarette smoking9 and obesity10 are both independent risk factors for fibrosis in ALD and must also be addressed. Although a patient may fit the definition of obesity (body mass index >30), they may still have concurrent nutritional deficiencies in macronutrients (e.g., protein) or micronutrients (e.g., zinc).
Nutrition Therapy
Many patients with ALD are malnourished, and disease severity correlates with degree of malnutrition. Data from two VA Cooperative studies showed the importance of high caloric intake, as patients who consumed more calories had improved survival.11
While visceral proteins (albumin, prealbumin, and retinol binding protein) are the most common laboratory tests used to assess a patient's nutritional status, these results can be confounded by the underlying liver disease or superimposed infections. Evaluating clinical findings such as muscle wasting, edema, loss of subcutaneous fat, and glossitis/cheilosis are helpful in subjectively identifying protein energy malnutrition. Nutritional assessments of alcoholic patients can reveal adequate calorie intake. Indeed, in some studies, almost 50% of patients' energy intake was from alcohol alone, leading to deficient protein and micronutrient intake.12 The most common vitamin deficiencies are folate, vitamin B6, vitamin A, and thiamine. Mineral deficiencies include selenium, zinc, copper, and magnesium.13 We advise that patients take a multivitamin and be supplemented with zinc sulfate 220 mg/day, as well as magnesium oxide 400 mg/day.
There are not adequate data to definitively determine ideal nutritional support. However, American College of Gastroenterology and American Association for the Study of Liver Diseases guidelines recommend 1.2‐1.5 g/kg of protein and 35‐45 kcal/kg of body weight in patients with ALD.14 For a 175‐pound patient, that is approximately 96‐120 g/day of protein and 2,800‐3,600 cal/day. These high numbers often come as a surprise to physicians and patients alike, but it should be stressed that ALD patients are often malnourished. Therefore, efforts should be made to gain lean body weight. A bedtime snack of 700 cal with 26 g of protein (e.g., a can of nutritional supplement) can prevent nocturnal amino acid breakdown for gluconeogenesis and improve the nitrogen balance. Evening meals improve nutritional status and cell immunity and may reduce hospital admissions.15 Adherence to sodium restriction is vital in patients starting to retain fluid (peripheral edema, ascites), which is usually seen in more advanced disease. Table 1 provides a summary of nutritional recommendations for ALD patients.
Table 1.
Nutritional Recommendations for ALD Patients
| Evaluate for clinical signs of malnutrition in all ALD patients |
| Daily caloric intake: 35‐40 kcal/kg |
| Daily protein intake: 1.2‐1.5 g/kg |
| Evening snack of 700 cal and 26 g protein |
| Avoid unsaturated fats |
| Zinc sulfate 220 mg daily |
| Magnesium oxide 400 mg daily |
Gut‐derived endotoxin (lipopolysaccharide) plays a critical role in the pathogenesis of ALD. Improving the gut barrier function to prevent absorption of lipopolysaccharide is an active area of research. Fasting can impair gut barrier function. In a mouse model of ALD, unsaturated fat (corn oil/linoleic acid) worsened the gut barrier function and exacerbated ALD compared with diets rich in saturated fats.16 Similarly, zinc deficiency impaired gut barrier function in an animal model of ALD. In summary, food intake helps maintain barrier function, and alterations in dietary fat and zinc may worsen alcohol‐induced gut barrier dysfunction and endotoxemia.
Determining endpoints in nutritional support in ALD has not been studied adequately. In general, the more severe the ALD, the longer the patient will need to be replenished. Using the subjective global assessment—which is based on clinical findings such as muscle wasting, edema, loss of subcutaneous fat, and glossitis/cheilosis—could help guide changes to nutritional support.17 The Malnutrition Universal Screening Tool (MUST) has been used for alcoholic inpatients.18 Although it has not been validated prospectively, the MUST score could be used to reassess a patient's nutritional status while on nutritional support. A MUST score of 2 or more would indicate that a patient is malnourished.
Drug Therapy
Despite the prevalence and morbidity of ALD, there is no US Food and Drug Administration–approved therapy for any form of ALD. In patients who show disease progression despite alcohol cessation and efforts to improve nutritional status, off‐label drug therapy or complementary and alternative therapy may be considered (Table 2).
Table 2.
Medications and Complementary Therapy for ALD Patients
| Pentoxifylline 400 mg 3 times daily (prescription) |
| Silybum marianum (milk thistle) 200 mg 2‐3 times daily (complementary) |
| SAMe 400 mg 3‐4 times daily (complementary) |
| Probiotics as directed (complementary) |
Pentoxifylline is a nonselective phosphodiesterase inhibitor that has been shown in clinical trials to improve mortality in alcoholic hepatitis, primarily through the prevention of hepatorenal syndrome.3 Trials with pentoxifylline in patients with moderate ALD have not been performed. Because it has a very good safety profile, it may be used in patients with moderate ALD if they can tolerate the common side effect of nausea. Pentoxifylline is prescribed at a dose of 400 mg three times daily.
Basic evidence supports the use of Silybum marianum (milk thistle), and many of our patients are already taking this agent. Silybum has anti‐inflammatory and antioxidative properties resulting in antifibrotic and immunomodulating effects.19 It is safe to use in patients with liver disease and is widely used in Europe. A frequently used dose is 200 mg two to three times daily, but its efficacy has not been established in ALD.
S‐adenosylmethionine (SAMe) is a major methylating agent that has important epigenetic and anti‐inflammatory effects. In animal studies, SAMe is depleted in the early stages of ALD, leading to early fatty liver infiltration and mitochondrial damage. This damage can be reversed with SAMe supplementation.20 SAMe is available at health food stores and is recommended at a dose of 400 mg three to four times daily.
Altered intestinal bacterial composition, impaired gut barrier function, and gut‐associated endotoxemia are increasingly recognized as critical components of ALD. Probiotics are live microorganisms that, when consumed in adequate amounts, confer a health benefit to the host. There are many mechanisms by which probiotics enhance intestinal health and influence the gut‐liver axis, including modulation of the intestinal microflora, modification of intestinal barrier function, and immunomodulation. Probiotics have been shown to have beneficial effects in multiple studies in experimental ALD, and their effects are now being evaluated in a multicenter trial in ALD that is sponsored by the National Institutes of Health.21
Conclusion
Moderate ALD should be considered an illness requiring medical attention for improved survival. To best help ALD patients, physicians can increase the likelihood of alcohol cessation though brief interventions, provide specific nutrition recommendations, and add medical therapy as needed.
Potential conflict of interest: Nothing to report.
References
- 1. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57: 399‐420. [DOI] [PubMed] [Google Scholar]
- 2. Skude G, Wadstein J. Amylase, hepatic enzymes and bilirubin in serum of chronic alcoholics. Acta Med Scand 1977; 201: 53‐58. [DOI] [PubMed] [Google Scholar]
- 3. Lucey MR. Management of alcoholic liver disease. Clin Liver Dis 2009; 13: 267‐275. [DOI] [PubMed] [Google Scholar]
- 4. Lieber CS, Weiss DG, Groszmann R, Paronetto F, Schenker S. I. Veterans Affairs Coperative Study of polyenylphosphatidylcholine in alcoholic liver disease: effects on drinking behavior by nurse/physician teams. Alcohol Clin Exp Res 2003; 27: 1757‐1764. [DOI] [PubMed] [Google Scholar]
- 5.Alcoholic Anonymous. Membership Survey. New York: Alcoholic Anonymous; 2007 [cited 2012]; Available from: aa.org/pdf/products/p‐48_07 survey.pdf.
- 6. Muzyk AJ, Rivelli SK, Gagliardi JP. Defining the role of baclofen for the treatment of alcohol dependence: a systematic review of the evidence. CNS Drugs 2012; 26: 69‐78. [DOI] [PubMed] [Google Scholar]
- 7. Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Improvement of physical health and quality of life of alcohol‐dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med 2008; 168: 1188‐1199. [DOI] [PubMed] [Google Scholar]
- 8. Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006; 295: 2003‐2017. [DOI] [PubMed] [Google Scholar]
- 9. Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol 1992; 136: 1248‐1257. [DOI] [PubMed] [Google Scholar]
- 10. Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol‐induced liver disease. Hepatology 2002; 35: 635‐638. [DOI] [PubMed] [Google Scholar]
- 11. Mendenhall C, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res 1995; 19: 635‐641. [DOI] [PubMed] [Google Scholar]
- 12. Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology 1993; 17: 564‐576. [DOI] [PubMed] [Google Scholar]
- 13. Halsted CH. Nutrition and alcoholic liver disease. Semin Liver Dis 2004; 24: 289‐304. [DOI] [PubMed] [Google Scholar]
- 14. McCullough AJ, O'Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol 1998; 93: 2022‐2036. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch S, de la Maza MP, Gattas V, Barrera G, Petermann M, Gotteland M, et al. Nutritional support in alcoholic cirrhotic patients improves host defenses. J Am Coll Nutr 1999; 18: 434‐441. [DOI] [PubMed] [Google Scholar]
- 16. Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll‐like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res 2012; 36: 835‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res 2011; 35: 815‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teixeira J, Mota T, Fernandes JC. Nutritional evaluation of alcoholic inpatients admitted for alcohol detoxification. Alcohol Alcohol 2011; 46: 558‐560. [DOI] [PubMed] [Google Scholar]
- 19. Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol‐induced hepatic fibrosis in baboons. J Clin Gastroenterol 2003; 37: 336‐339. [DOI] [PubMed] [Google Scholar]
- 20. Anstee QM, Day CP. S‐adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility. J Hepatol 2012; 57: 1097‐1109. [DOI] [PubMed] [Google Scholar]
- 21. Kirpich IA, McClain CJ. Probiotics in the treatment of the liver diseases. J Am Coll Nutr 2012; 31: 14‐23. [DOI] [PubMed] [Google Scholar]


